Abstract
In lekking species, the allocation of effort into mate attraction signals is not uniform over time, and signalers may expend the greatest effort when potential mates are nearby. Close-range courtship interactions are critical determinants of male fitness and the study of these interactions can therefore answer important questions in sexual selection. In anurans, attention has largely focused on long-range mate attraction mediated by acoustic signaling. However, many species also engage in courtship behaviors at close range, and the cues that elicit these behaviors are unknown but likely to be non-acoustic. I performed an experiment in which I assessed the role of female visual cues in eliciting courtship calls by males of the nocturnal treefrog Hyla versicolor. Males that could see an approaching female were more likely to give courtship calls than those that could not. These results provide some of the first evidence for an effect of vision on calling behavior in a nocturnal anuran and demonstrate that multiple sensory modalities are involved in the final stages of mate attraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal courtship involves intense behavioral displays, and animals may regulate the expression of these often costly displays based on cues associated with the likelihood of mating (Patricelli et al. 2002). For example, displaying males may increase the expression of a costly display when receptive females are nearby (Kelso and Verrell 2002; Byrne 2008; Patricelli and Krakauer 2010). These close-range courtship interactions likely are critical determinants of male fitness and it is therefore of interest to determine the cues that lead males to increase their investment in signaling during courtship. In nocturnal anurans, this problem is not straightforward because most social interactions take place in the acoustic modality, yet females in most species do not advertise their presence by producing vocalizations of their own (Gerhardt and Huber 2002). Nonetheless, male anurans exhibit unique behaviors when females are at close range such as producing distinctive vocalizations (courtship calls) and grasping females in amplexus, implying that cues from other sensory modalities elicit these behaviors (Fellers 1979; Wells 1988). Anurans are sensitive to stimuli in multiple modalities, but little is known about how these cues are used by males in courtship (Taylor et al. 2008; Caldwell et al. 2010; Akre and Ryan 2011). Close-range courtship interactions likely have a strong impact on male fitness, and therefore, studies of sexual selection in anurans should consider the possibility that other sensory modalities, in addition to sound, play important roles in mediating these processes. Here, I describe an experiment in which I tested the role of vision on the production of courtship calls in the grey treefrog, Hyla versicolor.
The primary call type given by males in most anuran species, and thus the focus of most studies of anuran communication, is the advertisement call. Males’ advertisement calls attract females and are often involved in mediating interactions with neighboring males as well (Wells 1977; Wells and Schwartz 2006; Wells 2007). Advertisement calls generally are broadcast irrespective of cues indicating that a female is nearby. In contrast, courtship calls are a vocalization type that males typically produce only when a female is approaching at close range (Wells and Schwartz 2006; Wells 2007). The courtship calls of many anurans are modifications of the advertisement call such that calling performance reaches an extreme level (Rosen and Lemon 1974; Wells 1980; Owen and Tucker 2006). Courtship calls in H. versicolor have the same spectral and fine-temporal structure as advertisement calls but are substantially longer in duration (Fellers 1979; Wells and Taigen 1986; Klump and Gerhardt 1987). In fact, calling efforts during courtship calling approach the upper limits on acoustic signal performance measured for H. versicolor (Reichert and Gerhardt 2012). The increase in call duration during courtship calls corresponds to female preferences for longer duration calls (Klump and Gerhardt 1987; Gerhardt et al. 1996). However, the substantial energetic costs that are likely to be associated with courtship calling render long-term production of courtship calls unsustainable (Wells and Taigen 1986). Thus, individuals are expected to limit production of courtship calls to contexts in which they are especially likely to be effective; that is, when there are cues indicating the presence of a receptive female.
Previous studies of anurans demonstrated that males increase vocal production in the presence of females, although the particular cues used by males to detect female presence are rarely described (Morris and Yoon 1989; Byrne 2008; Akre and Ryan 2011). Visual cues are an especially likely candidate because females make conspicuous movements during phonotaxis (Rheinlaender et al. 1979), frogs are highly sensitive to motion (Ewert 2004), and several studies have demonstrated the importance of vision in mate choice in nocturnal anurans (Taylor et al. 2008; Gomez et al. 2009; Gomez et al. 2010; Sztatecsny et al. 2010). I tested whether vision plays a role in eliciting courtship calls in male H. versicolor by recording their vocal behavior in an experiment in which I manipulated their ability to see an approaching female. This experiment is one of the first tests of the importance of nocturnal vision on male mating behaviors in an anuran and provides important information on the cues used in the critical final stages of courtship.
Methods
Subject males and females were captured from local ponds in Boone County, Missouri (USA). Gravid females were captured in amplexus and maintained at 4 °C to prevent oviposition prior to testing. On testing days, I released 20–80 males at approximately 1,600 h from a temporary animal-care facility into an artificial pond located within a greenhouse (details on the artificial pond facility are given by Schwartz et al. 2001; Reichert and Gerhardt 2011). I stimulated males with an artificial rainstorm and broadcasts of synthetic chorus noise, which generally resulted in strong nightly choruses. I chose test subject males from among these chorusing individuals. I performed experiments between 2100 and 0100 hours from May to July of 2011.
Male test subjects were placed into a wire-mesh cage and transported to an arena located 2 m outside of the artificial pond. The pond was surrounded by opaque cloth that prevented test subjects from obtaining visual cues from any frog in the pond. The arena itself consisted of a PVC pipe skeleton surrounded by a dark cloth screen (2 m in length by 0.5 m in width). I placed the male at one end of the arena, on a platform elevated 10 cm above the ground. Female test subjects were acclimated to the testing temperature for at least 30 min. At the time of testing I placed the female beneath a cup at the opposite end of the arena from the male.
Subjects were divided randomly into two treatment groups. In the opaque treatment, a glass screen (0.5 m wide, 0.3 m tall) was placed immediately next to the male’s platform in between the platform and the female release point. The screen had an opaque backing that effectively blocked the male’s view of the runway and thus removed any potential visual cues from the female, and prevented the two from contacting one another. In the clear treatment, I removed the opaque backing from the screen so that only the transparent glass remained. In this treatment physical contact was again prevented, but the male could potentially see the female as she approached. Only visual cues were manipulated, thus a difference between the treatment groups indicates that vision played a role in eliciting courtship calling, irrespective of any contribution of other sensory modalities. Tests were performed at night under ambient light levels within the clear-roofed greenhouse. I did not control for irradiance and these tests were performed across nights with different ambient light conditions ranging from full moon to completely overcast; therefore, I do not attempt to specify the range of light levels at which visual detection of females may be possible. Nevertheless, the experimental light levels were likely within the range of those expected to be experienced in the field, and this experimental design is sufficient to demonstrate that males can use visual cues when ambient conditions allow.
While the female was restrained in the arena, I recorded 10 calls from the subject male to serve as a baseline of its calling behavior using a Tascam DR-680 digital-audio recorder (16-bit PCM files, 44.1 kHz sampling rate) and a Sennheiser ME-67 directional microphone mounted on a boom above the male. I then released the female by pulling on a rope attached to her container, allowing her to move freely about the arena. I recorded male calls until the female had spent 30 s at the barrier that separated her from the male. I observed female movements from a position immediately adjacent to the arena using the night-vision feature of a Sony DCR-SR85 camcorder positioned so that the entire arena floor was visible on the video display. I only included tests in the dataset for which the female showed robust phonotaxis (i.e., directed movements towards the calling male including head and body scanning; Rheinlaender et al. 1979) and the male continued calling throughout (3 of 43 experimental trials were excluded because males did not call consistently). Individual males were only tested in one of the two treatment groups (N= 19 males in the opaque treatment and N= 21 males in the clear treatment) and were only tested once within a treatment group. Some females were used in tests with multiple males, but I randomized male treatment to ensure that female identity did not bias the results.
Call analyses
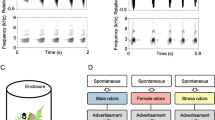
To examine courtship calling, I used Raven Pro 1.3 software (Cornell Laboratory of Ornithology) to measure the number of pulses per call in each recording. I used pulse number as a proxy for call duration because call duration itself is heavily affected by individual body temperature (Gerhardt 1978). Thus, comparisons between males recorded at different temperatures, and the development of standard criteria to define courtship calling (see below), are facilitated by using the more temperature-independent pulse numbers. I compiled these data separately for the baseline and experimental periods. Courtship calls in H. versicolor differ only quantitatively from advertisement calls (Fig. 1) and thus there is no objective criterion to differentiate the two. To ensure robust results, I used two different criteria that were both based on the observation from previous studies that courtship calls are longer in duration than normal advertisement calls (e.g., Fellers 1979). (a) For the relative threshold criterion, I defined a courtship call as any call given during the experimental period with a pulse number at least 50 % greater than the average pulse number of advertisement calls given during that same individual’s baseline calling period. (b) For the absolute threshold criterion, I defined a courtship call as any call that had a pulse number of 30 or greater. Such calls are approximately 3 standard deviations longer than average advertisement calls measured in the field (Gerhardt et al. 1996).
In addition to examining differences in the propensity to give courtship calls, I also examined whether males in the opaque and clear treatments differed in overall calling output. Thus, in addition to measuring pulse numbers, I analyzed each male’s recordings to measure two temporal call characteristics related to calling effort: (a) call duration, here measured as the time from the start to the end of the call and (b) call period, defined as the amount of time between the onsets of two consecutive calls. I averaged these call characteristics separately for each male’s baseline and experimental recordings. I performed a mixed MANOVA to examine the effects of treatment (opaque or clear, a between-subjects variable), time period (baseline or experimental recording period, a within-subjects variable), and the interaction between treatment and time period on the four dependent variables: call duration, call period, average pulse number and pulse number of the longest call. All call characteristics used in these analyses other than pulse number were temperature-corrected to 20 °C using regressions of male body temperatures on average values of each call characteristic. In order to meet the assumption of multivariate normality required for the MANOVA, I used a reciprocal transformation on both call duration and call period. I assessed whether the data met the assumptions of the MANOVA analysis using the methods of DeCarlo (1997); these assumptions were met following the reciprocal transformation described above.
Results
Males were significantly more likely to give courtship calls, defined by either criterion, when they were in the clear treatment group (Table 1; Relative criterion χ 2 = 10.6, P< 0.001; Absolute criterion χ 2 = 6.5, P= 0.011). Very few males gave courtship calls in the opaque treatment, and this proportion was similar to the proportion of males that gave courtship calls under the absolute criterion during the baseline recording period (3 of 40 males gave calls defined as courtship calls in baseline recordings). The pulse number of the longest call given in the experimental period was greater for males in the clear treatment than in the opaque treatment (Table 2; Fig. 2; t test, t 38 = 3.4, P< 0.001). There was no difference between the treatments in the average pulse number of calls given during the baseline period (Table 2; t 38 = 0.2, P= 0.83).
I found a significant effect of the interaction between treatment and time period on the four calling variables measured in this study (F 4,35 = 4.09, P= 0.008). The main effects of both time period and treatment were also significant (time period, F 4,35 = 7.71, P< 0.001; treatment: F 4,35 = 3.11, P= 0.027). When the call variables were examined individually, there were significant interaction effects between time period and treatment on the maximum pulse number (F 1,38 = 15.62, P< 0.001), average pulse number (F 1,38 = 4.69, P= 0.037) and call duration (F 1,38 = 4.50, P= 0.04), but not on call period (F 1,38 = 0.24, P= 0.63). Call durations and pulse numbers were largest during the experimental period of the clear treatment and were relatively uniform in the other three combinations of time period and treatment (Table 2).
Discussion
Male anurans attend to various aspects of their social environment, although most previous descriptions of this behavior have involved responses to acoustic cues (Wells 1988). This experiment confirms that visual cues elicit changes in calling behavior in male frogs. In particular, males were substantially more likely to give courtship calls when they were able to see an approaching female. As a result, when males could see the female, their average call durations and pulse numbers were higher than in the baseline period or in the opaque treatment in which they were unable to see an approaching female. Although average calling efforts did not differ depending on the experimental treatment, I did not continue recording after females had arrived at the barrier separating her from the male and therefore may not have been able to detect such differences in the context in which they are most likely to be expressed. It is likely that at this close range males increase their overall vocal performance, and indeed an increased calling effort following the production of courtship calls has been found in a previous study (Reichert and Gerhardt 2012). Nonetheless, the courtship calling response to visual cues is a significant behavioral difference between the two treatments and has important implications for an understanding of both anuran sensory ecology and the behaviors associated with the final stages of mate attraction.
Many recent studies emphasize that animals are responsive to cues in multiple sensory modalities (Hebets and Papaj 2005). These data add to a growing number of studies that demonstrate that vision plays a role in the behavioral interactions of nocturnal anurans (Hödl and Amézquita 2001; Amézquita and Hödl 2004; Taylor et al. 2007), a group that is better known for its acoustic communication system. Most previous experimental studies of nocturnal vision in anurans focused on female attraction to visual cues or signals produced by males (e.g., Taylor et al. 2008; Gomez et al. 2009; Taylor et al. 2011). I found that males were highly responsive to visual cues associated with an approaching female and altered their calling behavior in a way that is likely to be even more attractive to the female. Similarly, Akre and Ryan (2011) found that male túngara frogs increased the production of costly ‘chuck’ notes in response to what were likely visual cues associated with female movements during phonotaxis. Thus, there is now evidence that both sexes not only are attentive to and evaluate properties of acoustic signals, but also that they are responsive to visual cues. These findings open the door for future studies of interactions between the two modalities. In particular, based on the results of this study, it will be interesting to determine the timing relationship between female movements and male courtship calling, the threshold, in terms of amount of movement or distance to the signaler, at which female cues elicit courtship calling behavior, and the effects of such variables as ambient light levels and the density of competitors on the likelihood that female cues elicit courtship calling.
Female mate preferences are often tested in playback tests that measure a female’s response to the broadcast of synthetic signals (Gerhardt and Huber 2002). These tests are assumed to measure a proxy for female mate choice because similar female behavior in a natural chorus normally results in her mating with the calling male towards whom she moves (Gerhardt 1982). I do not question the utility of phonotaxis tests, but my data underscore the additional complications that occur in natural interactions that are less easily simulated by simpler laboratory procedures. Specifically, I found that males are highly responsive to their surroundings and are especially attentive to visual cues in a way that an approaching female is likely to elicit an abrupt change in male calling behavior including the production of courtship calls and an overall increased vocal performance (see also Reichert and Gerhardt 2012). The effects such behavior might have on mating preferences measured in standard laboratory phonotaxis tests are unknown, but potentially of great importance to the interpretation of female preference functions. Specifically, preference functions are often based on the time a female takes to approach a target signal (e.g., Bush et al. 2002). My data suggest that in a natural context, females approaching a signaling male would elicit courtship calls. These courtship calls, being especially attractive to females (Klump and Gerhardt 1987), may alter the speed with which a female approaches the signaling male. These effects may be particularly pronounced for males with advertisement calls that are generally less attractive to females. Previous studies of female preference functions indicate that females’ preferences are asymmetrically biased against less attractive calls (Gerhardt et al. 2000; Schwartz et al. 2002); in other words, a highly unattractive male may benefit to a greater extent from a given amount of change in a call characteristic in the direction of the female preference than would a male whose calls were initially more attractive. Because of this, courtship calling may be especially beneficial to males with relatively unattractive advertisement calls. If males can detect females by visual cues, their courtship call production may therefore tend to equalize female approach times in the natural context, suggesting that preference functions measured in the laboratory may over-exaggerate female preferences as they are expressed in nature. This in turn could be a partial explanation for the common observation that strong female preferences measured in the laboratory often are not detected in field studies (Dyson et al. 1992; Sullivan and Hinshaw 1992; Friedl 2006).
Finally, these data provide additional evidence that males modulate their vocal performance in response to cues in the social environment. Frogs are known to be highly sensitive to cues indicating increased competition and respond by adjusting properties of their own vocalizations (e.g., Lopez et al. 1988; Bosch and Marquez 2001; Schwartz et al. 2002; Reichert 2011b). While these responses to competition often increase the attractiveness of males’ calls to females (e.g., Wells and Schwartz 1984; Wells and Taigen 1986; Baugh and Ryan 2010; Baugh and Ryan 2011; Reichert 2011a), less attention has been given to the causes and consequences of males’ vocal responses to females themselves (Morris and Yoon 1989; Byrne 2008; Akre and Ryan 2011). The high effort courtship calls given by males are likely to be energetically costly and to limit future calling opportunities (Wells and Taigen 1986; Runkle et al. 1994), yet are extremely effective in attracting females (Klump and Gerhardt 1987). I found that males are most likely to perform such behaviors when visual cues indicating a nearby female are available. In the noisy chorus environment, males can only attract females from a limited distance; thus, a strategy of monitoring the environment for female cues and only producing the highest performance calls when females are present should balance the costs of high performance calls while maximizing the likelihood of attracting a mate.
References
Akre KL, Ryan MJ (2011) Female túngara frogs elicit more complex mating signals from males. Behav Ecol 22:846–853
Amézquita A, Hödl W (2004) How, when, and where to perform visual displays: the case of the Amazonian frog Hyla parviceps. Herpetologica 60:420–429
Baugh A, Ryan M (2011) The relative value of call embellishment in túngara frogs. Behav Ecol Sociobiol 65:359–367
Baugh AT, Ryan MJ (2010) Mate choice in response to dynamic presentation of male advertisement signals in túngara frogs. Anim Behav 79:145–152
Bosch J, Marquez R (2001) Call timing in male-male acoustical interactions and female choice in the midwife toad Alytes obstetricans. Copeia 2001:169–177
Bush SL, Gerhardt HC, Schul J (2002) Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim Behav 63:7–14
Byrne PG (2008) Strategic male calling behavior in an Australian terrestrial toadlet (Pseudophryne bibronii). Copeia 2008:57–63
Caldwell MS, Johnston GR, McDaniel JG, Warkentin KM (2010) Vibrational signaling in the agonistic interactions of red-eyed treefrogs. Curr Biol 20:1012–1017
DeCarlo LT (1997) On the meaning and use of kurtosis. Psychol Methods 2:292–307
Dyson ML, Passmore NI, Bishop PJ, Henzi SP (1992) Male behavior and correlates of mating success in a natural population of African painted reed frogs (Hyperolius marmoratus). Herpetologica 48:236–246
Ewert J-P (2004) Motion perception shapes the visual world of amphibians. In: Prete FR (ed) Complex worlds from simpler nervous systems. MIT Press, Cambridge, MA, pp 117–160
Fellers GM (1979) Aggression, territoriality, and mating behaviour in North American treefrogs. Anim Behav 27:107–119
Friedl TWP (2006) Individual male calling pattern and male mating success in the European treefrog (Hyla arborea): Is there evidence for directional or stabilizing selection on male calling behaviour? Ethology 112:116–126
Gerhardt HC (1978) Temperature coupling in the vocal communication system of the gray tree frog, Hyla versicolor. Science 199:992–994
Gerhardt HC (1982) Sound pattern recognition in some North American treefrogs (Anura: Hylidae): implications for mate choice. Am Zool 22:581–595
Gerhardt HC, Dyson ML, Tanner SD (1996) Dynamic properties of the advertisement calls of gray tree frogs: patterns of variability and female choice. Behav Ecol 7:7–18
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans. The University of Chicago Press, Chicago
Gerhardt HC, Tanner SD, Corrigan CM, Walton HC (2000) Female preference functions based on call duration in the gray tree frog (Hyla versicolor). Behav Ecol 11:663–669
Gomez D, Richardson C, Lengagne T, Derex M, Plenet S, Joly P, Léna J-P, Théry M (2010) Support for a role of colour vision in mate choice in the nocturnal European treefrog (Hyla arborea). Behaviour 147:1753–1768
Gomez D, Richardson C, Lengagne T, Plenet S, Joly P, Léna J-P, Théry M (2009) The role of nocturnal vision in mate choice: females prefer conspicuous males in the European tree frog (Hyla arborea). Proc R Soc Lond B 276:2351–2358
Hebets EA, Papaj DR (2005) Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57:197–214
Hödl W, Amézquita A (2001) Visual signaling in anuran amphibians. In: Ryan MJ (ed) Anuran communication. Smithsonian Institution Press, Washington, D.C., pp 121–141
Kelso EC, Verrell PA (2002) Do male veiled chameleons, Chamaeleo calyptratus, adjust their courtship displays in response to female reproductive status? Ethology 108:495–512
Klump GM, Gerhardt HC (1987) Use of non-arbitrary acoustic criteria in mate choice by female gray tree frogs. Nature 326:286–288
Lopez PT, Narins PM, Lewis ER, Moore SW (1988) Acoustically induced call modification in the white-lipped frog, Leptodactylus albilabris. Anim Behav 36:1295–1308
Morris MR, Yoon SL (1989) A mechanism for female choice of large males in the treefrog Hyla chrysoscelis. Behav Ecol Sociobiol 25:65–71
Owen PC, Tucker JK (2006) Courtship calls and behavior in two species of chorus frogs, genus Pseudacris (Anura: Hylidae). Copeia 2006:137–144
Patricelli GL, Krakauer AH (2010) Tactical allocation of effort among multiple signals in sage grouse: an experiment with a robotic female. Behav Ecol 21:97–106
Patricelli GL, Uy JAC, Walsh G, Borgia G (2002) Sexual selection: male displays adjusted to female’s response. Nature 415:279–280
Reichert MS (2011a) Aggressive calls improve leading callers’ attractiveness in the treefrog Dendropsophus ebraccatus. Behav Ecol 22:951–959
Reichert MS (2011b) Effects of multiple-speaker playbacks on aggressive calling behavior in the treefrog Dendropsophus ebraccatus. Behav Ecol Sociobiol 65:1739–1751
Reichert MS, Gerhardt HC (2011) The role of body size on the outcome, escalation and duration of contests in the grey treefrog, Hyla versicolor. Anim Behav 82:1357–1366
Reichert MS, Gerhardt HC (2012) Trade-offs and upper limits to signal performance during close-range vocal competition in gray tree frogs Hyla versicolor. Am Nat 180:425–437
Rheinlaender J, Gerhardt HC, Yager DD, Capranica RR (1979) Accuracy of phonotaxis by the green treefrog Hyla cinerea. J Comp Physiol A 133:247–255
Rosen M, Lemon RE (1974) Vocal behavior of spring peepers, Hyla crucifer. Copeia 1974:940–950
Runkle LS, Wells KD, Robb CC, Lance SL (1994) Individual, nightly, and seasonal variation in calling behavior of the gray tree frog, Hyla versicolor: implications for energy expenditure. Behav Ecol 5:318–325
Schwartz JJ, Buchanan B, Gerhardt HC (2002) Acoustic interactions among male gray treefrogs, Hyla versicolor, in a chorus setting. Behav Ecol Sociobiol 53:9–19
Schwartz JJ, Buchanan BW, Gerhardt HC (2001) Female mate choice in the gray treefrog (Hyla versicolor) in three experimental environments. Behav Ecol Sociobiol 49:443–455
Sullivan BK, Hinshaw SH (1992) Female choice and selection on male calling behaviour in the grey treefrog Hyla versicolor. Anim Behav 44:733–744
Sztatecsny M, Strondl C, Baierl A, Ries C, Hödl W (2010) Chin up: are the bright throats of male common frogs a condition-independent visual cue? Anim Behav 79:779–786
Taylor RC, Buchanan BW, Doherty JL (2007) Sexual selection in the squirrel treefrog Hyla squirella: the role of multimodal cue assessment in female choice. Anim Behav 74:1753–1763
Taylor RC, Klein BA, Stein J, Ryan MJ (2008) Faux frogs: multimodal signalling and the value of robotics in animal behaviour. Anim Behav 76:1089–1097
Taylor RC, Klein BA, Stein J, Ryan MJ (2011) Multimodal signal variation in space and time: how important is matching a signal with its signaler? J Exp Biol 214:815–820
Wells KD (1977) The social behaviour of anuran amphibians. Anim Behav 25:666–693
Wells KD (1980) Social behavior and communication of a dendrobatid frog (Colostethus trinitatis). Herpetologica 36:189–199
Wells KD (1988) The effect of social interactions on anuran vocal behavior. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 433–454
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Wells KD, Schwartz JJ (1984) Vocal communication in a Neotropical treefrog, Hyla ebraccata: advertisement calls. Anim Behav 32:405–420
Wells KD, Schwartz JJ (2006) The behavioral ecology of anuran communication. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Hearing and sound communication in amphibians. Springer handbook of auditory research. Springer, New York, pp 44–86
Wells KD, Taigen TL (1986) The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor). Behav Ecol Sociobiol 19:9–18
Acknowledgments
Members of the Gerhardt lab assisted with frog collection. Flavia Barbosa and two anonymous reviewers gave helpful comments on previous versions of this manuscript. Funding was provided by a Doctoral Dissertation Improvement Grant from the U.S. National Science Foundation, Dean E. Metter Memorial Award from the Society for the Study of Amphibians and Reptiles, and Graduate Assistance in Areas of National Need fellowship from the University of Missouri and the U.S. Department of Education.
Ethical standards
The Missouri Department of Conservation gave permission to collect frogs. The University of Missouri Animal Care and Use Committee approved the experimental procedures (protocol number 6546).
Conflict of interest
The author declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Gibbons
Rights and permissions
About this article
Cite this article
Reichert, M.S. Visual cues elicit courtship signals in a nocturnal anuran. Behav Ecol Sociobiol 67, 265–271 (2013). https://doi.org/10.1007/s00265-012-1446-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1446-9