Abstract
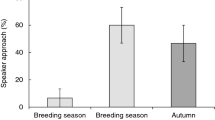
Black-capped chickadees Poecile atricapillus alter the number of D notes of their chick-a-dee call to reflect urgency and threat. Here, I tested whether heterospecific responses of an allopatric species to these mobbing calls occur. Heterospecific chickadee mobbing calls and songs from North America were broadcast to European great tits (Parus major) and compared with conspecific mobbing calls. During conspecific mobbing playbacks, all great tits approached the speaker, during the heterospecific “chick-a-dee” playbacks, 63.3% individuals approached the speaker, while during the song playback, only 31.3% of the great tits approached the speaker. Minimum distances of great tits were lower during conspecific mobbing calls compared to allopatric chick-a-dee calls and to allopatric chickadee song. Also, minimum distances were lower when comparing allopatric chick-a-dee calls and chickadee song. Great tits approached the speaker on average down to (mean ± SE) 20.0 ± 1.8 m during playbacks of 1–4 D elements, to 17.7 ± 2.0 m during playbacks of 5–7 D elements and down to 11.5 ± 2.0 m during playbacks of 8–11 D elements. The number of D notes was inversely related to minimum distance. Thus, the urgency message encoded in the D notes was perceived also by an allopatric but phylogenetically related European species, suggesting that the heterospecific response is possibly phylogenetically conserved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals use acoustic signals in a wide variety of contexts, such as mate choice, foraging, flock maintenance, and alarm or mobbing activities (Charrier and Sturdy 2005). Alarm calls are usually produced when animals encounter predators, and these calls are mainly addressed at conspecifics (Templeton and Greene 2007) and also at heterospecifics and at predators (e.g., Pavey and Smyth 1998; Caro 2005). In songbirds, such alarm calls are typically shorter and less complex than songs and used in a wider range of contexts (Charrier and Sturdy 2005).
Alarm call systems have been broadly classified into referential and risk-based systems. In species with more complex vocal abilities, referential signals label different categories of predators, which has been shown in mammals and in birds (see Caro 2005 for an overview), in which different call types distinguish between terrestrial and aerial predators or between high and low urgency. Species with a less complex alarm call system or limited vocal complexity often use the same (or a similar) call type for various predator species, threat levels, or situations. Their calls differ in call rate or production pattern to denote different threat levels. However, in some species, both systems exist (e.g., in Poecile tits, Templeton et al. 2005).
The level of urgency can be encoded in different ways, either by changes in acoustic structures of calls (Manser 2001) or in variation/increase in call rate (e.g., repetition of or different number of elements, Blumstein and Armitage 1997; Baker and Becker 2002; Templeton et al. 2005; Templeton and Greene 2007; Fallow and Magrath 2010). For example, in juvenile hyenas Crocuta crocuta, the reduction of intervals between whoop calls in a calling bout increased the likelihood of a conspecific to approach the caller (Theis et al. 2007). Yellow mongoose Cynictis penicillata use an urgency-based alarm calling system, indicating high and low urgency through two distinct call types (Roux et al. 2009). Black-capped Poecile atricapillus and Carolina chickadees Poecile carolinensis alter the number of D notes of their chick-a-dee call to reflect urgency and threat level with more D notes reflecting higher threat (Templeton et al. 2005; Soard and Ritchison 2009). Furthermore, different levels of arousal are expressed in call structure (e.g., frequency; Fichtel and Hammerschmidt 2002).
Mobbing calls can be seen as special recruitment calls to gather conspecifics, e.g., in suricates Suricata suricatta to mob snakes (Manser et al. 2001), or represent the affective state of the caller who wants the group to gather together (Manser 2001). In birds, and especially in passerines, heterospecific attraction and mobbing behavior has received much attention (Caro 2005). Different hypotheses have been explicated to explain mobbing behavior of passerines, e.g., driving the predator away (move-on hypothesis—Pettifor 1990; Flasskamp 1994), as cultural transmission of predator recognition from parents to juveniles (Curio et al. 1978; Frankenberg 1981), or as predator–prey communication (perception advertisement or quality advertisement—Curio et al. 1978; Ostreiher 2003). While mobbing, passerines mob a predator by emitting repeated, loud, and easily localizable calls that recruit other con- and heterospecific individuals (Curio et al. 1978; Hurd 1996; Forsman and Mönkkönen 2001; Baker and Becker 2002; Krams and Krama 2002).
Most of these alarm call systems are primarily denoted to conspecific receivers. However, this may represent a study bias because communication networks exist and have been under research in the last decades, mainly with an emphasis on conspecific communication networks (Dabelsteen 2005; McGregor 2005; Matessi et al. 2008). Interspecific information transfer within animal communities has recently received much attention (e.g., Magrath et al. 2009a, b; Goodale et al. 2010). Heterospecifics acquire information about threat levels or predator types. However, it is yet unclear whether these signals are explicitly addressed to both con- and heterospecifics, or whether heterospecifics recognize and make use of alarm calls by eavesdropping. Heterospecific recognition has been studied in a variety of species within and between animal classes (see e.g., Nuechterlein 1981; Ramakrishnan and Coss 2000; Randler 2006; Magrath et al. 2007; Templeton and Greene 2007; Lea et al. 2008; Fallow and Magrath 2010; Kitchen et al. 2010).
Heterospecific responses within animal classes have been found, e.g., in different primate species (Hauser and Wrangham 1990; Oda and Matasaka 1996; Ramakrishnan and Coss 2000; Zuberbühler 2000; Fichtel 2004), in bats (Russ et al. 2004), or within the sciurids (Blumstein and Armitage 1997). In birds, alarm calls of black-capped chickadees and tufted titmice (Parus bicolor) lead to an increase in vigilance in downy woodpeckers (Picoides pubescens—Sullivan 1984) and western grebes (Aechmophorus occidentalis) eavesdropped on the alarm calls of Forster’s terns (Sterna forsteri—Nuechterlein 1981).
Between animal classes, heterospecific alarm call recognition has been reported from vervet monkeys (Cercopithecus aethiops) responding to alarm calls of superb starlings (Spreo superbus—Hauser 1988; Seyfarth and Cheney 1990) and hornbills (Ceratogymna elata and Ceratogymna atrata) distinguish between different primate alarm calls (e.g., terrestrial vs. aerial predator—Rainey et al. 2004a, b). Müller and Manser (2008) reported that banded mongooses Mungos mungo responded to alarm calls of three plover Vanellus species suggesting that banded mongooses use heterospecific alarms for predator avoidance but do not use additional information provided in these signals (high vs. low urgency). Vitousek et al. (2007) found even a response of a non-vocal reptile to the alarm calls of a bird.
A recent study showed that heterospecifics are unable to recognize subtle differences between contact and mobbing calls from chaffinches (Fringilla coelebs) but rather assess urgency by the number of elements (Randler and Förschler 2011). Heterospecific alarm call recognition may be facilitated—at least partially—by a similar structure of different species’ mobbing calls (Ficken and Popp 1996; Hurd 1996; Johnson et al. 2003; Magrath et al. 2007; Fallow and Magrath 2010). However, heterospecific recognition can be based on both, an innate component and learning (see e.g., Ramakrishnan and Coss 2000; Shriner 1999; Magrath et al. 2009a; Fallow et al. 2011). Johnson et al. (2003) found an anti-predator response to unfamiliar calls of an allopatric species, while blue-gray tanagers (Thraupis episcopus) did not respond to unfamiliar mobbing calls (Nocera et al. 2009).
Most North American species of the family Paridae use a similar alarm-calling system, comprised of risk-based predator-mobbing alarms (chick-a-dee calls, with variation in D numbers) and distinct “seet” alarm calls to label aerial predators in flight (Langham et al. 2006; Templeton and Greene 2007; Sieving et al. 2010). Following Langham et al. (2006), this parid call system seems highly conserved and even works in allopatric taxa of the Paridae. In detail, the number of D notes of black-capped chickadee calls are a pattern that encodes threat (Templeton et al. 2005), and is a graded signal that informs conspecifics about the presence of a predator in Carolina chickadees (Soard and Ritchison 2009; Bartmess-LeVasseur et al. 2010) and tufted titmice Baeolophus bicolor (Courter and Ritchison 2010). There was a strong inverse relationship between the number of D notes per alarm call and the wingspan of raptors and predator body length, with the smallest predators eliciting calls with the most D notes (Templeton et al. 2005; Courter and Ritchison 2010). These mobbing calls of black-capped chickadees attract both, con- and heterospecific sympatric species (Hurd 1996; Turcotte and Desrochers 2002; Templeton and Greene 2007).
Apart from situations in a predator-related context, more D notes within a call lead to a higher recruitment (measured as latency to take food; Mahurin and Freeberg 2009). According to their note composition (e.g., the detailed composition and variation of the “chick-a-dee”), these various calls are given in situations of mild alarm, as contact calls for the pair and flock and in coordinating group movements (Ficken et al. 1978).
As mobbing calls within the family Paridae seem highly conserved (Langham et al. 2006), here I tested, whether these mobbing calls are innate versus learned by comparing alarm/mobbing calls from an allopatric taxa with their respective song, and I assessed the possible phylogenetic conservation of the chickadee alarm call system. In this study, chick-a-dee alarm/mobbing calls were broadcast to great tits Parus major in Central Europe and compared with their own conspecific mobbing call and the heterospecific song of the chickadee to control for novelty. Furthermore, the relationship between the number of D notes and the response was assessed.
Materials and methods
Great tits were used as focal species because they show a mobbing response during large—if not all—parts of the year (e.g., January until July—Hinde 1952). In a previous study, great tits were identified as one of the strongest responders to mobbing calls of a sympatric heterospecific species (Randler and Förschler 2011). Black-capped chickadee alarm calls and songs were obtained from the xeno-canto database (www.xeno-canto.org) and the website of the University of Washington. Great tit playbacks were obtained from own recordings, the xeno-canto database, and from Schulze (2003). I obtained vocalizations from 4 different individuals for song playback (frequency range approximately between 3 and 4 kHz; Ratcliffe and Weisman 1985), of 11 individuals for chick-a-dee playbacks, and of 4 different great tits for conspecific mobbing playbacks. The calls and songs were digitally edited to minimize disturbing noises using Avisoft SASLab Pro 4.3 (Raimund Specht). All chick-a-dee calls were used in their natural sequence (that is, all chick-a-dee calls contained all the elements but D notes were varied), but additionally, some calls were manipulated to obtain an equal number of playbacks (e.g., if a 5 D call sequence was needed, a 6 D call sequence was shortened by 1 D note by removing alternatively the last D note or one in the midst). Afterwards, the calls were copied to an analogous tape using a Grundig 437 CD player and AIWA CX-Z87M cassette recorder to produce the playback tapes. Calls were broadcast using a small portable SuperTech MCR 103 cassette recorder at about 76 (72–79) dB measured at 1 m from the loudspeaker using a PeakTech 5035 sound level meter. All stimuli were standardized on 5 min (which was the observation time). Three different playback tapes were constructed for each example and broadcast four times (thus, leading to a total of 12 playbacks of 1 D note, 12 playbacks of 2 D notes, …). This lead to a total of 120 playbacks with chick-a-dee calls (1, 2, 3, 4, 5, 6, 7, 8, 9, 11 D notes, respectively), of 16 playbacks of the “fee-bee” song of the black-capped chickadee (of four different stimuli), and of 14 playbacks of 4 conspecific great tit calls (“churr” calls) which served as a positive control. The number of playbacks was not equalized because two different questions were addressed. First, the differences between song, conspecific and heterospecific alarm calls were tested (and the number of song playbacks and conspecific playbacks were sufficient to obtain an effect), and in the second question, the number of D-syllables were varied, leading to a higher sample size of chick-a-dee alarm calls. The playbacks were broadcast to 150 different individual great tits: 14 were tested with conspecific playback, 120 with chickadee calls, and 16 with chickadee songs. The datasets are independent, i.e., every subject was tested only with one of the three stimuli. The study area is large (about 90 km2) and easy accessible by roads and trails, and I worked in more than 20 different parts of it to avoid using an individual tit more than once. Playback sites were separated by more than 500 m, but as the individuals were not marked, sampling the same individual twice would be possible but unlikely. Every trail was walked only once and covered during the same day.
Trials were conducted between February 16, 2011 and April 1, 2011 and between 0830 and 1400 hours. These dates correspond to the beginning of the territorial phase of great tits, but ended well before the mean date of egg-laying (Hölzinger 1997). Seasonal effects might have an influence on the response of tits but the playbacks were evenly distributed across the study period to avoid that, e.g., the territorial song of the chickadee would have been used in February and the alarm call in March. Broadcasting of playbacks started when there was a sequence of 5 min where no alarm or mobbing calls of the target bird had occurred. A focal great tit was selected when it was approximately 30 m away. Observations were made from a distance of 40 m from the playback source and there was no obvious influence on the birds’ behavior. The approach of the individual great tit (yes/no) and the minimum distance to the playback source was recorded. The basis of the analysis was playback site and each playback site was used only once. The distance between the different playback sites was far enough (at least 500 m) to minimize that a responding individual contributed more than once to the analyses. Playbacks were made in the Odenwald region of mixed deciduous forest northeast of Heidelberg, SW Germany. The song of the black-capped chickadee was used to control for novelty, i.e., to secure that the great tits do not simply respond to any novel sound.
For analyses, different approaches were used. First, chick-a-dee calls were compared with song and conspecific mobbing. A chi-square test based on the crosstab function in PASW 19.0 was used for this analysis, and data on conspecific alarm calls and on fee-bee song were skewed; therefore, non-parametric tests were used when comparing the three groups. Second, chick-a-dee calls were grouped arbitrarily into three groups: 1–4 D elements (N = 48), 5–7 D elements (N = 36), and 8–11 D elements (N = 36), suggesting a three different threat level. The general linear mixed models accounted for the four trials for each stimulus by using the “stimulus” and the “source” from which the original sounds were obtained as a random factor (stimulus nested within source). This accounted for the fact that all stimuli have been used four times and that some original sources were used more than once (see for similar methods, e.g., Barrera et al. 2011). Finally, the response was calculated as mean for each D playback. PASW 19.0 was used for analysis and the alpha-level was set at 0.05 (two-tailed).
Results
During the 14 conspecific playbacks (“churr” calls), all 14 great tits approached the speaker, and during the heterospecific “chick-a-dee” playbacks, 76 individuals (63.3%) approached the speaker and 44 did not, while during the song (“fee-bee”) playback, only 5 (31.3%) out of 16 individuals approached the speaker. This was significantly different (χ2 = 15.1, df = 2, p = 0.001). There were significant differences in minimum approach distance to the speaker among conspecific mobbing calls, allopatric mobbing calls, and allopatric song (Kruskal–Wallis test χ2 = 32.29, df = 2, p < 0.001, Fig. 1). Minimum distances of great tits were lower during conspecific calls (“churr” calls) compared to allopatric chick-a-dee calls (Mann–Whitney U test, Z = −4.743, p < 0.001, N 1 = 14, N 2 = 120) and to allopatric chickadee “fee-bee” song (Mann–Whitney U test, Z = −4.653, p < 0.001, N 1 = 14, N 2 = 16). Minimum approach distances were also lower when allopatric chick-a-dee calls and chickadee song were compared (Mann–Whitney U test, Z = −2.930, p = 0.003, N 1 = 120, N 2 = 16). Thus, chick-a-dee calls lead to a stronger heterospecific attraction of great tits compared to the response toward chickadee song playback.
Using a general linear mixed model based on minimum approach distance as dependent variable, playback tape/stimulus nested within source as random factor and number of D elements as fixed factor revealed a significant influence of the number of dee elements (F 9,110 = 2.86, p = 0.004). Using a general linear mixed model based on the groupings of D elements revealed that great tits approached the speaker on average down to (mean ± SE) 20.0 ± 1.8 m during playbacks of 1–4 D elements, to 17.7 ± 2.0 m during playbacks of 5–7 D elements, and down to 11.5 ± 2.0 m during playbacks of 8–11 D elements (F 2,27 = 4.88, p = 0.015). The number of D-syllables was inversely related to minimum distance (regression: F 1,8 = 9.20, p = 0.016, N = 10, R 2 = 0.535, Fig. 2).
Discussion
Great tits responded more strongly towards the allopatric chick-a-dee mobbing calls than towards the allopatric song which seems a plausible response because the song of the black-capped chickadee is usually directed towards conspecifics and the mobbing calls are addressed at both con- and heterospecifics. As expected, great tits reacted most strongly towards their own mobbing calls. However, the response that great tits showed towards the allopatric mobbing calls was rather similar compared to the reaction of conspecific playbacks of Parid mobbing calls in North America (Templeton et al. 2005; Soard and Ritchison 2009; Courter and Ritchison 2010), and great tits approached nearer to the speaker the more D notes per call have been broadcast. Thus, the urgency message encoded in these D notes was perceived also by an allopatric but phylogenetically related European species. This study is another one showing that heterospecific recognition of alarm calls exists. In addition, it is among the first studies addressing the issue of heterospecific responses of allopatric taxa (Nocera et al. 2009). One aspect that should be considered is that the approach effect might be a detection issue. However, as the individuals approached the speaker, but minimum distance varied significantly according to the D notes, it seems an urgency-based response following the rule “more D notes–more urgency” rather than being a detection issue. It would be interesting to particularly investigate this effect in black-capped chickadees because a recent study suggests that the duty cycle may also be responsible for approach effects (Wilson and Mennill 2011).
It is unclear whether the great tits perceived the mobbing calls as mobbing calls or as food calls. In chickadees, both calls attract conspecifics (Mahurin and Freeberg 2009), but in one case, food intake is a result and in the other case, mobbing results. It would be interesting to tease these two aspects apart in a further study on heterospecific responses of the great tits (by using both food calls and alarm calls). This could be done with a 2 × 2 design varying both the calls and the presentation of food. In this present study, only alarm calls of the chickadees were used, and therefore, the response could be considered as a response to alarm calls. In addition, the great tits showed typical mobbing behavior as response, such as using mobbing and/or alarm calls during their approach towards the speaker or wing flicking, supporting the suggestion that the heterospecific mobbing calls were perceived as mobbing calls. Also, there was no food provided in the vicinity of the playback sites. As the mobbing calls are loud, they impose a predation risk to the signaler because they may attract other predators (see discussion about loud and soft calls in Krama et al. 2008). But benefits may be that other individuals or species respond to the loud mobbing calls and join a flock to mob a predator. In addition, dominant individuals more often use loud calls (Krams 2000) suggesting it is also a signal of quality.
One explanation may be that these calls are acoustically similar as a result of calls retaining features from a common ancestor (de Kort and ten Cate 2001). Thus, acoustic similarity might facilitate heterospecific recognition. This could be studied by using manipulations of alarm calls to test to what extent which traits of the calls are responsible to elicit a reaction. Fallow et al. (2011) proposed that acoustic similarity can prompt responses to heterospecific alarm calls regardless of experience. It would be interesting to repeat the study with other European species and on other continents, e.g., in the Asia-Pacific region.
As a last explanation, we could assume that there is a simple general rule in heterospecific alarm calling across taxa: The more intense calling leads to more attraction. For some species, it is known that sympatric heterospecifics are more attracted to playback of mobbing calls with a higher calling rate (e.g., Templeton and Greene 2007; Fallow and Magrath 2010; Randler and Förschler 2011). This would be interesting to test with original and experimentally manipulated mobbing calls. However, as a cautionary note, Nocera et al. (2009) found that tanagers did not respond to playbacks of unfamiliar Poecile mobbing calls. Also, some European species do not respond to each other’s mobbing calls. This suggests that the “more threat/urgency evokes more calling” rule is not a general pattern across all species but might be more conserved in phylogenetically closely related species.
As a conclusion, the allopatric response to mobbing calls of black-capped chickadees by great tits shows that this response is either a phylogenetically conserved recognition mechanism within the family Paridae (Langham et al. 2006), or it may be a general rule in heterospecific attraction “the more intense calling represents higher threat/urgency.” Future work should include different species of the Paridae to assess differences in phylogenetic relationship and inherited heterospecific recognition (de Kort and ten Cate 2001).
References
Baker MC, Becker AM (2002) Mobbing calls of black-capped chickadees: effects of urgency on call production. Wilson Bull 114:510–516
Barrera JP, Chong L, Judy KN, Blumstein DT (2011) Reliability of public information: predators provide more information about risk than conspecifics. Anim Behav 81:779–787
Bartmess-LeVasseur J, Branch CL, Browning SA, Owens JL, Freeberg TM (2010) Predator stimuli and calling behavior of Carolina chickadees (Poecile carolinensis), tufted titmice (Baeolophus bicolor), and white-breasted nuthatches (Sitta carolinensis). Behav Ecol Sociobiol 64:1187–1198
Blumstein DT, Armitage KB (1997) Alarm calling in yellow-bellied marmots: I. The meaning of situationally variable alarm calls. Anim Behav 53:143–171
Caro T (2005) Anti-predator defence in mammals and birds. Chicago University Press, Chicago
Charrier I, Sturdy CB (2005) Call-based species recognition in black-capped chickadees. Behav Process 70:271–281
Courter JR, Ritchison G (2010) Alarm calls of tufted titmice convey information about predator size and threat. Behav Ecol 21:936–942
Curio E, Ernst U, Vieth W (1978) The adaptive significance of avian mobbing. II. Cultural transmission of enemy recognition in blackbirds: effectiveness and some constraints. Z Tierpsychol 48:184–202
Dabelsteen T (2005) Public, private or anonymous? Facilitating and countering eavesdropping. In: McGregor PK (ed) Animal Communication Networks. Cambridge University Press, Cambridge, pp 38–62
de Kort SR, ten Cate C (2001) Response to interspecific vocalizations is affected by the degree of phylogenetic relatedness in Streptopelia doves. Anim Behav 61:239–247
Fallow PM, Magrath RD (2010) Eavesdropping on other species: mutual interspecific understanding of urgency information in avian alarm calls. Anim Behav 79:411–417
Fallow PM, Gardner JL, Magrath RD (2011) Sound familiar? Acoustic similarity provokes responses to unfamiliar heterospecific alarm calls. Behav Ecol 22:401–410
Fichtel C (2004) Reciprocal recognition of sifaka (Propithecus verreauxi verreauxi) and redfronted lemur (Eulemur fulvus rufus) alarm calls. Anim Cogn 7:45–52
Fichtel C, Hammerschmidt K (2002) Responses of red-fronted lemurs to experimentally modified alarm calls: evidence for urgency-based changes in call structure. Ethology 108:763–777
Ficken MS, Ficken RW, Witkin SR (1978) Vocal repertoire of the black-capped chickadee. Auk 95:34–48
Ficken MS, Popp J (1996) A comparative analysis of passerine mobbing calls. Auk 113:370–380
Flasskamp A (1994) The adaptive significance of avian mobbing. V. An experimental test of the ‘move on’ hypothesis. Ethology 96:322–333
Forsman JT, Mönkkönen M (2001) Responses by breeding birds to heterospecific song and mobbing call playbacks under varying predation risk. Anim Behav 62:1067–1073
Frankenberg E (1981) The adaptive significance of avian mobbing. IV. “Alerting others” and “perception advertisement” in blackbirds facing an owl. Z Tierpsychol 55:97–118
Goodale E, Beauchamp G, Magrath RD, Nieh JC, Ruxton GD (2010) Interspecific information transfer influences animal community structure. Trends Ecol Evol 25:354–361
Hauser MD (1988) How infant vervet monkeys learn to recognize starling alarm calls. Behaviour 105:187–201
Hauser MD, Wrangham RW (1990) Recognition of predator and competitor calls in nonhuman primates and birds: a preliminary report. Ethology 86:116–130
Hinde RA (1952) The behaviour of the great tit (Parus major) and some other related species. Behaviour Suppl 2:1–201
Hölzinger J (1997) Die Vögel Baden-Württembergs. Singvögel 2. Ulmer. Stuttgart.
Hurd CR (1996) Interspecific attraction to the mobbing calls of black-capped chickadees (Parus atricapillus). Behav Ecol Sociobiol 38:287–292
Johnson FR, McNaughton EJ, Shelley CD, Blumstein DT (2003) Mechanisms of heterospecific recognition in avian mobbing calls. Aust J Zool 51:577–585
Kitchen DM, Bergman TJ, Cheney DL, Nicholson JR, Seyfarth RM (2010) Comparing responses of four ungulate species to playbacks of baboon alarm calls. Anim Cogn 13:861–870
Krama T, Krams I, Igaune K (2008) Effects of cover on loud trill-call and soft seet-call use in the crested tit, Parus cristatus. Ethology 114:656–661
Krams I (2000) Long-call use in dominance-structured crested tit Parus cristatus winter groups. J Avian Biol 31:15–19
Krams I, Krama T (2002) Interspecific reciprocity explains mobbing behaviour of the breeding chaffinches, Fringilla coelebs. Proc Biol Sci 269:2345–2350
Langham GM, Contreras TA, Sieving KE (2006) Why pishing works: titmouse (Paridae) scolds elicit a generalized response in bird communities. Ecoscience 13:485–496
Lea AJ, Barrera JP, Tom LM, Blumstein DT (2008) Heterospecific eavesdropping in a non-social bird. Behav Ecol 19:1041–1046
Magrath RD, Pitcher BJ, Gardner JL (2007) A mutual understanding? Interspecific responses by birds to each other’s aerial alarm calls. Behav Ecol 18:944–951
Magrath RD, Pitcher BJ, Gardner JL (2009a) Recognition of other species’ aerial alarm calls: speaking the same language or learning another? Proc Biol Sci 276:769–774
Magrath RD, Pitcher BJ, Gardner JL (2009b) An avian eavesdropping network: alarm signal reliability and heterospecific response. Behav Ecol 20:745–752
Mahurin EJ, Freeberg TM (2009) Chick-a-dee call variation in Carolina chickadees and recruiting flockmates to food. Behav Ecol 20:111–116
Manser MB (2001) The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proc Biol Sci 268:2485–2491
Manser MB, Bell MB, Fletcher LB (2001) The information that receivers extract from alarm calls in suricates. Proc Biol Sci 268:2315–2324
Matessi G, Matos RJ, Dabelsteen T (2008) Communication in social networks of territorial animals: networking at different levels in birds and other systems. In d’Ettorre P, Hughes DP Sociobiology of communication—an interdisciplinary perspective. Oxford University Press, Oxford, pp 33–53
McGregor PK (2005) Animal Communication Networks. Cambridge University Press, Cambridge
Müller CA, Manser MB (2008) The information banded mongooses extract from heterospecific alarms. Anim Behav 75:897–904
Nocera JJ, Taylor PD, Ratcliffe LM (2009) Inspection of mob-calls as sources of predator information: response of migrant and resident birds in the Neotropics. Behav Ecol Sociobiol 62:1769–1777
Nuechterlein GL (1981) ‘Information parasitism’ in mixed colonies of Western Grebes and Forster’s Terns. Anim Behav 29:985–989
Oda R, Matasaka N (1996) Interspecific responses of ringtailed lemurs to playback of antipredator alarm calls given by Verreaux’s sifakas. Ethology 102:441–452
Ostreiher R (2003) Is mobbing altruistic or selfish behaviour? Anim Behav 66:145–149
Pavey CR, Smyth AK (1998) Effects of avian mobbing on roost use and diet of powerful owls, Ninox strenua. Anim Behav 55:313–318
Pettifor RA (1990) The effects of avian mobbing on a potential predator, the European kestrel, Falco tinnunculus. Anim Behav 39:821–827
Rainey HJ, Zuberbühler K, Slater PJB (2004a) Hornbills can distinguish between primate alarm calls. Proc Biol Sci 271:755–759
Rainey HJ, Zuberbühler K, Slater PJB (2004b) The responses of black-casqued hornbills to predator vocalisations and primate alarm calls. Behaviour 141:1263–1277
Ramakrishnan U, Coss RG (2000) Recognition of heterospecific alarm vocalizations by bonnet macaques (Macaca radiata). J Comp Psych 114:3–12
Randler C (2006) Red squirrels (Sciurus vulgaris) respond to alarm calls of Eurasian jays (Garrulus glandarius). Ethology 112:411–416
Randler C, Förschler MI (2011) Heterospecifics do not respond to subtle differences in chaffinch mobbing calls—message is encoded in number of elements. Anim Behav 82:725–730
Ratcliffe L, Weisman RG (1985) Frequency shift in the fee bee song of the black-capped chickadee. Condor 87:555–556
Roux AL, Cherry MI, Manser MB (2009) The vocal repertoire in a solitary foraging carnivore, Cynictis penicillata, may reflect facultative sociality. Naturwissenschaften 96:575–584
Russ JM, Jones G, Mackie IJ, Racey PA (2004) Interspecific responses to distress calls in bats (Chiroptera: Vespertilionidae): a function for convergence in call design? Anim Behav 67:1005–1014
Schulze A (2003) Die Vogelstimmen Europas, Nordafrikas und Vorderasiens. Germering, Edition Ample
Seyfarth RM, Cheney DL (1990) The assessment by vervet monkeys of their own and another species’ alarm calls. Anim Behav 40:754–764
Shriner WM (1999) Antipredator responses to a previously neutral sound by free-living adult golden-mantled ground squirrels, Spermophilus lateralis (Sciuridae). Ethology 105:747–757
Sieving KE, Hetrick SA, Avery ML (2010) The versatility of graded acoustic measures in classification of predation threats by the tufted titmouse Baeolophus bicolor: exploring a mixed framework for threat communication. Oikos 119:264–276
Soard CM, Ritchison G (2009) ‘Chick-a-dee’ calls of Carolina chickadees convey information about degree of threat posed by avian predators. Anim Behav 78:1447–1453
Sullivan K (1984) Information exploitation by downy woodpeckers in mixed-species flocks. Behaviour 91:294–311
Templeton CN, Greene E (2007) Nuthatches eavesdrop on variations in heterospecific chickadee mobbing alarm calls. Proc Natl Acad Sci U S A 104:5479–5482
Templeton CN, Greene E, Davis K (2005) Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308:1934–1937
Theis KR, Greene KM, Benson-Amram SR, Holekamp KE (2007) Sources of variation in the long-distance vocalizations of spotted hyenas. Behaviour 144:557–584
Turcotte Y, Desrochers A (2002) Playback of mobbing calls of black-capped chickadees help estimate the abundance of forest birds in winter. J Field Ornithol 73:303–307
Vitousek MN, Adelman JS, Gregory NC, St Clair JJH (2007) Heterospecific alarm call recognition in a non-vocal reptile. Biol Lett 3:632–634
Wilson DR, Mennill DJ (2011) Duty cycle, not signal structure, explains conspecific and heterospecific responses to the calls of black-capped Chickadees (Poecile atricapillus). Behav Ecol 22:784–790
Zuberbühler K (2000) Interspecies semantic communication in two forest primates. Proc Biol Sci 267:713–718
Acknowledgments
I am grateful to Indrikis Krams and an anonymous reviewer for their helpful comments that improved the manuscript.
Ethical standards
The experiments comply with the current laws of Germany.
Conflict of interest
The author declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Brumm
Rights and permissions
About this article
Cite this article
Randler, C. A possible phylogenetically conserved urgency response of great tits (Parus major) towards allopatric mobbing calls. Behav Ecol Sociobiol 66, 675–681 (2012). https://doi.org/10.1007/s00265-011-1315-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1315-y