Abstract
In altricial birds, parental feeding is essential, and its amount may depend on the quality of both parents. A relationship between parental quality and feeding rate is generally attributed to an active adjustment by parents in order to retain good quality mates or ensure high fitness through raising high-quality offspring. However, the behaviour and need of young may also change with parental quality, and this may affect parental behaviour. A further problem is that most studies have investigated post-hatching parental investment in relation to the secondary sexual signals of males, but not females. In a cross-fostering experiment, we examined the feeding rates of rearing parents in relation to the size and ornamentation of both original and rearing parents in collared flycatchers (Ficedula albicollis). Using this setup, we could examine whether the observed feeding patterns were the results of the decision of the parents based on their own and their partner’s traits or the constraints imposed by the behaviour or need of offspring. When correcting for clutch size and year, we found that feeding rate of both foster parents correlated with the wing patch size of the original female. This implies that original maternal quality had an offspring-mediated indirect effect on investment of foster parents, that is intrinsic nestling quality may constrain parental feeding decisions. This explanation should not be overlooked in future studies on preferential parental investment, and our results also point out that maternal ornaments deserve more attention in such studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life history theory suggests that the energy expenditure of animals is shared between self-maintenance and reproductive investment. Because nestlings of altricial birds are incapable of acquiring food for themselves, their survival depends entirely on their parents’ feeding. Parental investment requires enormous energy investment, thereby affecting parents’ survival (Cichoń et al. 1998) and reproductive success in future breeding attempts (Gustafsson and Sutherland 1988). Therefore, in order to maximise their lifetime reproductive success, parents may alter their investment based on the expected value of current and future breeding attempts. The quality of individuals or their mates could be important factors to predict the value of the current brood.
Ornamental traits may indicate the quality or attractiveness of individuals (Andersson 1994) and, as a consequence, the direct or indirect fitness benefits individuals may obtain by mating with a given partner. Potential indirect benefits include ‘good genes’, which result in high quality and viability of offspring and thereby increases the reproductive success of the parents (Petrie 1994; Sheldon et al. 1997). A mate with elaborate traits may provide direct benefits as well, such as high-quality territory with abundant food or good breeding site (Keyser and Hill 2000). Secondary sexual traits may also signal the level of parental care (see below).
Recently, many studies have focused on the relationship between plumage signals and parental investment (e.g. Sanz 2001; Limbourg et al. 2004; DeMory et al. 2010). The results are often contradictory, but this is not surprising given the opposing predictions of the prevailing hypotheses. For example, the ‘good parent’ hypothesis suggests that good quality individuals with elaborate traits will invest more in their offspring because they are able to do so (Hoelzer 1989; Linville et al. 1998). On the other hand, highly ornamented males should invest less into their first brood if they have the opportunity to increase their reproductive success via extra-pair copulations or by obtaining more partners (Magrath and Komdeur 2003; Mitchell et al. 2007). The situation is further complicated by the fact that the quality of the reproductive partner may also influence the decision of an individual about the investment in its current reproductive event. One may argue that an individual mated to a good quality partner should invest more in its offspring because they inherit the good genes of the partner and therefore have higher expected fitness (Mazuc et al. 2003; Johnsen et al. 2005). On the contrary, the ‘compensation hypothesis’ suggests that individuals mated to a less ornamented partner should compensate for the worse quality of their mates and enhance the viability of their nestlings by providing more care (Gowaty et al. 2007; Ratikainen and Kokko 2010). Finally, Burley’s original differential allocation hypothesis predicts that parents should adjust their investment according to their own attractiveness relative to that of the partners rather than according to their own or their partner’s attractiveness per se, that is individuals mated to a more attractive partner should invest more into the offspring to retain their mate (Burley 1986).
Despite the relatively large number of studies, we lack important information concerning the role of parental quality in parental feeding decisions. As we outlined above, most hypotheses argue that parents adjust their feeding rate to retain good quality mates or because the future reproductive success of the offspring may be related to parental quality. However, the behaviour, growth rate and therefore energetic requirement of the offspring may also depend on parental quality (Silva et al. 2007), either due to genetic reasons or due to early maternal effects. Begging behaviour of the offspring is suggested to be a reliable signal of need (Cotton et al. 1996; Rodriguez-Girones et al. 2001), and parents of many species are known to respond to this signal in terms of both feeding rate (Ottosson et al. 1997; Moreno-Rueda et al. 2009) and food allocation to individual offspring (Kölliker et al. 1998; Leonard and Horn 2001; Rosivall et al. 2005). Therefore, it is quite plausible to assume that a correlation between a parental trait and feeding rate is the result of a correlation between this parental trait and nestling behaviour/need, rather than an active decision of parents based on their own or their partner’s traits. In other words, it is possible that parents do not adjust their investment directly to parental traits (along the lines of the aforementioned hypotheses); rather, their investment is constrained by nestling behaviour/need (hereafter ‘offspring constraint hypothesis’) and the relationship between parental investment and the given parental traits is indirect. To our knowledge, this hypothesis has not yet been tested.
A further problem with the literature on post-hatching parental investment is that despite biparental care in many bird species, most studies focused on parental investment in relation to male quality or attractiveness (e.g. Mazuc et al. 2003; Johnsen et al. 2005; Osorno et al. 2006), while only few have investigated the relationship between parental investment and ornamental traits of females. In addition, the outcome of these studies is quite mixed. Some studies have not found any correlation between paternal feeding rate and female ornaments (Pilastro et al. 2003; Matessi et al. 2009; Maguire and Safran 2010), while others have found positive association between female ornament and male brood defence (Griggio et al. 2003; Matessi et al. 2009) or male feeding rate (Mahr et al. 2012).
We studied parental investment in a Hungarian population of the collared flycatcher. Collared flycatchers have two sexually selected plumage traits. In our population, both male and female quality is related to the condition-dependent wing patch size, a heritable plumage signal (Török et al. 2003; Hegyi et al. 2008b). This trait has been reported to play a role in the territorial aggression of males (Garamszegi et al. 2006) and the competitive interactions of females (Hegyi et al. 2008a). Males also have a conspicuous forehead patch. Its size is not condition dependent, but heritable (Hegyi et al. 2002, 2006) and might signal the quality of males. Males with larger forehead patches bred earlier in the season (Hegyi et al. 2006), and after an immune challenge, their song rate decreased less than that of small-patched males (Garamszegi et al. 2004a). Furthermore, there was a positive association between the forehead patch size of rearing males and the growth of nestling wing feathers (Szöllősi et al. 2009) and between the forehead patch size of both original and foster fathers and nestling mass growth rate (Hegyi et al. 2011b).
As we outlined above, male and female ornaments may correlate with feeding rate because (1) parents adjust their investment directly to their own or their partner’s traits (e.g. ‘good parent’, ‘compensation’ and ‘differential allocation’ hypotheses) or (2) parental quality-dependent nestling behaviour/need constrains parental investment (‘offspring constraint hypothesis’). The primary aim of this study was to investigate the relationship between parental investment and plumage signals of both males and females in a way that allows us to distinguish between these two main mechanisms. Therefore, we conducted a full-brood cross-fostering experiment. As rearing parents had no information on the traits of original parents, any correlation between the feeding rate of rearing parents and the traits of original parents had to be mediated by intrinsic nestling quality and would support the second mechanism. Correlations between feeding rate and the traits of rearing parents, however, indicate parental decisions based on their own or their partner’s traits and would therefore support the first mechanism.
Our secondary goal was to deepen our knowledge concerning the role of female ornaments in parental investment decisions after hatching, because only very few papers have been published on this issue.
Finally, we aimed to find an explanation for the previously found positive associations between growth of nestlings and forehead patch size of males in our population (Szöllősi et al. 2009; Hegyi et al. 2011b). We predicted a positive association between forehead patch size of males and feeding rate of either or both of the parents.
Methods
Study species and field methods
Our study was conducted in a Hungarian population of collared flycatchers. Our nestbox plots are located in an oak-dominated woodland in the Pilis Mountains (47°43′ N, 19°01′ E), a protected area of Duna-Ipoly National Park. The collared flycatcher is a small, hole-breeding, insectivorous species with wintering sites in Sub-Saharan Africa (Cramp and Perrins 1993). Males return to the breeding grounds and occupy nestboxes in the middle of April. Females arrive a few weeks later and, after mate choice, they build the nest, lay and incubate the eggs (six to seven on average) alone. After hatching, both parents feed the nestlings, but the brooding of ectothermic (0–6 days old) young is the exclusive task of the female.
Nestboxes were continuously monitored after the arrival of birds. Full broods with similar brood size were cross-fostered 2 days after hatching. Four days after hatching, video recordings of approximately 1.5-h (82.9 ± 16.8 min) were taken inside the nestboxes to estimate parental feeding effort. One day before the video recording, we exchanged the nestboxes for special ones, which had the same inner sizes, but had a special back chamber (hidden from the parents) for the videocamera. This method had previously been successfully applied in this population (Rosivall et al. 2005). The video records were taken between 0830 and 2000 hours, but we tried to avoid the midday time (1200–1530 hours) when feeding activity may be reduced. There was no difference in the feeding rates between the morning and afternoon hours (female: t = 0.78; df = 23; p = 0.45; male: t = −1.27; df = 23; p = 0.22), and feeding activity did not change within these periods either (morning/ female: df = 1,8; F = 1.01, p = 0.34; male: df = 1,8; F = 0.38, p = 0.55; afternoon/ female: df = 1,17; F = 0.62, p = 0.44; male: df = 1,17; F = 0.0, p = 0.98).
When the chicks became 10 days old, we caught the parents with spring traps and measured their morphological traits. The binary age of males (yearling or older) was determined based on the colour of remiges (Svensson 1992). The size of the forehead patch was estimated as the product of maximum width and maximum height (Hegyi et al. 2002). We estimated the wing patch size of both parents by the sum of the lengths of non-covered white bars on the fourth to eighth primaries (Török et al. 2003). Body size was estimated by tarsus length. All of these traits were measured with calliper to the nearest 0.1 mm.
Statistical analysis
We used altogether 36 broods (16 in 2002 and 20 broods in 2003) in our experiment, but excluded 2 secondary broods of polygynous males and 3 broods with five chicks because of low sample size in this brood size category. One brood was removed to avoid pseudoreplication as the female was included in the experiment in both years. In three cases, brood-predation occurred before the chicks were 10 days old; therefore, the parents were not caught. The sample size may differ between analyses, because some measurements were occasionally missing (in the final models, it was 25).
We used general linear models to investigate the effect of rearing and original parental traits on the feeding rate of rearing parents. The proportion of time females spent with brooding during the video recording varied considerably. Because our recording times were relatively short (82.9 ± 16.8 min), differences in the incubation times were more likely to be the result of mere chance than biologically meaningful differences between the females. Therefore, the feeding rates of females were calculated for the period when they were not incubating. Feeding rates of the males were calculated for the whole period. In both cases, feeding rate was calculated as the number of feedings per hour. Year-standardised laying date, tarsus length, forehead patch size of males and female wing patch size were used in our analyses as covariates (in case of laying date, used the deviation from the yearly median; for all other variables, we used the deviation from the yearly mean divided by SD). Wing patch size of males was year- and also age-standardised, because it strongly differs between adults and yearlings (Török et al. 2003). Year, brood size and age of males were used as fixed factors.
To avoid overparameterization, we performed two analyses with backward stepwise model selection. First, we analysed the effect of laying date, brood size and the traits of original parents on the feeding rate of rearing parents. Second, we added the traits of rearing parents to the final model (i.e. which included only significant variables) of the first analysis. Values indicated for the non-significant terms are derived from analyses, in which the given terms were re-entered to the final model one by one (Hegyi and Garamszegi 2011). We also performed our analyses using an information theoretic approach, by calculating the Akaike information criterion (AICc) parameter weights of our independent variables (not shown). The parameter weight is analogous to the probability that the given variable is a component of the AICc best model (see details in Burnham and Anderson 2002; Symonds and Moussalli 2011). All variables included in the final models of the stepwise regressions received high parameter weights (ranging from 0.622 to 0.885), thereby confirming the results presented below.
Given that the feeding strategy of a parent may depend on the feeding effort of its mate (Linville et al. 1998; Mitchell et al. 2007; Maguire and Safran 2010), we also investigated the relationship between the feeding rate of male and female parents. For this analysis, we used a general linear model. In each above-mentioned analysis, model residuals were normally distributed. We used Statistica 6.1 (StatSoft, Inc. 2003.Tulsa, Oklahoma, USA) and SAS 9.1 (SAS Institute Inc., Cary, North Carolina, USA) for the statistical analyses.
Results
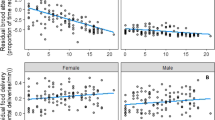
The provisioning rate of rearing males increased with the wing patch size of original females (Table 1, Fig. 1). None of the remaining variables (traits of original males and rearing parents, brood parameters) correlated with the feeding activity of males (Table 1). The feeding rate of females was significantly higher when rearing more chicks (seven compared to six; Table 1). It also differed between years (Table 1), and similarly to males, it was significantly positively correlated with the wing patch size of original females (Table 1, Fig. 1). However, just like in males, laying date, the traits of the original males and that of the rearing parents did not correlate with the feeding activity (Table 1). There was no correlation between the feeding rates of males and females (F 1,28 = 0.09; p = 0.927).
Discussion
In our whole-brood fostering experiment, we investigated whether the feeding activity of parents was related to ornamental traits of the original and rearing parents while controlling for clutch size, laying date and study years. The experimental design allowed us to examine whether foster parents adjusted their feeding activity to their own or their partners’ traits or rather to nestling quality/behaviour which is dependent on the quality of the original parents. We found that the wing patch size of original females positively correlated with the feeding rate of both rearing parents. This result suggests a nestling-mediated indirect association between the ornamentation of the original female and feeding rate of the foster parents and thereby supports the ‘offspring constraint hypothesis’. Though in this study, we did not investigate the need or behaviour of nestlings, the offspring of more ornamented females might differ in behaviour from the chicks of small-patched mothers. Thus, one possible explanation would be that, as a result of inherited maternal genes, nestlings of high-quality females were larger and begged more. However, the body mass of nestlings on the day of video recording was not related to the wing patch size of the original female (our unpublished data). Nonetheless, this result does not preclude the possibility that inherited genes influence the begging intensity of nestlings per se or via their growth rate. Though there is so far no clear experimental evidence for genetic effects of female ornaments on offspring growth, a study of male ornaments suggests that such effects may exist (Parker 2003). Alternatively, females may allocate different amounts of nutrients or hormones into their eggs depending on their quality (Navara et al. 2006), which in turn may affect the begging intensity of nestlings and the feeding activity of rearing parents. For example, it is known of several yolk steroid hormones such as corticosterone (Loiseau et al. 2008), androgens (Eising and Groothuis 2003) or specifically testosterone (Quillfeldt et al. 2006), that they affect the begging activity of nestlings. An earlier study in our collared flycatcher population found no correlation between female wing patch size and the concentration of testosterone in the eggs (Hegyi et al. 2011a). However, yolk androstenedione level significantly increased with laying order in small-patched females, while it did not change in females with large wing patch (Hegyi et al. 2011a). When the interaction between laying order and wing patch size was removed from the model, the overall effect of wing patch size became significant: there was on average more androstenedione in eggs of females with smaller wing patches (Hegyi et al., unpublished results). The same study found that nestlings from eggs with less androstenedione hatched with smaller mass and grew faster (Hegyi et al. 2011a). If, in line with these results, nestlings of large patched females hatched with smaller mass and grew faster in our study, they may have required more food during early development, and this could explain our results. Unfortunately, we could not test this, because we had no information on hatching mass in this study.
Though, as we have shown, female ornamentation may correlate with post-hatching parental investment, only very few studies have investigated this possibility so far. Even these are hard to compare, because some of them focused on brood defence, while others on feeding rate. Two studies (Pilastro et al. 2003; Maguire and Safran 2010) found no correlation between female colouration and the feeding rate of males, while there was a non-significant tendency for female feeding rate to correlate positively with female attractiveness in the study of Pilastro et al. (2003). Griggio et al. (2003) found a positive relationship between male brood defence and female ornamentation. Interestingly, the two studies which estimated parental investment in both ways came to mixed results. Male rock sparrows (Petronia petronia) defended but did not feed their chicks more when paired with reduced breast patched females (Matessi et al. 2009). On the contrary, a blue tit (Cyanistes caeruleus) study showed that males invested less in feeding, but did not defend the brood less, when paired to UV-reduced females (Mahr et al. 2012). Thus, it seems (based on the above results) that the investment of males is either unrelated to or positively correlated with female ornaments, and the authors suggested that the latter result supported the differential allocation hypothesis. However, as our results show, this is not necessarily the case. Positive correlation may also occur if males do not directly adjust their investment to female ornaments (as suggested e.g. by the ‘differential allocation hypothesis’), but rather respond to the need/behaviour of the nestlings (as suggested by the ‘offspring constraint hypothesis’).
Many more studies focused on the relationship between male ornamentation and parental feeding behaviour, though none of them considered the possibility that such a relationship may be constrained by nestling quality. The results are again quite mixed. Some studies showed a positive correlation between male attractiveness and male feeding rate (Buchanan and Catchpole 2000), while others found a negative (Sanz 2001) or no relationship (Maguire and Safran 2010). In addition, in species with multiple colour signals, the two feather ornaments may show contrasting relationship with male feeding rate (Johnsen et al. 2005). The association between male ornaments and female feeding rate also varies (positive: Maguire and Safran 2010; none: Mazuc et al. 2003; Sanz 2001; negative: Limbourg et al. 2004).
The fact that we found no correlation between the rearing parents’ feather ornaments and their feeding rate is still surprising for the following reasons. First, in a Swedish population of collared flycatchers, males with an experimentally enlarged forehead patch reduced their feeding rate because they had to defend their territory more intensively against other males (Qvarnström 1997). Given that in our population the wing patch size but not the forehead patch size has an important role in intrasexual competition (Garamszegi et al. 2006; Hegyi et al. 2008a), we expected a negative correlation between wing patch size and feeding rate, something we did not observe. Second, earlier studies in our population have found positive correlations between nestling growth and the forehead patch size of the original males (Szöllősi et al. 2009), or both original and rearing males (Hegyi et al. 2011b). Therefore, we predicted that, contrary to results in the Swedish population, males with larger forehead patch (or their mates) would feed their nestlings more. However, in our study, feeding rate of the rearing parents did not change with the forehead patch size of rearing males. The growth patterns found earlier are therefore the result of either attractive males or their partners feeding the chicks with higher quality prey (Sejberg et al. 2000; Grieco 2002) or the offspring of large patched males being of superior genetic quality (Petrie 1994).
The feeding rate of females differed between years. The abundance of caterpillar, which is a major food type for developing chicks (Török 1986), was much higher in the year when females had higher feeding rates (our unpublished data). This suggests that females increased their feeding rate when surplus food was available, while this was not true for males. It is possible that, when chicks are young (feeding rate was recorded 4 days after hatching), males do not invest as much energy into parental care and do not respond as readily to environmental conditions as females do, because the value of the brood is not equal for males and females. Though less than 10 % of males were socially polygynous (Garamszegi et al. 2004b) in our population, 55.7 % of broods contained offspring sired by extra-pair males (Rosivall et al. 2009). This means that males have a chance to mate with a secondary female or to sire extra-pair young when their primary brood is young (Magrath and Elgar 1997; Magrath and Komdeur 2003). For females, in contrast, the number of progeny in a breeding season is limited by the number of eggs laid and chicks reared (there is no evidence for intraspecific brood parasitism in this species).
The brood value argument may apply also to the effect of brood size, because females rearing seven nestlings fed more frequently than those rearing six, while there was no relationship between brood size and feeding rate of the rearing males. However, our results are in contrast with an earlier brood-size manipulation experiment in the same population, which found that feeding rate of both parents were influenced by brood size (Török and Tóth 1990). Nevertheless, it should be noted that in the previous study, brood size was manipulated with two nestlings, and feeding rate was measured at an older nestling age when the value of the brood may be higher for the males (Michl et al. 2000).
In summary, the main finding of our study is an association between a condition-dependent plumage ornament of the original mother and the provisioning rate of the rearing parents. This indirect effect is important to understand the factors shaping parental investment. Our results indicate that a relationship between parental traits and feeding rate may be explained not only by direct parental adjustment of feeding effort to these traits but also by differences in the need or behaviour of the nestlings. Further studies should examine the generality of such offspring quality constraints and explore their potential mechanisms. Our results also show that the role of female ornaments in parental investment decisions deserves more attention than it has received so far.
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, New Jersey
Buchanan KL, Catchpole CK (2000) Song as an indicator of male parental effort in the sedge warbler. Proc R Soc Lond B 267:321–326
Burley N (1986) Sexual selection for aesthetic traits in species with biparental care. Am Nat 127:415–445
Burnham KP, Anderson DR (2002) Model selection and multi-model inference. Springer, New York
Cichoń M, Olejniczak P, Gustafsson L (1998) The effect of body condition on the cost of reproduction in female collared flycatchers Ficedula albicollis. Ibis 140:128–130
Cotton PA, Kacelnik A, Wright J (1996) Chick begging as a signal: are nestlings honest? Behav Ecol 7:178–182
Cramp S, Perrins CM (1993) The birds of the Western Palearctic, vol VII. Oxford University Press, Oxford
DeMory ML, Thompson CF, Sakaluk SK (2010) Male quality influences male provisioning in house wrens independent of attractiveness. Behav Ecol 21:1156–1164
Eising CM, Groothuis TGG (2003) Yolk androgens and begging behaviour in black-headed gull chicks: an experimental field study. Anim Behav 66:1027–1034
Garamszegi LZ, Møller AP, Török J, Michl G, Péczely P, Richard M (2004a) Immune challenge mediates vocal communication in a passerine bird: an experiment. Behav Ecol 15:148–157
Garamszegi LZ, Török J, Michl G, Møller AP (2004b) Female survival, lifetime reproductive success and mating status in a passerine bird. Oecologia 138:48–56
Garamszegi LZ, Rosivall B, Hegyi G, Szöllősi E, Török J, Eens M (2006) Determinants of male territorial behavior in a Hungarian collared flycatcher population: plumage traits of residents and challengers. Behav Ecol Sociobiol 60:663–671
Gowaty PA, Anderson WW, Bluhm CK, Drickamer LC, Kim YK, Moore AJ (2007) The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc Natl Acad Sci USA 104:15023–15027
Grieco F (2002) Time constraint on food choice in provisioning blue tits, Parus caeruleus: the relationship between feeding rate and prey size. Anim Behav 64:517–526
Griggio M, Matessi G, Pilastro A (2003) Male rock sparrow (Petronia petronia) nest defence correlates with female ornament size. Ethology 109:659–669
Gustafsson L, Sutherland WJ (1988) The cost of reproduction in the collared flycatcher Ficedula albicollis. Nature 335:813–815
Hegyi G, Garamszegi LZ (2011) Using information theory as a substitute for stepwise regression in ecology and behavior. Behav Ecol Sociobiol 65:69–76
Hegyi G, Török J, Tóth L (2002) Qualitative population divergence in proximate determination of a sexually selected trait in the collared flycatcher. J Evol Biol 15:710–719
Hegyi G, Török J, Tóth L, Garamszegi LZ, Rosivall B (2006) Rapid temporal change in the expression and age-related information content of a sexually selected trait. J Evol Biol 19:228–238
Hegyi G, Garamszegi LZ, Eens M, Török J (2008a) Female ornamentation and territorial conflicts in collared flycatchers (Ficedula albicollis). Naturwissenschaften 95:993–996
Hegyi G, Rosivall B, Szöllősi E, Hargitai R, Eens M, Török J (2008b) Phenotypic plasticity in a conspicuous female plumage trait: information content and mating patterns. Anim Behav 75:977–989
Hegyi G, Herényi M, Szöllősi E, Rosivall B, Török J, Groothuis TGG (2011a) Yolk androstenedione, but not testosterone, predicts offspring fate and reflects parental quality. Behav Ecol 22:29–38
Hegyi G, Rosivall B, Szöllősi E, Eens M, Török J (2011b) Context-dependent effects of nestling growth trajectories on recruitment probability in the collared flycatcher. Behav Ecol Sociobiol 65:1647–1658
Hoelzer GA (1989) The good parent process of sexual selection. Anim Behav 38:1067–1078
Johnsen A, Delhey K, Schlicht E, Peters A, Kempenaers B (2005) Male sexual attractiveness and parental effort in blue tits: a test of the differential allocation hypothesis. Anim Behav 70:877–888
Keyser AJ, Hill GE (2000) Structurally based plumage coloration is an honest signal of quality in male blue grosbeaks. Behav Ecol 11:202–209
Kölliker M, Richner H, Werner I, Heeb P (1998) Begging signals and biparental care: nestling choice between parental feeding locations. Anim Behav 55:215–222
Leonard ML, Horn AG (2001) Begging calls and parental feeding decisions in tree swallows (Tachycineta bicolor). Behav Ecol Sociobiol 49:170–175
Limbourg T, Mateman AC, Andersson S, Lessells CM (2004) Female blue tits adjust parental effort to manipulated male UV attractiveness. Proc R Soc Lond B 271:1903–1908
Linville SU, Breitwisch R, Schilling AJ (1998) Plumage brightness as an indicator of parental care in northern cardinals. Anim Behav 55:119–127
Loiseau C, Sorci G, Dano S, Chastel O (2008) Effects of experimental increase of corticosterone levels on begging behavior, immunity and parental provisioning rate in house sparrows. Gen Comp Endocrinol 155:101–108
Magrath MJL, Elgar MA (1997) Paternal care declines with increased opportunity for extra-pair matings in fairy martins. Proc R Soc Lond B 264:1731–1736
Magrath MJL, Komdeur J (2003) Is male care compromised by additional mating opportunity? Trends Ecol Evol 18:424–430
Maguire SE, Safran RJ (2010) Morphological and genetic predictors of parental care in the North American barn swallow Hirundo rustica erythrogaster. J Avian Biol 41:74–82
Mahr K, Griggio M, Granatiero M, Hoi H (2012) Female attractiveness affects paternal investment: experimental evidence for male differential allocation in blue tits. Front Zool 9:14
Matessi G, Carmagnani C, Griggio M, Pilastro A (2009) Male rock sparrows differentially allocate nest defence but not food provisioning to offspring. Behaviour 146:209–223
Mazuc J, Chastel O, Sorci G (2003) No evidence for differential maternal allocation to offspring in the house sparrow (Passer domesticus). Behav Ecol 14:340–346
Michl G, Török J, Garamszegi LZ, Tóth L (2000) Sex-dependent risk taking in the collared flycatcher, Ficedula albicollis, when exposed to a predator at the nestling stage. Anim Behav 59:623–628
Mitchell DP, Dunn PO, Whittingham LA, Freeman-Gallant CR (2007) Attractive males provide less parental care in two populations of the common yellowthroat. Anim Behav 73:165–170
Moreno-Rueda G, Soler M, Martín-Vivaldi M, Palomino JJ (2009) Brood provisioning rate and food allocation rules according to nestling begging in a clutch-adjusting species, the Rufous-tailed Scrub-robin Cercotrichas galactotes. Acta Ornithol 44:167–175
Navara KJ, Badyaev AV, Mendonça MT, Hill GE (2006) Yolk antioxidants vary with male attractiveness and female condition in the house finch (Carpodacus mexicanus). Phys Biochem Zool 79:1098–1105
Osorno JL, Morales J, Moreno J, Merino S, Tomás G, Vásquez RA (2006) Evidence for differential maternal allocation to eggs in relation to manipulated male attractiveness in the pied flycatcher (Ficedula hypoleuca). J Ornithol 147:605–611
Ottosson U, Backman J, Smith HG (1997) Begging affects parental effort in the pied flycatcher, Ficedula hypoleuca. Behav Ecol Sociobiol 41:381–384
Parker TH (2003) Genetic benefits of mate choice separated from differential maternal investment in red junglefowl (Gallus gallus). Evolution 57:2157–2165
Petrie M (1994) Improved growth and survival of offspring of peacocks with more elaborate trains. Nature 371:598–599
Pilastro A, Griggio M, Matessi G (2003) Male rock sparrows adjust their breeding strategy according to female ornamentation: parental or mating investment? Anim Behav 66:265–271
Quillfeldt P, Masello JF, Strange IJ, Buchanan KL (2006) Begging and provisioning of thin-billed prions, Pachyptila belcheri, are related to testosterone and corticosterone. Anim Behav 71:1359–1369
Qvarnström A (1997) Experimentally increased badge size increases male competition and reduces male parental care in the collared flycatcher. Proc R Soc Lond B 264:1225–1231
Ratikainen I, Kokko H (2010) Differential allocation and compensation: who deserves the silver spoon? Behav Ecol 21:195–200
Rodriguez-Girones MA, Zuniga JM, Redondo T (2001) Effects of begging on growth rates of nestling chicks. Behav Ecol 12:269–274
Rosivall B, Török J, Szöllősi E (2005) Food allocation in collared flycatcher (Ficedula albicollis) broods: do rules change with the age of nestlings? Auk 122:1112–1122
Rosivall B, Szöllősi E, Hasselquist D, Török J (2009) Effects of extrapair paternity and sex on nestling growth and condition in the collared flycatcher, Ficedula albicollis. Anim Behav 77:611–617
Sanz JJ (2001) Experimentally reduced male attractiveness increases parental care in the pied flycatcher Ficedula hypoleuca. Behav Ecol 12:171–176
Sejberg D, Bensch S, Hasselquist D (2000) Nestling provisioning in polygynous great reed warblers (Acrocephalus arundinaceus): do males bring larger prey to compensate for fewer nest visits? Behav Ecol Sociobiol 47:213–219
Sheldon BC, Merilä J, Qvarnström A, Gustafsson L, Ellegren H (1997) Paternal genetic contribution to offspring condition predicted by size of male secondary sexual character. Proc R Soc Lond B 264:297–302
Silva MC, Boersma PD, Mackay S, Strange I (2007) Egg size and parental quality in thin-billed prions, Pachyptila belcheri: effects on offspring fitness. Anim Behav 74:1403–1412
Svensson L (1992) Identification guide to European passerines. Published by the author, Stockholm
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behav Ecol Sociobiol 65:13–21
Szöllősi E, Rosivall B, Hasselquist D, Török J (2009) The effect of parental quality and malaria infection on nestling performance in the collared flycatcher (Ficedula albicollis). J Ornithol 150:519–527
Török J (1986) Food segregation in three hole-nesting bird species during the breeding season. Ardea 74:129–136
Török J, Tóth L (1990) Costs and benefits of reproduction of the collared flycatcher, Ficedula albicollis. In: Blondel J, Gosler A, Lebreton D-J, McCleery R (eds) Population biology of passerine birds: an integrated approach (NATO ASI series). Springer, Berlin Heidelberg New York, pp 307–319
Török J, Hegyi G, Garamszegi LZ (2003) Depigmented wing patch size is a condition-dependent indicator of viability in male collared flycatchers. Behav Ecol 14:382–388
Acknowledgments
We thank Rita Hargitai, Márton Herényi, Beáta Szigeti and Eszter Szöllősi for their help in the field, and Orsolya Molnár, Marty Leonard and two anonymous reviewers for helpful comments on the manuscript. The study was supported by Hungarian Scientific Research Fund (OTKA) grants (K75618 to JT; PD75481 and F68295 to BR; and PD72117 and K101611 to GH), the Erdők a Közjóért Alapítvány, the Eötvös Loránd University and the Pilis Park Forestry.
Ethical standards
Work at the study site was done under permits from Duna-Ipoly National Park. All experiments comply with the laws of Hungary.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Leonard
Rights and permissions
About this article
Cite this article
Kiss, D., Hegyi, G., Török, J. et al. The relationship between maternal ornamentation and feeding rate is explained by intrinsic nestling quality. Behav Ecol Sociobiol 67, 185–192 (2013). https://doi.org/10.1007/s00265-012-1437-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1437-x