Abstract
Reproductive success of brood parasites varies considerably both among and within host species, mainly due to differences in host egg-rejection rates and survival of parasitic chicks. Here, we investigated the breeding success of the cuckoo (Cuculus canorus) in one of its major hosts, the great reed warbler (Acrocephalus arundinaceus), with respect to host social mating status. In this passerine, polygynous males provide less parental care to their young per nest than monogamous males. Consequently, their less-assisted females may fledge lower numbers of nestlings than monogamous females. This may be especially true for secondary females, which often receive limited or no paternal help with young at all. Based on these findings, we expected higher cuckoo reproductive success in nests of socially monogamous than polygynous great reed warbler males. More specifically, we predicted lower fledging success of cuckoo young in nests of secondary than primary or monogamous females. In line with the prediction, we found higher cuckoo fledging success in nests of monogamous than polygynous males, monogamous nests being more than twice as successful as secondary nests. We detected, however, only a tendency to lower cuckoo success in primary compared to monogamous nests and no differences between primary and secondary nests. Moreover, neither parasitism nor host egg-rejection rates differed among the nests of different status. Our results show, for the first time, that the social mating status of a host may influence the overall reproductive success of a brood parasite and thus should be considered in further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Avian brood parasitism is an alternative reproductive strategy where parasitic females lay their eggs into the nests of other individuals (hosts), avoiding thus subsequent parental duties (Payne 1977; Rothstein 1990). In this way, brood parasites may dramatically reduce host fitness (Øien et al. 1998; Hauber 2003). Therefore, during their long-term interactions, both the brood parasites and their hosts have evolved intricate adaptations and counter-adaptations, which make the system an illustrative example of coevolution (Schulze-Hagen et al. 2009; Davies 2011).
However, not only do the brood parasites affect host fitness but also hosts may influence the reproductive output of brood parasites. Several previous studies have shown that the reproductive success of brood parasites varies considerably both among and within host species (Brooke and Davies 1987; Soler et al. 1995; Scott and Lemon 1996; Rutila et al. 2002; Mermoz and Reboreda 2003; Grim et al. 2011). This variation can be ascribed mainly to the differences in host egg-rejection rates (Davies 2000; Stokke et al. 2008) and in predation pressure on host nests (Dearborn 1999; Kleven et al. 2004; Hannon et al. 2009; de Mársico and Reboreda 2010). Furthermore, because parasites are dependent on hosts, their reproductive success may also relate to host parental quality. Chicks of many brood parasites are larger and require longer periods of host parental care compared to host own young (Wyllie 1981; Kilpatrick 2002; Numerov 2003). Raising the alien nestling may thus be more exhausting for the foster parents, especially for small passerines, than raising their own offspring (but see Brooke and Davies 1989). It is then natural to expect that survival of parasitic chicks will vary with the amount and quality of parental care provided by the hosts.
Generally, the intensity of parental care varies considerably among species (Trivers 1972; Clutton-Brock 1991). A lot of this variation can be explained by the differences in species-specific mating systems (Thomas and Székely 2005; Olson et al. 2008). In many polygynous birds with biparental nestling care, females sharing the same male usually receive less male assistance with parental duties than monogamous females (Webster 1991; Pinxten and Eens 1994; Trnka and Prokop 2010), resulting in their lower direct reproductive success (Johnson et al. 1993; Lubjuhn et al. 2000; Pribil 2000). Based on this scenario, survival of parasitic chicks in host nests may also vary with host social mating status.
A well-known example of a cuckoo (Cuculus canorus) host is the great reed warbler (Acrocephalus arundinaceus) (Moskát and Honza 2002; Kleven et al. 2004). It is a facultatively polygynous, open-nesting passerine with biparental nestling care (Cramp 1992; Hasselquist 1998). The rate of polygyny in this species varies between 8 and 43 % (Hasselquist 1998; Leisler and Wink 2000; Trnka et al. 2010; Honza et al. 2011), and cuckoo parasitism rate ranges between 0 and 68 % (e.g. Moksnes et al. 1993; Moskát et al. 2002; Moskát and Honza 2002). Previous studies have demonstrated that polygynous great reed warbler males provide significantly less parental care (feeding as well as nest defence) to their offspring than monogamous males (Dyrcz 1986; Bensch and Hasselquist 1994; Sejberg et al. 2000; Trnka and Prokop 2010).
While a recent study of Trnka and Prokop (2011) compared frequencies of hatched cuckoos among great reed warbler nests of different status, here we made an important step forward. In the present study, we investigated whether there is an association between great reed warbler social mating status and cuckoo reproductive success. We used within-species variability in presumed host quality in contrast to between-species variability that was examined in previous studies (Kleven et al. 2004; Grim 2006; Sklepowicz and Hałupka 2009; Remeš 2010).
Some studies have shown that cuckoos may parasitize nests non-randomly within a host population according to nest position, host parental quality, egg appearance or nesting activity (Moskát and Honza 2000; Avilés et al. 2006; Cherry et al. 2007; Polačiková et al. 2009; but see Avilés et al. 2009; Antonov et al. 2012). As these characteristics can differ between monogamous and polygynous hosts, we separately explored cuckoo parasitism rate, host egg-rejection rate and survival of cuckoo chicks in great reed warbler nests of different status. We expected higher cuckoo reproductive success in nests of monogamous than polygynous males. Since secondary great reed warbler females often fledge a lower number of young than monogamous females (Catchpole et al. 1985; Dyrcz 1986; Bensch and Hasselquist 1991), we specifically predicted lower fledging success of cuckoo young in secondary than in monogamous or primary nests. According to our best knowledge, the influence of host social mating status on the reproductive output of a brood parasite has not yet been studied.
Methods
Study sites and populations
The study was carried out on two colour-ringed great reed warbler populations: at fishponds near Štúrovo (47°51′ N 18°36′ E) in Slovakia (site 1) and in a fishpond area between Hodonín (48°51′ N 17°07′ E) and Mutěnice (48°54′ N 17°02′ E), Czech Republic (site 2). The two sites are located about 155 km apart. For their detailed descriptions, see Trnka and Prokop (2010) and Honza et al. (2002), respectively. The studied populations consist of 40–60 (site 1) and 80–100 breeding pairs (site 2). On both sites, great reed warblers breed in relatively narrow belts of littoral vegetation surrounding the fishponds. The overall rate of male polygyny (i.e. percent of polygynous males) was 26 % (21–29 %) and did not differ between years within sites (site 1: χ 2 = 0.04, df = 1, P = 0.849; site 2: χ 2 = 1.22, df = 1, P = 0.270) or between the sites (χ 2 = 0.28, df = 1, P = 0.595).
Data collection
We conducted the fieldwork during the breeding seasons of 2009 and 2010. From early May to late July, we systematically searched for great reed warbler nests and checked them daily from the beginning of egg laying until clutch completion. During the nest controls, we numbered each egg with a waterproof pen according to the laying sequence. These regular checks enabled us to ascertain the incidence of cuckoo parasitism: if the nest contained a cuckoo egg, we considered it as parasitized. We regarded cuckoo eggs as accepted if hosts did not reject them (by ejection or nest desertion) within 6 days after parasitism (see also Øien et al. 1998; Moskát and Honza 2002). After clutch completion, we checked each active nest two to three times during egg incubation until hatching and then other two to three times until fledging or nest failure.
In total, we found 335 nests (site 1, 102; site 2, 233). We determined the social mating status of each female and her mate based on their captures at the nests during the nest-building or egg-laying stages and confirmed it repeatedly throughout a season by direct observations of colour-ringed birds defending their nests or feeding their young. Male and female mating status may change in time due to settlement of other females or because of nest predation/desertion (Bensch 1996; Sejberg et al. 2000; Trnka and Prokop 2011). Therefore, we used the current mating status of nest owners either during egg laying and early incubation (to assess its effect on parasitism and host egg-rejection rates) or when caring for young (to examine its consequences for cuckoo fledging success). At both of these stages, we classified each nest as belonging to a monogamous, primary or secondary female. A monogamous female was the only female of a monogamous male. A primary female was the first female of a polygynous male, which started her breeding at the time when the secondary female has already been present in the same territory. Analogically, the secondary female was the second female of the polygynous male, breeding after, but overlapping in time with, the primary female (see also Bensch 1996). Out of 335 nests included into analyses, 231 were initially monogamous, 41 primary and 63 secondary. In 33 nests, the initial status changed later in the course of breeding season (15 from monogamous to primary, 5 from primary to monogamous, 2 from primary to secondary, 9 from secondary to monogamous and 2 from secondary to primary).
Previous studies have shown that females of polygynous males in some species may be of lower quality than monogamous females or, more specifically, that secondary females are of lower quality than primary females (Griggio et al. 2003). On the other hand, the opposite may also be true (Forstmeier et al. 2001a, b) or there may be no differences in female quality at all (Honza et al. 2011; Trnka and Prokop 2011; Trnka et al. 2012). To check for the potential effect of this confounding variable, we compared body condition (expressed as residuals from the linear regression of body mass on wing length) of the monogamous, primary and secondary females (n = 114) caring for 8- to 10-day-old nestlings. We found no differences in female condition in relation to her mating status (after controlling for female identity, generalised linear mixed-effects model (GLMM): F = 1.04; df = 2, 17; P = 0.375).
Statistical analysis
We fitted GLMMs to test whether (1) cuckoo parasitism (1 = parasitized, 0 = non-parasitized; n = 335), (2) host rejection of parasitic eggs (1 = rejected, 0 = accepted; n = 116 parasitized clutches) and (3) cuckoo fledging success (1 = fledged, 0 = not fledged; n = 50 nests with accepted cuckoo eggs) differ in relation to nest status (monogamous, primary and secondary; a fixed effect) and laying date (date of the first egg laid per clutch, a covariate). To control for the possible relationship between nest status and laying date, we centred the laying date around the mean in each nest status category. Apart from laying date, in model 2, we also used actual number of host eggs in the nest at time of parasitism (as a covariate), as this factor was shown to have a pronounced effect on great reed warbler egg-rejection rates (Moskát and Hauber 2007; Moskát et al. 2010). To cope with the issue of pseudoreplications, in models 1 and 3, we included both male and female identity as random effects. Only female identity as a random effect entered the model 2 because it is the female who is responsible for the rejection of parasitic eggs in the great reed warbler (Požgayová et al. 2009, 2011). As we did not have any year- and site-specific predictions, we included year and study site as categorical random effects (in all models) to take potential spatiotemporal variation in the data into account. We fitted the models with binary response variables using the glmer function from the package lme4 (Bates et al. 2008) and estimated the GLMM parameters using Laplace approximation (Bolker et al. 2009).
Based on the initial models 1–3 (Table 1), we specified sets of candidate models with all possible combinations of fixed effects and identical random effects. Then, we determined the best-fit model based on Akaike’s information criterion (AIC), corrected for low sample size (AICc) where appropriate, following the recommendations of Burnham and Anderson (2002). Models were ranked from the best to the worst using Δ i (Δ i = AICc(i) − AICc(min)), and the Akaike weights (w i) were calculated to give the relative support for a given model compared with the others. For the top candidate models that provided substantial support (Δi ≤ 2), we applied model averaging to identify the relative importance of each model term in predicting the response variable and to estimate effect sizes of the predictors. Model selection and averaging procedures were carried out in the package MuMIn (Bartoń 2011). All statistical analyses were performed in R 2.12.0 (R Development Core Team 2010).
Results
Out of 335 great reed warbler nests, 128 (38 %) were parasitized. Hosts rejected the parasitic egg in 66 (57 %) nests, most frequently by ejection (45 cases). In 20 cases, the parasitized clutch was deserted; one cuckoo egg was buried into nest lining. The cuckoo egg was accepted in 50 (43 %) nests; in 12 other nests, host reaction could not be evaluated due to nest failure within our temporal criterion of egg acceptance. For site- and year-specific data, see Table 2.
Incidence of cuckoo parasitism was best explained by two models: one that included no fixed term and the other that included only laying date. However, model averaging showed that laying date was not an important predictor of cuckoo parasitism (95 % confidence interval (CI) of its model-averaged estimate contained zero, results not shown).
Two models explained the variation in host rejection of parasitic eggs. The best model included only laying date, and the second one, laying date and host clutch size at time of parasitism. Model averaging showed that only laying date was important in predicting host egg-rejection behaviour (model-averaged estimate ± SE = −0.043 ± 0.019, 95 % CI −0.080 to −0.006). Thus, host egg-rejection probability decreased with advancing clutch initiation date. Host clutch size at time of parasitism was not important as 95 % CI of its model-averaged estimate included zero.
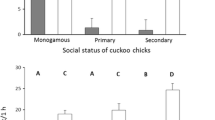
Only one model substantially explained the variation in cuckoo fledging success. This model contained only nest status and showed that cuckoo young were less likely to fledge from nests of polygynous than monogamous males (estimate ± SE = −1.805 ± 0.671, 95 % CI −2.912 to −0.442). The most pronounced differences in cuckoo fledging success were between monogamous and secondary nests, monogamous nests being more than twice as successful as secondary nests (Table 3, Fig. 1). Moreover, there was a tendency to lower cuckoo success in primary compared to monogamous nests and no differences between primary and secondary nests (Table 3, Fig. 1).
Out of 50 nests with accepted cuckoo eggs, 30 (60 %) were successful. Of the seven unsuccessful monogamous nests, three nest failures were due to predation, one due to starvation and three cases of nest failure were due to other causes. Of the five unsuccessful primary nests, two were predated, two cuckoo chicks starved to death and one case of nest failure was due to other causes. Of the eight unsuccessful secondary nests, four were predated, two cuckoo chicks starved to death and two cases were due to other causes. There were no significant differences among the three category of nests with respect to the cause of nest failure (Fisher exact probability test: P = 0.822).
Discussion
In the present study, we detected 60 % overall fledging success of cuckoo young in nests of their great reed warbler hosts, which is comparable to 69.2 % reported by Kleven et al. (2004). Nevertheless, we found high variation in cuckoo fledging success with respect to the host social mating status. As we predicted, cuckoo chicks in nests of monogamous males had higher fledging success than chicks in the nests of polygynous males. We found that cuckoos had more than two times higher fledging success in monogamous nests than in secondary nests (i.e. 76 and 33 %, respectively; Fig. 1). However, cuckoo fledging success between monogamous and primary nests only tended to differ, and we detected no difference when comparing primary and secondary nests.
In the light of studies conducted by Sejberg et al. (2000) and Trnka and Prokop (2010), we suppose that lower fledging success of cuckoos in secondary nests may result from their higher mortality, caused by starvation and predation due to insufficient assistance with feeding of young or nest defence rendered by polygynous males. Starvation of cuckoo chicks may, however, also be a result of host anti-parasite defence (Grim et al. 2003; Grim 2007). Although it may hardly apply to the cuckoos raised only in secondary nests (see “Results”), this possibility should be tested more rigorously in future studies. In addition, differences in growth rates among the cuckoo chicks raised in nests of different status may also stand behind lower cuckoo fledging success in the secondary nests. Neither our study nor the others, unfortunately, have explored growth rates, parental food provisioning and defence of cuckoo chicks in host nests of different status. Only Honza et al. (2010) showed that great reed warblers defended cuckoo chicks with similar intensity as their own offspring, but they did not consider host social mating status. Moreover, there may be other variables related to host social mating status that could explain our results, including female physical condition or phenotypic quality, female age, nest location or territory quality (Verner and Willson 1966; Orians 1969; Slagsvold and Lifjeld 1994; Forstmeier et al. 2001a, b; Grønstøl et al. 2003). Such relationships, however, have not been found either in our or other great reed warbler populations (Leisler et al. 1995; Bensch 1996; Hansson et al. 2000; Honza et al. 2011; Trnka and Prokop 2011; Trnka et al. 2012, this study). Therefore, to support our assumptions mentioned above, further field studies are needed. Only carefully designed experiments may discern between the contribution of different components of host parental care to the differential survival rates of cuckoo nestlings in nests of monogamous and polygynous hosts. Such studies would greatly improve our understanding of the costs of polygyny for the brood parasites as well as for their hosts.
In contrast to the variation in fledging success of cuckoos fostered by hosts of different social mating status, rejection rates of cuckoo eggs were similar on monogamous, primary and secondary nests. We found, however, that early breeders (regardless of their mating status) exhibited higher egg-rejection rates than later breeding birds. Previous studies on the closely related oriental reed warbler (Acrocephalus orientalis) demonstrated that rejection rates of parasitic eggs relate to host age (Lotem et al. 1992, 1995). Young breeders are usually naive (inexperienced), start breeding later in the season (i.e. they have lower renesting potential) than old and more experienced birds. Thus, younger great reed warbler females could be also more likely to accept cuckoo eggs than older females. However, due to the difficulty of ageing great reed warblers after complete moult and because only a few ringed nestlings returned to our study sites, we could not determine the age of all breeding birds and thus evaluate its effect on egg-rejection behaviour. Therefore, we could not decide which factor is more relevant to egg rejection, whether host age (in terms of egg recognition abilities) or time constraints associated with late breeding.
Although several previous studies documented non-random distribution of parasitized nests within a host population (e.g. Soler et al. 1995; Avilés et al. 2006; Polačiková et al. 2009, but see Antonov et al. 2012), we did not find any differences in parasitism rates among the nests of different status. This seems to contradict the previous study of Trnka and Prokop (2011), where nests of polygynous great reed warbler males suffered more from cuckoo parasitism than monogamous nests. However, Trnka and Prokop (2011) considered only successfully parasitized nests, i.e. those where the cuckoo chicks hatched and evicted host offspring. In the present study, on the other hand, we investigated the levels of initial cuckoo parasitism (i.e. the percentage of naturally parasitized nests) in monogamous and polygynous hosts. When we re-analysed both data sets using the same measure of parasitism (i.e. the rate of successful cuckoo parasitism), we found the same pattern as above. In Trnka and Prokop’s study, successful cuckoo parasitism differed with nest status (Fisher exact probability test: P = 0.003), while in the present study, it did not (χ 2 = 4.22, df = 2, P = 0.121). It seems that these results are driven by the site-specific differences as rates of successful parasitism differed at site 1 (χ 2 = 14.61, df = 2, P < 0.001; data from both studies pooled), but not at site 2 (χ 2 = 1.96, df = 2, P = 0.375).
Considering that cuckoo females that parasitize monogamous great reed warbler nests achieve much higher breeding success than those parasitizing nests of polygynous males, we suppose that monogamous pairs are more suitable as cuckoo hosts than polygynous. However, cuckoos most probably do not differentiate between the nests of different status and parasitize them perhaps following a simple nest-visibility rule (see also Moskát and Honza 2000; Avilés et al. 2009; V. Jelínek et al. in prep.). Nonetheless, our study suggests that the actual level of social polygyny in a host population may considerably influence the overall reproductive success of a local cuckoo population. Therefore, social mating status of a host may be an important confounding variable affecting the outcomes of studies on cuckoo parasitism and population dynamics of cuckoo–host associations and, as such, should be controlled for in field experiments.
References
Antonov A, Stokke BG, Fossøy F, Ranke PS, Liang W, Yang C, Moksnes A, Shykoff J, Røskaft E (2012) Are cuckoos maximizing egg mimicry by selecting host individuals with better matching egg phenotypes? PLoS ONE 7(2):e31704. doi:10.1371/journal.pone.0031704
Avilés JM, Stokke BG, Moksnes A, Røskaft E, Åsmul M, Møller AP (2006) Rapid increase in cuckoo egg matching in a recently parasitized reed warbler population. J Evol Biol 19:1901–1910. doi:10.1111/j.1420-9101.2006.01166.x
Avilés JM, Moskát C, Bán M, Hargitai R, Parejo D (2009) Common cuckoo (Cuculus canorus) do not rely on indicators of parental abilities when searching for host nests: the importance of host defenses. Auk 126:431–438. doi:10.1525/auk.2009.08162
Bartoń K (2011) MuMIn: multi-model inference. R package version 1.3.6. http://CRAN.R-project.org/package=MuMIn
Bates D, Maechler M, Dai B (2008) lme4: linear mixed-effects models using S4 classes. R package version 0.999375-28. http://lme4.r-forge.r-project.org/
Bensch S (1996) Female mating status and reproductive success in the great reed warbler: is there a potential cost of polygyny that requires compensation? J Anim Ecol 65:283–296. doi:10.2307/5875
Bensch S, Hasselquist D (1991) Nest predation lowers the polygyny threshold: a new compensation model. Am Nat 138:1297–1306. doi:10.1086/285287
Bensch S, Hasselquist D (1994) Higher rate of nest loss among primary than secondary females: infanticide in the great reed warbler? Behav Ecol Sociobiol 35:309–317. doi:10.1007/BF00184420
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. doi:10.1016/j.tree.2008.10.008
Brooke M de L, Davies NB (1987) Recent changes in host usage by cuckoos Cuculus canorus in Britain. J Anim Ecol 56:873–883. doi:10.2307/4954
Brooke M de L, Davies NB (1989) Provisioning of nestling cuckoos Cuculus canorus by reed warbler Acrocephalus scirpaceus hosts. Ibis 131:250–256. doi:10.1111/j.1474-919X.1989.tb02767.x
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: A practical information-theoretic approach. Springer, Berlin
Catchpole C, Leisler B, Winkler H (1985) Polygyny in the great reed warbler, Acrocephalus arundinaceus—a possible case of deception. Behav Ecol Sociobiol 16:285–291. doi:10.1007/BF00310992
Cherry MI, Bennett ATD, Moskát C (2007) Host intraclutch variation, cuckoo egg matching and egg rejection by great reed warblers. Naturwissenchaften 94:441–447. doi:10.1007/s00114-007-0216-4
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Cramp S (1992) The birds of the western Palearctic, vol 6. Oxford University Press, Oxford
Davies NB (2000) Cuckoos, cowbirds and other cheats. Academic Press, London
Davies NB (2011) Cuckoo adaptations: trickery and tuning. J Zool 284:1–14. doi:10.1111/j.1469-7998.2011.00810.x
De Mársico MC, Reboreda JC (2010) Brood parasitism increases mortality of bay-winged cowbird nests. Condor 112:407–417. doi: http://dx.doi.org/10.1525/cond.2010.090118
Dearborn DC (1999) Brown headed cowbird nestling vocalizations and risk of nest predation. Auk 116:448–457
Dyrcz A (1986) Factors affecting facultative polygyny and breeding results in the great reed warbler (Acrocephalus arundinaceus). J Ornithol 127:447–461. doi:10.1007/BF01640260
Forstmeier W, Kuijper DPJ, Leisler B (2001a) Polygyny in the dusky warbler, Phylloscopus fuscatus: the importance of female qualities. Anim Behav 62:1097–1108. doi:10.1006/anbe.2001.1859
Forstmeier W, Leisler B, Kempenaers B (2001b) Bill morphology reflects female independence from male parental help. Proc R Soc Lond B 268:1583–1588. doi:10.1098/rspb.2001.1692
Griggio M, Tavecchia G, Biddau L, Mingozzi T (2003) Mating strategies in the rock sparrow Petronia petronia: the role of female quality. Ethol Ecol Evol 15:389–398
Grim T (2006) Cuckoo growth performance in parasitized and unused hosts: not only host size matters. Behav Ecol Sociobiol 60:716–723. doi:10.1007/s00265-006-0215-z
Grim T (2007) Experimental evidence for chick discrimination without recognition in a brood parasite host. Proc R Soc Lond B 274:373–381. doi:10.1098/rspb.2006.3731
Grim T, Kleven O, Mikulica O (2003) Nestling discrimination without recognition: a possible defence mechanism for hosts towards cuckoo parasitism? Proc R Soc Lond B 270:S73–S75. doi:10.1098/rsbl.2003.0017
Grim T, Samaš P, Moskát C, Kleven O, Honza M, Moksnes A, Røskaft E, Stokke BG (2011) Constraints on host choice: why do parasitic birds rarely exploit some common potential hosts? J Anim Ecol 80:508–518. doi:10.1111/j.1365-2656.2010.01798.x
Grønstøl GB, Byrkjedal I, Fiksen Ø (2003) Predicting polygynous settlement while incorporating varying female competitive strength. Behav Ecol 14:257–267. doi:10.1093/beheco/14.2.257
Hannon SJ, Wilson S, McCallum CA (2009) Does cowbird parasitism increase predation risk to American redstart nests? Oikos 118:1035–1043. doi:10.1111/j.1600-0706.2008.17383.x
Hansson B, Bensch S, Hasselquist D (2000) Patterns of nest predation contribute to polygyny in the great reed warbler. Ecology 81:319–328. doi:10.1890/0012-9658(2000) 081[0319:PONPCT]2.0.CO;2
Hasselquist D (1998) Polygyny in great reed warblers: a long-term study of factors contributing to male fitness. Ecology 79:2376–2390. doi:10.1890/0012-9658(1998) 079[2376:PIGRWA]2.0.CO;2
Hauber ME (2003) Hatching asynchrony, nestling competition, and the cost of interspecific brood parasitism. Behav Ecol 14:224–235. doi:10.1093/beheco/14.2.227
Honza M, Taborsky B, Taborsky M, Teuschl Y, Vogl W, Moksnes A, Røskaft E (2002) Behaviour of female common cuckoos, Cuculus canorus, in the vicinity of host nests before and during egg laying: a radiotelemetry study. Anim Behav 64:861–868. doi:10.1006/anbe.2002.1969
Honza M, Procházka P, Šicha V, Požgayová M (2010) Nest defence in a cuckoo host: great reed warblers risk themselves equally for their own and parasitic chicks. Behaviour 147:741–756. doi:10.1163/000579510X491081
Honza M, Požgayová M, Procházka P, Cherry MI (2011) Blue-green eggshell coloration is not a sexually selected signal of female quality in an open-nesting polygynous passerine. Naturwissenschaften 98:493–499. doi:10.1007/s00114-011-0790-3
Johnson LS, Kermott LH, Lein MR (1993) The cost of polygyny in the house wren Troglodytes aedon. J Anim Ecol 62:669–682. doi:10.2307/5387
Kilpatrick AM (2002) Variation in growth of brown-headed cowbird (Molothrus ater) nestlings and energetic impacts on their host parents. Can J Zool 80:145–153. doi:10.1139/Z01-217
Kleven O, Moksnes A, Røskaft E, Rudolfsen G, Stokke BG, Honza M (2004) Breeding success of common cuckoos Cuculus canorus parasitizing four sympatric species of Acrocephalus warblers. J Avian Biol 35:394–398. doi:10.1111/j.0908-8857.2004.03359.x
Leisler B, Wink M (2000) Frequencies of multiple paternity in three Acrocephalus species (Aves, Sylviidae) with different mating systems (A. palustris, A. arundinaceus, A. paludicola). Ethol Ecol Evol 12:237–249
Leisler B, Beier J, Heine G, Siebenrock KH (1995) Age and other factors influencing mating status in German great reed warblers (Acrocephalus arudinaceus). Jpn J Ornithol 44:169–180
Lotem A, Nakamura H, Zahavi A (1992) Rejection of cuckoo eggs in relation to host age: a possible evolutionary equilibrium. Behav Ecol 3:128–132. doi:10.1093/beheco/3.2.128
Lotem A, Nakamura H, Zahavi A (1995) Constraints on egg discrimination and cuckoo–host co-evolution. Anim Behav 49:1185–1209. doi:10.1006/anbe.1995.0152
Lubjuhn T, Winkel W, Epplen JT, Brün J (2000) Reproductive success of monogamous and polygynous pied flycatchers Ficedula hypoleuca. Behav Ecol Sociobiol 48:12–17. doi:10.1007/s002650000208
Mermoz ME, Reboreda JC (2003) Reproductive success of shiny cowbirds (Molothrus bonariensis) parasitizing the larger brown-and-yellow marshbird (Pseudoleistes virescens). Auk 120:1128–1139. doi: http://dx.doi.org/10.1642/0004-8038(2003)120[1128:RSOSCM]2.0.CO;2
Moksnes A, Røskaft E, Bičík V, Honza M, Øien IJ (1993) Cuckoo Cuculus canorus parasitism on Acrocephalus warblers in southern Moravia in the Czech Republic. J Ornithol 134:425–434. doi:10.1007/BF01639833
Moskát C, Hauber ME (2007) Conflict between egg recognition and rejection decisions in common cuckoo (Cuculus canorus) hosts. Anim Cogn 10:377–386. doi:10.1007/s10071-007-0071-x
Moskát C, Honza M (2000) Effect of nest and nest site characteristics on the risk of cuckoo Cuculus canorus parasitism in the great reed warbler Acrocephalus arundinaceus. Ecography 23:335–341. doi:10.1034/j.1600-0587.2000.d01-1642.x
Moskát C, Honza M (2002) European cuckoo Cuculus canorus parasitism and host’s rejection behaviour in a heavily parasitized great reed warbler Acrocephalus arundinaceus population. Ibis 144:614–622. doi:10.1046/j.1474-919X.2002.00085.x
Moskát C, Szentpéteri J, Barta Z (2002) Adaptations by great reed warblers to brood parasitism: a comparison of populations in sympatry and allopatry with the common cuckoo. Behaviour 139:1313–1329. doi:10.1163/156853902321104181
Moskát C, Bán M, Székely T, Komdeur J, Lucassen RWG, van Boheemen LA, Hauber ME (2010) Discordancy or template-based recognition? Dissecting the cognitive basis of the rejection of foreign eggs in hosts of avian brood parasites. J Exp Biol 213:1976–1983. doi:10.1242/jeb.040394
Numerov AD (2003) Interspecific and intraspecific brood parasitism in birds. Federal State Unitary Enterprise Publish and Polygraph Corporation, Voronezh
Øien IJ, Moksnes A, Røskaft E, Honza M (1998) Costs of cuckoo Cuculus canorus parasitism to reed warblers Acrocephalus scirpaceus. J Avian Biol 29:209–215. doi:10.2307/3677102
Olson VA, Liker A, Freckleton RP, Székely T (2008) Parental conflict in birds: comparative analyses of offspring development, ecology and mating opportunities. Proc R Soc Lond B 275:301–307. doi:10.1098/rspb.2007.1395
Orians GH (1969) On the evolution of mating systems in bird and mammals. Am Nat 103:589–603
Payne RB (1977) The ecology of brood parasitism in birds. Annu Rev Ecol Syst 8:1–28. doi:10.1146/annurev.es.08.110177.000245
Pinxten R, Eens M (1994) Male feeding of nestlings in the facultatively polygynous European starling: allocation patterns and effect on female reproductive success. Behaviour 129:113–140. doi:10.1163/156853994X00389
Polačiková L, Procházka P, Cherry MI, Honza M (2009) Choosing suitable hosts: common cuckoos Cuculus canorus parasitize great reed warbler Acrocephalus arundinaceus of high quality. Evol Ecol 23:879–891. doi:10.1007/s10682-008-9278-9
Požgayová M, Procházka P, Honza M (2009) Sex-specific defence behaviour against brood parasitism in a host with female-only incubation. Behav Process 81:34–38. doi:10.1016/j.beproc.2008.12.019
Požgayová M, Procházka P, Polačiková L, Honza M (2011) Closer clutch inspection—quicker egg ejection: timing of host responses toward parasitic eggs. Behav Ecol 22:46–51. doi:10.1093/beheco/arq163
Pribil S (2000) Experimental evidence for the cost of polygyny in the red-winged blackbird Agelaius phoeniceus. Behaviour 137:1153–1173. doi:10.1163/156853900502574
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Remeš V (2010) Explaining postnatal growth plasticity in a generalist brood parasite. Naturwissenschaften 97:331–335. doi:10.1007/s00114-009-0635-5
Rothstein SI (1990) A model system for coevolution: avian brood parasitism. Annu Rev Ecol Syst 21:81–508. doi:10.1146/annurev.ecolsys.21.1.481
Rutila J, Latja R, Koskela K (2002) The common cuckoo Cuculus canorus and its cavity nesting host, the redstart Phoenicurus phoenicurus: a peculiar cuckoo-host system? J Avian Biol 33:414–419. doi:10.1034/j.1600-048X.2002.02937.x
Schulze-Hagen K, Stokke BG, Birkhead TR (2009) Reproductive biology of the European cuckoo Cuculus canorus: early insights, persistent errors and the acquisition of knowledge. J Ornithol 150:1–16. doi:10.1007/s10336-008-0340-8
Scott DM, Lemon RE (1996) Differential reproductive success of brown-headed cowbirds with northern cardinals and three other hosts. Condor 98:259–271. doi:10.2307/1369144
Sejberg D, Bensch S, Hasselquist D (2000) Nestling provisioning in polygynous great reed warblers (Acrocephalus arundinaceus): do males bring larger prey to compensate for fewer nest visits? Behav Ecol Sociobiol 47:213–219. doi:10.1007/s002650050658
Sklepowicz B, Hałupka L (2009) The use of sympatric reed warblers Acrocephalus scirpaceus and marsh warblers Acrocephalus palustris as breeding hosts: parasitism rates and breeding success of common cuckoos Cuculus canorus. Acta Ornithol 44:177–184. doi:10.3161/000164509X482759
Slagsvold T, Lifjeld JT (1994) Polygyny in birds: the role of competition between females for male parental care. Am Nat 143:59–80. doi:10.1086/285596
Soler JJ, Soler M, Møller AP, Martínez JG (1995) Does the great spotted cuckoo choose magpie hosts according to their parenting ability. Behav Ecol Sociobiol 36:201–206. doi:10.1007/BF00177797
Stokke BG, Hafstad I, Rudolfsen G, Moksnes A, Møller AP, Røskaft E, Soler M (2008) Predictors of resistance to brood parasitism within and among reed warbler populations. Behav Ecol 19:612–620. doi:10.1093/beheco/arn007
Thomas GH, Székely T (2005) Evolutionary pathways in shorebird breeding systems: sexual conflict, parental care, and chick development. Evolution 59:2222–2230. doi:10.1111/j.0014-3820.2005.tb00930.x
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man, 1871–1971. Aldine, Chicago, pp 136–179
Trnka A, Prokop P (2010) Does social mating system influence nest defence behaviour in great reed warbler Acrocephalus arundinaceus males? Ethology 116:1075–1083. doi:10.1111/j.1439-0310.2010.01821.x
Trnka A, Prokop P (2011) Polygynous great reed warblers Acrocephalus arundinaceus suffer more cuckoo Cuculus canorus parasitism than monogamous pairs. J Avian Biol 42:192–195. doi:10.1111/j.1600-048X.2010.05193.x
Trnka A, Prokop P, Batáry P (2010) Infanticide or interference: does the great reed warbler selectively destroy eggs? Ann Zool Fenn 47:272–277
Trnka A, Prokop P, Kašová M, Sobeková K, Kocian Ľ (2012) Hatchling sex ratio and female mating status in the great reed warbler, Acrocephalus arundinaceus (Aves, Passeriformes): further evidence for offspring sex ratio manipulation. Ital J Zool 79:212–217. doi:10.1080/11250003.2011.631945
Verner J, Willson MF (1966) The influence of habitats on mating systems of North American passerine birds. Ecology 47:143–147. doi:10.2307/1935753
Webster MS (1991) Male parental care and polygyny in birds. Am Nat 137:274–280. doi:10.1086/285161
Wyllie I (1981) The cuckoo. Batsford, London
Acknowledgments
We thank T. Bolcková, M. Čapek, V. Jelínek, M. Kašová, K. Morongová, Z. Šebelíková, M. Šulc and B. Trnková for their invaluable assistance in the field. Suggestions of three anonymous referees helped to substantially improve the manuscript. The study was supported by the Slovak Grant Agency for Science (grant number 1/0566/09), the Grant Agency of the Academy of Sciences of the Czech Republic (grant number IAA600930903), the Czech Science Foundation (grant number P506/12/2404) and the Institutional Research Plan (RVO: 68081766). We are obliged to the Slovak Fishing Association, management of the Fish Farm Hodonín and local conservation authorities for the permissions to conduct the fieldwork.
Ethical standards
The work described here was done under licence and complied with the current laws of the countries in which it was performed. Licences to conduct the research and bird ringing in Slovakia were issued by the Ministry of Environment of the Slovak Republic (licences number 269/132/05-5.1pil and 7230/2008-2.1pil). The fieldwork in the Czech Republic adhered to the Animal Care Protocol of the Academy of Sciences of the Czech Republic (licence number 0008/98-M103) and current Czech Law on the Protection of Animals against Mistreatment.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Soler
Rights and permissions
About this article
Cite this article
Trnka, A., Požgayová, M., Procházka, P. et al. Breeding success of a brood parasite is associated with social mating status of its host. Behav Ecol Sociobiol 66, 1187–1194 (2012). https://doi.org/10.1007/s00265-012-1372-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1372-x