Abstract
Upon encountering predators, many animals produce specific vocalisations that alert others and sometimes dissuade the predators from hunting. Callicebus monkeys are known for their large vocal repertoire, but little is known about the function and meaning of most call types. We recorded a large number of natural predator responses from five different groups of black-fronted titi monkeys in their Atlantic forest habitat in South Eastern Brazil. When detecting predatory threats, adult group members responded with call sequences that initially consisted of two brief, high-pitched calls with distinct frequency contours. Call A was mainly given to raptors but also to predatory capuchin monkeys and other threats within the canopy, while call B was given to predatory or non-predatory disturbances on the ground. In later parts of the sequences, we also recorded a high-pitched unmodulated call C and various low-pitched loud calls. Results therefore suggest that calls A and B provide listeners with rapid and reliable information about the general classes of danger experienced by the caller, while obtaining more specific information through other call types and combinations and behavioural responses. We discuss these findings in relation to current evolutionary theory of primate communication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many birds and mammals produce specific vocalisations to predators, a behaviour that can function to alert conspecifics and to communicate detection to the predator (Caro 2005). Some species produce several acoustically distinct alarm calls in response to different predator types (Seyfarth et al. 1980a, b; Manser et al. 2002; Templeton et al. 2005), but in others, the nature of the danger encountered is reflected by the number of calls per sequence (Schel et al. 2009), the rate of call delivery (Lemasson et al. 2010), the intensity of calls (Blumstein 1999), or by combinations of calls (Arnold and Zuberbühler 2006a, b).
If predator-induced calls evoke specific and adaptive responses in recipients, researchers typically conclude that the utterance conveys something about the event experienced by the caller, although the nature of this experience has remained controversial (e.g., Seyfarth et al. 1980b; Zuberbühler et al. 1997; Zuberbühler 2001; Rendall et al. 2009). Related to this, it is not clear whether primates intend to produce calls that refer to specific external events, or whether they merely respond to “evolutionarily important” events that place them into different motivations. One way to address this has been by investigating whether associated variables, such as the level of threat experienced by the caller, can explain the caller's behaviour better than the predatory category (e.g., California ground squirrels: Owings and Virginia 1978). In some other species, it has been argued that alarm calls refer to both the level and type of threat (Manser et al. 2002; Templeton et al. 2005; Sieving et al. 2010). Chickadees (Poecile atricapilla), for instance, produce “seet” alarm call in response to flying raptors and a “chick-a-dee” alarm call in response to perched or stationary raptors, but their calls also provide information about the threat level (Templeton et al. 2005). Within the primate lineage, the predator type appears to have an overriding influence on alarm calling behaviour, with little evidence that variation in distance or direction has a major impact (e.g., vervet monkeys: Cheney and Seyfarth 1990, Diana monkeys: Zuberbühler 2000b).
Another line of research in animal alarm calling concerns the evolution of the acoustic morphology of alarm signals. Marler (1955) proposed that low-pitched, broadband calls were more conspicuous and easier to localise for predators than high-pitched, narrowband calls. One prediction from Marler's hypothesis was that the acoustic structure of alarm calls should reflect whether warning or signalling detection is the adaptive anti-predator strategy pursued by the caller. High-pitched alarm calls have usually been interpreted as the product of natural selection having favoured behaviour that alerts others without putting the caller at risk (Campbell and Snowdon 2007). For example, many birds produce high-pitched alarm calls that are difficult to locate. In contrast, many primate alarm calls are loud and conspicuous (e.g., Zuberbühler 2000a; Eckardt and Zuberbühler 2004; Schel et al. 2009), suggesting that callers are less concerned about being located. Indeed, in some species, listeners respond in part by looking towards the caller, thereby demonstrating that calls are easily localisable. In some cases, there is direct evidence that these calls are also directed at the predator (Zuberbühler et al. 1997; Caro 2005). Communicating to a predator can be adaptive if the signal indicates detection and so interferes with an ambush and surprise-based hunting strategy (Zuberbühler et al. 1997, 1999; Clarke et al. 2006).
Callicebus monkeys are known for their complex vocal system with numerous high- and low-pitched calls, which can be uttered singly or combined in more complex structures (Moynihan 1966; Robinson 1979a). Early experimental work has documented that the monkeys are sensitive to call order (Robinson 1979a), but since then, little progress has been made concerning the function, meaning, and context-specific use of their vocal utterances. The most studied vocal behaviour is “duetting”, long and loud sequences of calls uttered by the mated pair in a coordinated way. Duets can be produced spontaneously or in response to the duets by other breeding pairs, a behaviour that seems to function in delineating or enforcing territorial boundaries (Moynihan 1966; Kinzey et al. 1977; Kinzey 1981; Robinson 1979b, a, 1981; Kinzey and Robinson 1983; Robinson et al. 1987; Müller 1995; Müller and Anzenberger 2002).
Apart from these studies, very little work has been conducted on titi monkey vocal behaviour and virtually nothing is known about their vocalisations in the predation context (Cisneros-Heredia et al. 2005; Sampaio and Ferrari 2005; Ferrari 2009; de Luna et al. 2010). Although predator-specific alarm calls are well described in Old World monkeys (see Zuberbühler 2009 for a review), this is not the case for most New World monkeys (but see Digweed et al. 2005; Fichtel et al. 2005; Kirchhof and Hammerschmidt 2006; Wheeler 2010), which besides having undergone an independent radiation within the primate lineage also differ in essential life-history and socio-ecological characteristics from cercopithecines and lemurs (Strier 2007). Therefore, discovering whether and how titi monkeys use specific vocal signals when interacting with predators thus has considerable theoretical implications for evolutionary theories of primate communication and cognitive process underlying call production. To this end, we conducted a detailed observational study on five groups of black-fronted titi monkeys (Callicebus nigrifrons) in their natural Atlantic forest habitat in South Eastern Brazil. Our goal was to systematically describe the vocal and locomotor behaviour of free-ranging titi monkeys in response to natural disturbances.
Methods
Study site and species
The study was conducted during two field seasons (May–October 2009; May–July 2010) at the “Serra do Caraça” study site, an 11,000-ha private reserve in the State of Minas Gerais, South-Eastern Brazil (20°05S; 43°29 W). A few additional recordings were made in 2008. The habitat can be described as a mountainous transition zone that separates the savannah (“Cerrado”) from the Atlantic forest in the south, and a transition zone from Cerrado to Atlantic Forest to a xeric forest of small thorny trees and shrubs in the north (Derby 1966; Giulietti and Pirani 1988; Giulietti et al. 1997). Altitudes range from 850 to 2,070 m with a rainy season from October to March and a dry season from April to September. As a consequence of timber extraction and previous “slash-and-burn” agricultural practices, the vegetation is in different stages of ecological succession (Silva and Talamoni 2003; Coelho et al. 2008).
Callicebus monkeys live in socially monogamous family groups, consisting of a pair of reproductive adults and up to four generations of offspring (Mendoza and Mason 1986; Kinzey and Becker 1983; Valeggia et al. 1999). Individuals are considered to be adults around 30 months of age when they become sexually reproductive (Valeggia et al. 1999). For this study, we considered adults as any fully-grown individual (>30 months), sub-adults as slightly smaller individuals than adults (18–30 months), juveniles as individuals about half the size of adults (6–18 months), and infants as smaller than juveniles (0–6 months) (adapted from Moynihan 1966; de Luna et al. 2010). Group identification and composition during the study are presented in Table 1.
Sub-adult individuals of both sexes disperse when they are approximately 3 years old (Bossuyt 2002). However, under certain conditions, offspring delay migration, while already dispersed individuals sometimes temporarily return to their natal group, which can increase group sizes to seven individuals (Table 1; see also Bicca-Marques et al. 2002, for a different group composition). There is no sexual dimorphism in the adults (max. weight, 1,650 g; Rowe 1996).
At our study site, black-fronted titi monkeys coexisted with four other primate species; black-tufted marmosets (Callithrix penicillata), white-fronted marmosets (Callithrix geoffroyi), capuchin monkeys (Cebus nigritus) and, in the recent past, southern brown howler monkeys (Alouatta clamitans) (Hirsch 2003).
Predators
There are a number of potential primate predators in the reserve, including several species of raptors and mammalian carnivores. Potentially dangerous raptors include the crowned eagle (Harpyhaliaetus coronatus), the black-chested buzzard-eagle (Geronoaetus melanoleucus) and the black hawk-eagle (Spizaetus tyrannus), along with several species of hawks (e.g., Accipiter sp.) and owls (Vasconcelos and Melo Junior 2001; Vasconcelos 2001). For several genera (Harpia, Spizaetus, Accipiter, Morphnus, Leucopternis, Spizastur), there is direct evidence of predation on Neotropical primates (Klein et al. 1988 ; Robinson 1994; Boinski and Chapman 1995; Oversluijs Vasquez and Heymann 2001; Miller and Treves 2007; Ferrari 2009; de Luna et al. 2010). The area is also inhabited by several mammalian carnivores, including tayras (Eira barbara) and at least four species of cats; ocelots (Leopardus pardalis), oncillas (Leopardus tigrinus), jaguarondis (Herpailurus yagouaroundi), pumas (Puma concolor), and possibly jaguars (Panthera onca). All but oncilla and jaguarondis are confirmed primate predators (Miranda et al. 2006; Bianchi and Mendes 2007; Ludwig et al. 2007; Bezerra et al. 2009; Ferrari 2009; de Luna et al. 2010). Finally, titi monkeys have also been observed being preyed upon by capuchin monkeys (in Freese and Oppenheimer 1981; Lawrence 2003; Sampaio and Ferrari 2005). Raptors are likely to represent the greatest predatory threat because they can attack at all heights, whereas most mammalian carnivores are terrestrial and rely on ambush. As generally true for field studies, the presence of human observers is likely to have a bigger dissuasive effect on terrestrial than aerial predators (de Luna et al. 2010).
Data collection
Habituated groups of monkeys were located and followed continuously throughout the day and responses to potential predators were recorded on an all occurrence basis (Martin and Bateson 2007). In most cases, we were not able to identify the first individual that called to the potential predator so that our data consist of group reactions, a common procedure in research on arboreal forest monkeys living in visually dense habitat (e.g. Zuberbühler et al. 1997). Information on predator type and location were collected whenever possible. During each predator encounter, the observer (CC) recorded the vocal behaviour of one or more group members and noted all correlated behaviour as well the total duration of the vocal responses.
Recordings were made with a SENNHEISER K6/M66 directional microphone and MARANTZ PMD660 solid-state recorder (44.1 kHz sampling rate; 16 bits accuracy). Any additional verbal comments were later transcribed. All recordings were transferred digitally onto a Dell laptop computer.
Acoustic analyses
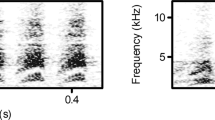
Based on previous reports and our pilot data, we were able to discriminate among three main types of soft, high-pitched calls based on frequency contours produced by all five groups. A calls were arch-shaped with a down-sweep modulation. B calls were S-shaped with an upsweep modulation. C calls were flat with a slight up or down modulation (Fig. 1).
To determine whether this qualitative categorisation was valid, we carried out an acoustic analysis. For each call, we measured its duration and fundamental frequency F0 (“pitch”) at the beginning, middle, and end of the call, as well as the number of harmonics (number of bands at integer multiples of the fundamental frequency, Rendell et al. 1999). Because these calls were very similar in fundamental frequency, we compared their modulation (or shape) by splitting the calls into two equal segments and calculating the transition onset, the transition offset, and the overall transitions of F0. These variables were chosen both for representing the main features of the three calls and because they could be easily measured manually from a spectrogram. All measurements and spectrographic illustrations were conducted with PRAAT acoustic analysis software (version 5.1; www.praat.org). Figure 2 illustrates how the parameters were obtained.
Temporal and frequency variables extracted from predator-associated calls: call duration (s) = c − a; fundamental frequency, F0 (Hz) = d; N harmonics (1 in this call) = e; frequency of maximum energy at call onset = a; frequency of maximum energy at call middle = b; frequency of maximum energy at call end = c; transition onset (ΔHz) = a − b; transition offset (ΔHz) = b – c; overall transition (ΔHz) = c − a. Depicted is a time-frequency spectrogram of a “chirp” vocalization made by an adult female
Statistical analyses
When carrying out statistical analyses of acoustic variables it is important to select measurements that are only moderately correlated with each other. A standard way of determining this set of variables is by regressing all parameters to check for co-linearity and removing parameters with a variance inflation factor greater than 4 (Glantz and Slinker 2001). Following this procedure, we looked for outliers by producing standardised Z scores for all values and rejecting all cases in which at least one parameter had a Z score of greater than 3.29 (Tabachnick and Fidell 2001). We then conducted a discriminant function analysis (DFA) to determine whether the set of acoustic variables, when combined in one model, could discriminate among the three main high-pitched calls types given in response to predators. We used the leave-one-out classification procedure to cross-validate the discriminant function that was generated. Since the acoustic data for alarm call types were two-factorial (group ID; call type), it has been argued that conventional DFA does not allow for a valid estimation of the overall significance of discriminability (Mundry and Sommer 2007). Therefore, we used a permutated discriminant function analysis (pDFA), using a macro written by Mundry and Sommer (2007), to estimate the cross-validated significance of the number of correctly classified calls. We then ran one-way related-samples analysis of variance tests to examine whether each of the acoustic parameters varied statistically with each call type. We conducted post hoc, pair-wise and Sidak-corrected comparisons to examine whether any of the acoustic parameters could discriminate between the call types.
We also carried out an inter-observer reliability test between CC and a second rater, who was naïve to the hypotheses. After completing training on N = 20 pre-classified calls (randomly selected, equivalent to 5% of the full call set), the second observer classified another 20 calls, again randomly selected. We calculated Cohen's Kappa coefficients to determine whether the levels of observer agreement reached the required reliability level (Cohen's ĸ = 0.80).
In a second major analysis, we examined the proportions of different calls given by the focal group in sequence. Ideally, this would have been carried out separately for each caller but, as this study was carried out in a natural forest habitat with difficult visual conditions, it was not possible to reliably observe all individuals during calling. We therefore report the calling response as a combined effort by all group members. Although this was a conservative measure, in 85% of cases, we were able to identify the caller. These observations showed that, in most cases, calling activity was due to one individual only, suggesting that the data represent call sequences produced by individual callers.
We coded all call types during the first minute, which allowed us to calculate the relative proportion and rate of each call type in the combined sequence. Some vocal responses were less than a minute, in which case, we used the actual duration to calculate call rates. Calls that could not be classified with confidence as either A, B, or C were coded as “other”. Rare types, such as grunts, trills, and moans, were also coded as “other”.
Statistical analyses were conducted with the statistical package PASW, version 18.0 (SPSS Inc., Chicago, IL, U.S.A.), except for the pDFA, which was conducted with R 2.13.1 (The R Foundation for statistical Computing, Vienna, Austria). All tests were two-tailed with a significance level set at 0.05, unless corrections were needed. We used non-parametric Kruskal–Wallis and Mann–Whitney tests with Bonferroni-corrected alpha values in case of multiple comparisons.
Results
General responses
During approximately 730 h of continuous observations, we registered 287 vocal responses to potential predator species from five habituated groups. Most cases (n = 132, 46%) were responses to raptors (n = 123 flying, n = 4 perched, n = 5 calling; see Appendix). Only events with sufficient recording quality were further analysed (n = 81).
Upon encountering raptors and other threats from the canopy or sky, monkeys usually called for significantly shorter periods than when encountering a disturbance on the ground (Mann–Whitney U test, U = 49.0, n 1 = 69, n 2 = 12, p = <0.001). The exception was one encounter with two eagles trying to perch close to the group in which case the monkeys called continuously for almost 11 min. The duration of vocal responses was significantly related to the stimulus type experienced (Kruskal–Wallis, χ 2 = 47.631, df = 6, p < 0.001; Fig. 3a; post hoc Mann–Whitney U tests, Bonferroni p value adjusted for multiple comparisons, Table 2).
Box plots indicating a the duration of calling behaviour and b the call rate during the first minute after encountering different types of predators or threats (medians, upper and lower quartiles, whiskers = adjacent values, asterisks = outliers). The two identified threats on the ground were responses from the same group (GM). Lines separate between predatory and other disturbances on the canopy/sky and on the ground. Call rate is expressed as the square root (SQRT) of number of calls produced during the first minute to correct for differences in the number of individuals per group
Similarly, call rates were significantly smaller to raptors and other threats from the canopy or sky than to disturbances on the ground (Mann–Whitney U test, U = 5.000, n 1 = 69, n 2 = 12, p = <0.001). Likewise, call rates were significantly related to stimulus type (Kruskal–Wallis, χ 2 = 48.789, df = 6, p < 0.001; Fig. 3b; post hoc Mann–Whitney U tests, Bonferroni p value adjusted for multiple comparisons, Table 2).
The typical response pattern to raptors was for the detecting individual to call while observing the predator and freezing, rapidly descending or moving to a protected location. Nearby group members typically remained silent, while scanning the canopy or sky and freezing, rapidly descending or moving towards a protected place as well. Distant group members, who could probably not hear the caller and detected the predator independently, produced the same call type as the initial caller.
To terrestrial disturbances, the first animal to call usually attracted other group members to the site, who then also called. This was accompanied by alert, approach, or mobbing behaviour. In one case, a spotted cat was mobbed for over 20 min (see Fig. 3a). The caller's behaviour included gazing at the cat and producing visual displays, such as arch postures, pilo-erection, tail lashing (swinging tail sideways), head swaying, and rapid erratic movements towards and away from the disturbance, while maintaining visual fixation. The behaviour of others included calling, looking towards or approaching the caller, scanning the forest ground or lower canopy, producing visual displays and mobbing the predator. Mobbing was also observed to tayras, but not to a non-predatory disturbance (deer), although the monkeys were agitated in all situations.
Call structure
An inter-rater reliability test suggested that our type classification was reliable (93.3% agreement; Cohen's Kappa coefficient ĸ = 0.865). To further verify whether our classification was justified, we selected the first five exemplars of A, B, and C calls from each group for acoustic analyses. Following checks for multi-colinearity and singularity, we subjected five of the eight original acoustic parameters to a discriminant function analysis (n = 75 calls, five calls of each type per group): total duration, frequency at the end of the fundamental frequency, early transition, late transition, and number of harmonics. We excluded two outliers (two C and one A call), resulting in a final sample of n = 72. One recommendation for discriminant function analyses is that the number of variables must be smaller than the number of objects in the smallest class of variables (Tabachnick and Fidell 2001). We thus excluded the variable “total duration”, because its contribution in a first DFA was least significant.
Two functions explained a significant amount of the variation in the acoustic structure of the call types (Fig. 4). The first function explained 92.5% of the variation (Wilks' lambda = 0.075, χ 28 = 174.785, p < 0.001), while the second function, explained the remaining 7.5% of the variation (Wilks' lambda = 0.627, χ 23 = 31.490, p < 0.001). In a cross-validated analysis, the functions successfully classified 94.4% (68/72) of the calls into the three categories. The success rate of classification was highest for C (100%), followed by B (92%), and A (91.7%). Disagreements were two A calls classified as C calls and two B calls classified as C calls. We then used a pDFA (Mundry and Sommer 2007) to estimate the significance of the number of correctly classified calls (cross-validated). Results from the pDFA indicated a highly significant level of discrimination (p < 0.001). Acoustic measures of calls A, B, and C from all five groups in natural contexts are presented in Table 3.
To examine whether each of the uncorrelated acoustic parameters varied statistically between call types, we conduced one-way related-samples analysis of variance tests with call type as the fixed factor and group as the random factor. Two of the four acoustic features varied significantly between call types (transition offset, F 2,8 = 96.193, p < 0.001 and number of harmonics, F 2,8 = 17.221, p < 0.001; Table 5; Fig. 5). Post hoc, pair-wise, Sidak-corrected comparisons discriminated among all call types (Fig. 5). The other two variables (end frequency and transition onset) did not vary significantly between call types (F 2,8 = 0.400, p = 0.683, and F 2,8 = 1.260, p = 0.334, respectively). Nonetheless, post hoc Sidak-corrected comparisons revealed that end frequencies of calls A and B were significantly lower that in call C (Fig. 5). Group identity did not affect any of the differences between call types (Table 4).
Box plots indicating the median, inter-quartiles, and range for each of five uncorrelated acoustic parameters describing black-fronted titi monkeys calls: a call duration, b frequency of maximum energy at call end, c transition onset (ΔHz), d transition offset (ΔHz), and e N harmonics. P values represent results of post hoc, pair-wise, and Sidak-corrected comparisons
Context specificity
To raptors, the first call in each sequence was always an A call, regardless of the raptors' behaviour (Table 5; Fig. 6). A calls were also the only or main calls during the first 30 calls produced (χ 2 = 36.105, n = 19, df = 2, p < 0.001; Fig. 6). This was observed to crowned eagles, black-chested buzzard-eagles, black hawk-eagles, caracaras, vultures, and several species of hawks. Monkeys did not give A calls to other bird species, except when surprised by medium-sized flying birds. On one occasion, an adult male gave A calls to a big bird flying by before perching in a nearby tree. The monkey kept on giving A calls while trying to locate the bird in the vegetation but then stopped immediately after identifying it as a dusky-legged guan (Penelope obscura), a common non-predatory bird. Additionally, A calls were given in response to the presence of capuchin monkeys, and other (unidentified) threats within the canopy, but never to disturbances on the ground (Fig. 6). In contrast, to raptor responses call sequences to Capuchin monkeys contained a large number of B calls following an initial sequence of A calls.
Sequential analyses of the first 30 calls produced in predatory contexts (see Fig. 4 for spectrograms of the calls). Terrestrial context includes one response to an unidentified spotted cat and one adult deer from the same group (GM)
To disturbances on the ground, the first call per sequence was always a B call. This was observed to a spotted cat, tayra, deer, and several unidentified disturbances. Subsequent calls were also always B calls sometimes mixed with C calls later on in the sequence, but never A calls (χ 2 = 19.436, n = 12, df = 2, p < 0.001, Fig. 6). Although B calls were used in higher proportions to terrestrial threats than to capuchins, the p value was not significant after a Bonferroni correction (z = −2.207, p = 0.031). Importantly, B calls were also often produced in non-predatory contexts and sometimes in the absence of external events, especially when monkeys were descending or foraging close to the ground, when an observer was blocking their intended path, during inter-group encounters and, for unhabituated groups, in response to humans.
Call type C was the least common and produced in almost all contexts, but especially to capuchin monkeys and deer (Fig. 6; Table 5). In non-predatory contexts, it was given during intra-specific disputes, in response to other groups calling and during movements towards or away from significant events, such inter-group encounters.
Sequence composition during the first minute
The proportion of A, B, and C calls within the first minute were all significantly dependent on the type of stimuli (A, χ 2 = 53.061, df = 6, p < 0.001; B, χ 2 = 59.845, df = 6, p = 0.000; C, χ 2 = 24.632, df = 6, p < 0.001, Fig. 7). The proportion of A calls was significantly higher for raptors (median = 1.0) than capuchins (median = 0.378, Mann–Whitney U = 4.0, n 1 = 29, n 2 = 6, p < 0.001) or terrestrial threats (median = 0, U = 0.0, n 1 = 29, n 2 = 12, p = 0.000). The proportion of B calls was significantly higher for terrestrial threats (median = 0.9058) than capuchins (median = 0, U = 0.0, n 1 = 12, n 2 = 6, p < 0.001) or raptors (median = 0, U = 0.0, n 1 = 12, n 2 = 29, p < 0.001). The proportion of call C was significantly higher for capuchins (median = 0.3875) than terrestrial threats (median = 0.0, U = 6.0, n 1 = 6, n 2 = 12, p = 0.002) or raptors (median = 0.0, U = 124.0, n 1 = 6, n 2 = 29, p = 0.032, Fig. 7).
Proportion of each call type during the first minute, expressed as call rates. BS = combined utterance of B call immediately followed by a low-pitched vocal unit, which changed the amplitude and acoustic appearance, previously termed “chirrup” and “chuck” by Moynihan (1966) and “chirrup” by Robinson (1979a). BS utterances seemed to be produced in the later parts of sequences, suggesting different behavioural motivations or intentions
Discussion
Black-fronted titi monkeys produce different call types in response to a variety of disturbances, including predators. Our groups reliably uttered A calls in response to raptors, with the number of calls varying from one to many, depending on the birds' behaviour. One or few A calls were given in response to flying raptors, several calls in response to perched or calling raptors, with calling often only stopping after the predator flew away (Figs. 3a and 6). However, A calls do not qualify as “eagle alarms” or even aerial predator alarms since the monkeys produced the same call type also when encountering capuchin monkeys or other threats within the canopy. Instead, A calls appear to indicate that the caller detected a threat within the canopy, while later parts of the sequence reveal something about the nature of this disturbance. While raptors elicited series of A calls, depending on their behaviour, capuchin monkeys triggered B and C calls, despite the fact that they were encountered in the canopy.
Similarly, B calls do not qualify as terrestrial predator alarm because they are also given in a variety of situations where the caller has not detected a typical ground predator but is about to engage in risky behaviour, such as descending towards the ground or when foraging close to the ground. The fact that arboreal Capuchin monkeys also trigger B calls in later parts of the sequence further illustrate this point. In this context, group members often gather and descend, which may explain their increased rates of B calls. Context-specific differences are apparent in later parts of the sequence, however. For instance, in response to cats, deer, tayras, and other terrestrial threats, the monkeys consistently produced sequences of B calls, sometimes followed by low-pitched “other” calls later in the sequence, but never A calls.
Call type C was given least specifically, although regularly to capuchins, deer, and when neighbouring groups were in proximity, suggesting that it functions as a general alert call or that it is related to the caller's intention to move.
Calling responses sometimes lasted for several minutes, particularly to terrestrial predators. In the later parts of such sequences, we identified loud calls that were structurally very different from the first calls, similar to what has been described by Moynihan (1966) and Robinson (1979a) for the Amazonian titi monkey species. Due to their low occurrence, we did not describe them any further here. Nonetheless, we noticed that most low-pitched loud calls were produced in response to a terrestrial predator (cat) but not a non-predatory disturbance (deer). These responses suggest that titi monkeys differentiate between different types of terrestrial threats, despite the fact that all call sequences begin with long series of B calls.
A somewhat special case was the monkeys' responses to capuchin monkeys. Here, the monkeys' first calls were always A calls, but callers then switched to B calls, sometimes interspersed by C calls and other calls. Interactions with capuchin monkeys were usually very disruptive and monkeys were very agitated. After a few calls, they often moved downwards, stayed quiet, or ran away, sometimes pursued by the capuchin monkeys. The production of B and C calls may thus be a reflection of to the callers' intention to move in specific ways or directions.
These findings are consistent with the current theory of primate alarm calls, which states that aerial and terrestrial predators elicit acoustically distinct vocal behaviour (e.g., Seyfarth and Cheney 1980; Macedonia and Evans 1993; Zuberbühler 2000b; Digweed et al. 2005; Fichtel et al. 2005; Kirchhof and Hammerschmidt 2006; Schel et al. 2009; Wheeler 2010). However, they are also at odds with this general theory in a number of ways. Firstly, titi monkeys regularly produce B calls not only to terrestrial predators but also in non-predatory contexts, something that has also been observed in other New World primates, particularly during inter-group encounters (Digweed et al. 2005; Fichtel et al. 2005; Kirchhof and Hammerschmidt 2006; Wheeler 2010). In putty-nosed monkeys (Cercopithecus nictitans martini), males regularly produce loud and conspicuous calls to predators (Arnold and Zuberbühler 2006a, b), but the same calls are also produced during non-predator events, such as during inter-group encounters, to falling branches, or to initiate group travel (Arnold and Zuberbühler 2006a, 2008). If B call sequences produced in predatory and non-predatory situations are acoustically identical, listeners will have to consider the external context and the behaviour of others in deciding how to respond. Another possibility is that there are acoustic variants within the B calls or that there are differences in call delivery that are context related. For instance, monkeys seem to begin B call sequences with quiet and high-pitched variants and then progressively increase amplitude and add suffices (see Ouattara et al. 2009 for similar observations in Campbell's monkeys). Whether or not these differences are communicatively relevant will have to be addressed by future research.
In sum, we found extensive use of vocalisations during predator encounters in context-specific ways, a pattern so far not described for Callicebus species. Previous work has typically assumed that the titi monkeys' main anti-predatory strategy is based on cryptic behaviour (Terborgh 1983; Ferrari 2009; de Luna et al. 2010). In our study, however, we witnessed cryptic behaviour only on a few occasions. In one typical case, a small semi-habituated group remained silent after detecting a nearby tayra, but this may have been caused by the presence of human observers.
The monkeys studied are confronted with an unusually large array of predators, both aerial and terrestrial, which raises interesting questions about the specificity (or “semantic precision”) of their alarm calls (R Seyfarth, personal communication). A relevant topic for future research therefore is whether there is a relation, across species, between the number of terrestrial (or aerial) predators a species interacts with and the importance of acoustic call variations and context-specific call combinations.
Overall, the patterns described in this study suggest that titi monkey alarm call sequences refer to the general location of danger, real or anticipated, but that listeners may also obtain information about the predator class. Alarm signals with multiple strands of information have previously been described in some non-primate species. In meerkats and chickadees, acoustically distinct alarm calls refer to both predator type and level of urgency (Manser 2001; Manser et al. 2002; Templeton et al. 2005). The results presented here are novel in that they provide evidence that animal alarm calling behaviour can refer to the location of threat in addition to the predator category. Field experiments are needed to test this hypothesis in more detail.
References
Arnold K, Zuberbühler K (2006a) The alarm-calling system of adult male putty-nosed monkeys, Cercopithecus nictitans martini. Anim Behav 72:643–653. doi:10.1016/j.anbehav.2005.11.017
Arnold K, Zuberbühler K (2006b) Semantic combinations in primate calls. Nature 441:303–303. doi:10.1038/441303a
Arnold K, Zuberbühler K (2008) Meaningful call combinations in a non-human primate. Curr Biol 18:R202–R203. doi:10.1016/j.cub.2008.01.040
Bezerra B, Barnett A, Souto A, Jones G (2009) Predation by the tayra on the common marmoset and the pale-throated three-toed sloth. J Ethol 27:91–96. doi:10.1007/s10164-008-0090-3
Bianchi RDC, Mendes SL (2007) Ocelot (Leopardus pardalis) predation on primates in Caratinga Biological Station, Southeast Brazil. Am J Primatol 69:1173–1178. doi:10.1002/ajp.20415
Bicca-Marques JC, Garber PA, Azevedo-Lopes MAO (2002) Evidence of three resident adult male group members in a species of monogamous primate, the red titi monkey (Callicebus cupreus). Mammalia 66:138–142
Blumstein DT (1999) Alarm calling in three species of marmots. Behaviour 136:731–757
Boinski S, Chapman CA (1995) Predation on primates: where are we and what's next? Evol Anthropol 4:1–3. doi:10.1002/evan.1360040102
Bossuyt F (2002) Natal dispersal of titi monkeys (Callicebus moloch) at Cocha Cashu, Manu National Park, Peru. Am J Phys Anthropol Supplement 34:47
Campbell M, Snowdon C (2007) Vocal responses of captive-reared Saguinus oedipus during mobbing. Int J Primatol 28:257–270. doi:10.1007/s10764-007-9123-y
Caro TM (2005) Antipredator defense in birds and mammals. Univ Chicago Press, Chicago, Antipredator Defense in Birds and Mammals
Cäsar C, Young RJ (2008) A case of adoption in a wild group of black-fronted titi monkeys (Callicebus nigrifrons). Primates 49:146–148. doi:10.1007/s10329-007-0066-x
Cäsar C, Franco ES, Soares GDN, Young RJ (2008) Observed case of maternal infanticide in a wild group of black-fronted titi monkeys (Callicebus nigrifrons). Primates 49:143–145. doi:10.1007/s10329-007-0067-9
Cheney DL, Seyfarth RM (1990) How monkeys see the world: Inside the mind of another species. Chicago University Press, Chicago
Cisneros-Heredia DF, León-Reyes A, Seger S (2005) Boa constrictor Predation on a Titi monkey, Callicebus discolor. Neotrop Primates 13:11–12. doi:10.1896/1413-4705.13.3.11
Clarke E, Reichard UH, Zuberbühler K (2006) The syntax and meaning of wild gibbon songs. PLoS One 1:e73
Coelho CM, De Melo LFB, Sábato MAL, Magni EMV, Hirsch A, Young RJ (2008) Habitat use by wild maned wolves (Chrysocyon brachyurus) in a transition zone environment. J Mammal 89:97–104. doi:10.1644/06-mamm-a-383.1
de Luna AG, Sanmiguel R, Di Fiore A, Fernandez-Duque E (2010) Predation and predation attempts on red titi monkeys (Callicebus discolor) and equatorial sakis (Pithecia aequatorialis) in Amazonian Ecuador. Folia Primatol 81:86–95
Derby OA (1966) The Serra of Espinhaço, Brazil. J Geol 14:374–401
Digweed SM, Fedigan LM, Rendall D (2005) Variable specificity in the anti-predator vocalizations and behaviour of the white-faced capuchin, Cebus capucinus. Behaviour 142:997–1021
Eckardt W, Zuberbuhler K (2004) Cooperation and competition in two forest monkeys. Behav Ecol 15:400–411. doi:10.1093/beheco/arh032
Ferrari SF (2009) Predation risk and antipredator strategies. In: Garber PA, Estrada A, Bicca-Marques JC, Heymann EW, Strier KB (eds) South American primates. Developments in primatology. Progress and Prospects, Springer New York, pp 251–277. doi:10.1007/978-0-387-78705-3_10
Fichtel C, Perry S, Gros-Louis J (2005) Alarm calls of white-faced capuchin monkeys: an acoustic analysis. Anim Behav 70:165–176. doi:10.1016/j.anbehav.2004.09.020
Freese CH, Oppenheimer JR (1981) The capuchin monkeys, genus Cebus. In: Coimbra-Filho A, Mittermeier RA (eds) Ecology and behavior of neotropical primates, vol 1. Academia Brasileira de Ciências, Rio de Janeiro, pp 331–390
Giulietti AM, Pirani JR (1988) Patterns of geographic distribution of some plant species from the Espinhaço Range, Minas Gerais and Bahia, Brazil. In: Vanzolini PE, Heyer WR (eds) Proceedings of a workshop on neotropical distribution. Academia Brasileira de Ciências, Rio de Janeiro, pp 39–69
Giulietti AM, Pirani JR, Harley RM (1997) Espinhaço Range region, Eastern Brazil. In: Davis SD, Heywood VH, Herrera-Macbryde O, Villa-Lobos J, Hamilton AC (eds) Centres of plant diversity, a guide and strategy for their conservation. Information Press, Oxford, pp 397–404
Glantz SA, Slinker BK (2001) Primer of Applied Regression and Analysis of Variance. 2nd ed. McGraw-Hill, New York NY
Hirsch A (2003) Habitat fragmentation and priority areas for primate conservation in the Rio Doce Basin, Minas Gerais. Neotrop Primates 11:195–196
Kinzey WG (1981) The titi monkeys, genus Callicebus. In: Coimbra-Filho AF, Mittermeier RA (eds) Ecology and behavior of neotropical primates, vol 1. Academia Brasileira de Ciências, Rio de Janeiro, pp 241–296
Kinzey W, Becker M (1983) Activity pattern of the masked titi monkey, Callicebus personatus. Primates 24:337–343. doi:10.1007/bf02381979
Kinzey WG, Robinson JG (1983) Intergroup loud calls, range size, and spacing in Callicebus torquatus. Am J Phys Anthropol 60:539–544. doi:10.1002/ajpa.1330600416
Kinzey W, Rosenberger A, Heisler P, Prowse D, Trilling J (1977) A preliminary field investigation of the yellow handed titi monkey, Callicebus torquatus torquatus, in Northern Peru. Primates 18:159–181. doi:10.1007/bf02382957
Kirchhof J, Hammerschmidt K (2006) Functionally referential alarm calls in tamarins (Saguinus fuscicollis and Saguinus mystax)—evidence from playback experiments. Ethology 112:346–354. doi:10.1111/j.1439-0310.2006.01165.x
Klein BC, Harper LH, Bierregaard RO, Powell GVN (1988) The nesting and feeding behavior of the ornate hawk-eagle near Manaus, Brazil. Condor 90:239–245
Lawrence JM (2003) Preliminary report on the natural history of brown titi monkeys (Callicebus brunneus) at the Los Amigos Research Station, Madre de Dios, Peru. Am J Phys Anthropol Supplement 36:136
Lemasson A, Ouattara K, Bouchet H, Zuberbühler K (2010) Speed of call delivery is related to context and caller identity in Campbell's monkey males. Naturwissenschaften 97:1023–1027. doi:10.1007/s00114-010-0715-6
Ludwig G, Aguiar L, Miranda J, Teixeira G, Svoboda W, Malanski L, Shiozawa M, Hilst C, Navarro I, Passos F (2007) Cougar predation on black-and-gold howlers on Mutum Island, Southern Brazil. Int J Primatol 28:39–46. doi:10.1007/s10764-006-9103-7
Macedonia JM, Evans CS (1993) Variation among mammalian alarm call systems and the problem of meaning in animal signals. Ethology 93:177–197. doi:10.1111/j.1439-0310.1993.tb00988.x
Manser MB (2001) The acoustic structure of suricates' alarm calls varies with predator type and the level of response urgency. Proc Roy Soc Lond B 268:2315–2324
Manser MB, Seyfarth RM, Cheney DL (2002) Suricate alarm calls signal predator class and urgency. Trends Cogn Sci 6:55–57
Marler P (1955) Characteristics of some animal calls. Nature 176:6–8
Martin P, Bateson P (2007) Measuring behaviour: an introductory guide, 3rd edn. Cambridge University Press, Cambridge
Mendoza SP, Mason WA (1986) Parental division-of-labor and differentiation of attachments in a monogamous primate (Callicebus moloch). Anim Behav 34:1336–1347
Miller LE, Treves A (2007) Predation on primates: past studies, current challenges, and directions for the future. In: Campbell CJ, Fuentes A, Mackinnon KC, Panger M, Bearder SK (eds) Primates in perspective. Oxford University Press, Oxford, pp 535–547
Miranda J, Bernardi I, Moro-Rios R, Passos F (2006) Antipredator behavior of brown howlers attacked by black hawk-eagle in Southern Brazil. Int J Primatol 27:1097–1101. doi:10.1007/s10764-006-9062-z
Moynihan M (1966) Communication in the titi monkey, Callicebus. J Zool 150:77–127. doi:10.1111/j.1469-7998.1966.tb02999.x
Müller KH (1995) Ranging in masked titi monkeys (Callicebus personatus) in Brazil. Folia Primatol 65:224–228
Müller AE, Anzenberger G (2002) Duetting in the titi monkey Callicebus cupreus: structure, pair specificity and development of duets. Folia Primatol 73:104–115
Mundry R, Sommer C (2007) Discriminant function analysis with nonindependent data: consequences and an alternative. Anim Behav 74:965–976. doi:10.1016/j.anbehav.2006.12.028
Ouattara K, Lemasson A, Zuberbühler K (2009) Campbell’s monkeys use affixation to alter call meaning. Plos One 4:e7808. doi:10.1371/journal.pone.0007808
Oversluijs Vasquez MR, Heymann EW (2001) Crested eagle (Morphnus guianensis) predation on infant tamarins (Saguinus mystax and Saguinus fuscicollis, Callitrichinae). Folia Primatol 72:301–303
Owings DH, Virginia RA (1978) Alarm calls of California ground squirrels (Spermophilus beecheyi). Z Tierpsychol 46:58–70
Rendall D, Owren MJ, Ryan MJ (2009) What do animal signals mean? Anim Behav 78:233–240. doi:10.1016/j.anbehav.2009.06.007
Rendell LE, Matthews JN, Gill A, Gordon JCD, Macdonald DW (1999) Quantitative analysis of tonal calls from five odontocete species, examining interspecific and intraspecific variation. J Zool 249:403–410. doi:10.1111/j.1469-7998.1999.tb01209.x
Robinson JG (1979a) Analysis of the organization of vocal communication in the titi monkey Callicebus moloch. Z Tierpsychol 49:381–405
Robinson JG (1979b) Vocal regulation of use of space by groups of titi monkeys Callicebus moloch. Behav Ecol Sociobiol 5:1–15
Robinson JG (1981) Vocal regulation of inter- and intragroup spacing during boundary encounters in the titi monkey, Callicebus moloch. Primates 22:161–172. doi:10.1007/bf02382607
Robinson SK (1994) Habitat selection and foraging ecology of raptors in Amazonian Peru. Biotropica 26:443–458
Robinson JG, Wright PC, Kinzey WG (1987) Monogamous cebids and their relatives: intergroup calls and spacing. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) In primate societies. University of Chicago Press, Chicago, pp 44–53
Rowe N (1996) The pictorial guide to the living primates. Pogonias Press, East Hampton NY
Sampaio DT, Ferrari SF (2005) Predation of an infant titi monkey (Callicebus moloch) by a tufted capuchin (Cebus apella). Folia Primatol 76:113–115. doi:10.1159/000073617
Schel AM, Tranquilli S, Zuberbühler K (2009) The alarm call system of two species of black-and-white colobus monkeys (Colobus polykomos and Colobus guereza). J Comp Psychol 123:136–150. doi:10.1037/A0014280
Seyfarth RM, Cheney DL (1980) The ontogeny of vervet monkey alarm calling behavior: a preliminary report. Z Tierpsychol 54:37–56
Seyfarth RM, Cheney DL, Marler P (1980a) Monkey responses to three different alarm calls—evidence of predator classification and semantic communication. Science 210:801–803
Seyfarth RM, Cheney DL, Marler P (1980b) Vervet monkey alarm calls—semantic communication in a free-ranging primate. Anim Behav 28:1070–1094
Sieving KE, Hetrick SA, Avery ML (2010) The versatility of graded acoustic measures in classification of predation threats by the tufted titmouse Baeolophus bicolor: exploring a mixed framework for threat communication. Oikos 119:264–276. doi:10.1111/j.1600-0706.2009.17682.x
Silva JA, Talamoni SA (2003) Diet adjustments of maned wolves, Chrysocyon brachyurus (Illiger) (Mammalia, Canidae), subjected to supplemental feeding in a private natural reserve, Southeastern Brazil. Revista Brasileira de Zoologia 20:339–345
Strier KB (2007) Primate behavioral ecology. Allyn and Bacon, Boston
Tabachnick BG, Fidell LS (2001) Using multivariate statistics, 4th edn. Allyn & Bacon, Boston
Templeton CN, Greene E, Davis K (2005) Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308:1934–1937. doi:10.1126/science.1108841
Terborgh J (1983) Five New World primates: a study in comparative ecology. Princeton University Press, Princeton, N.J
Valeggia CR, Mendoza SP, Fernandez-Duque E, Mason WA, Lasley B (1999) Reproductive biology of female titi monkeys (Callicebus moloch) in captivity. Am J Primatol 47:183–195
Vasconcelos MF (2001) Adições à avifauna da Serra do Caraça, Minas Gerais. Atualidades Ornitológicas. Atualidades Ornitológicas 104:3–4
Vasconcelos MF, Melo Junior TA (2001) An ornithological survey of Serra do Caraça, Minas Gerais. Brazil Cotinga 15:21–31
Wheeler B (2010) Production and perception of situationally variable alarm calls in wild tufted capuchin monkeys (Cebus apella nigritus). Behav Ecol Sociobiol 64:989–1000. doi:10.1007/s00265-010-0914-3
Zuberbühler K (2000a) Causal knowledge of predators' behaviour in wild Diana monkeys. Anim Behav 59:209–220
Zuberbühler K (2000b) Referential labelling in Diana monkeys. Anim Behav 59:917–927
Zuberbühler K (2001) Predator-specific alarm calls in Campbell's monkeys, Cercopithecus campbelli. Behav Ecol Sociobiol 50:414–422
Zuberbühler K (2009) Survivor signals: the biology and psychology of animal alarm calling. Adv Stud Behav 40:277–322. doi:10.1016/S0065-3454(09)40008-1
Zuberbühler K, Noë R, Seyfarth RM (1997) Diana monkey long-distance calls: messages for conspecifics and predators. Anim Behav 53:589–604
Zuberbühler K, Jenny D, Bshary R (1999) The predator deterrence function of primate alarm calls. Ethology 105:477–490
Acknowledgments
We thank Luke Rendell and Will Hoppitt for their statistical advice and Robert Seyfarth and three other referees for their helpful comments. We are grateful to Priests Marcos, Lauro, Wilson, and Sebastião for allowing us to work in the reserve and to Aline Abreu for her support. We are thankful to Vandilso Farias for his assistance in the field and to Marina Bonde de Queiróz, Julianne Cosse de Azevedo, and Belmiro Damas for their help. This research was funded by FAPEMIG-Brazil and a doctoral dissertation scholarship by CAPES-Brazil to CC. Additional financial support was by the L.S.B. LEAKEY TRUST and the University of St Andrews. We thank John Robinson for helping with call classifications. Finally, our gratitude goes all previous members of this research project: Guedes, D., Ferreira, S.Q.C., Nahur, A.C., Assunção, M.L, Silva, P.H.N., Sena, M.L.C., Marcolino, C.P., Franco, E.S., Soares, G.C.N., Viegas, L,R.F., Afonso, C.G., Santos, R.V, Lima, B.S.S, Santos, G.P., Morais, C.M.F., Queiróz, M.B.
Ethical Standards
We received full ethical approval to conduct this study from the University of St Andrews' (School of Psychology) Ethics Committee.
Lawfulness
This work was performed in compliance with all Brazilian laws.
Conflict of interest
None of the authors have a financial relationship with the organisation that sponsored the research, i.e., FAPEMIG-Brazil and CAPES-Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Charpentier
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Cäsar, C., Byrne, R., Young, R.J. et al. The alarm call system of wild black-fronted titi monkeys, Callicebus nigrifrons . Behav Ecol Sociobiol 66, 653–667 (2012). https://doi.org/10.1007/s00265-011-1313-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1313-0