Abstract
Predators hunting for cryptic prey use search images, but how do prey search for cryptic predators? We address this question using the interaction between bumblebees and the colour-changing crab spider Misumena vatia which can camouflage itself on some flowers. In laboratory experiments, we exposed bumblebees to an array of flowers concealing robotic predators (a trapping mechanism combined with a 3D life-sized model of a crab spider or a circle). Groups of bees were trained to avoid either cryptic yellow spiders or yellow circles (equal area to the spiders) or remained predator naive. The bees were then exposed to a new patch of white flowers containing some cryptic predators (either white spiders, white circles or a mixture of both). We monitored individual foraging choices and used a 3D video tracking system to quantify the bees’ flight behaviour. The bees trained to avoid cryptic spiders, chose 40% fewer spider-harbouring flowers than expected by chance, but were indifferent to cryptic circles. They also aborted a higher proportion of landings on flowers harbouring spiders, ultimately feeding from half as many ‘dangerous’ flowers as naive bees. Previous encounters with cryptic spiders also influenced the flight behaviour of bees in the new flower patch. Experienced bees spent more time inspecting the flowers they chose to reject (both with and without concealed spiders) and scanned from side to side more in front of the flowers to facilitate predator detection. We conclude that bees disentangle shape from colour cues and thus can form a generalised search image for spider shapes, independent of colour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foraging animals must balance predator vigilance with foraging efficiency (Lima 1985). Thus, mechanisms which enhance predator detection should benefit foraging animals (Lima and Dill 1990). Indeed many animals possess predator avoidance responses that can be either innate (Veen et al. 2000; Berejikian et al. 2003; Turner et al. 2006) or learnt (Brown 2003; Kelley and Magurran 2003; Ings and Chittka 2008). Both innate and learned avoidance responses require an animal to recognise cues that signal the presence of their predators. In many cases, the cues that indicate predator presence are salient, e.g. passing shadows (Oliva et al. 2007; Cooper 2009), or chemical cues such as fish kairomones (reviewed in Wisenden 2000). However, in the case of cryptic predators, especially ambush or sit-and-wait predators, such cues are likely to be much less salient to prey (Troscianko et al. 2009).
When faced with cryptic prey, predators are known to utilise a prey-specific search image (Tinbergen 1960) defined as: “a transitory enhancement of detection ability for particular cryptic prey types or characteristics” (Ruxton et al. 2004). This strategy enables the predator to focus on the cryptic prey, even in the presence of distractors, but what strategies do prey use to recognise, and thus avoid, cryptic predators? Surprisingly, little attention has been given to this question. However, in our previous work (Ings and Chittka 2008), we showed that bumblebees are able to learn to reliably detect cryptic predators. As the bees were unable to detect any colour contrast between spider models and their background, we suggested that the bees must have been relying on shape-from-shading cues (Hershberger 1970; Ramachandran 1988). We were particularly intrigued by the observation that bees were able to rapidly learn to avoid spider models and that the avoidance response was maintained for at least 24 h. This led us to consider whether bees are developing a specific search image to enhance their detection of cryptic predators.
While it is widely known that bees possess advanced cognitive capabilities and can be trained to recognise and associate complex patterns with rewards or punishments (Stach et al. 2004; Chittka and Niven 2009; Avarguès-Weber et al. 2011) in both appetitive (rewarding or distasteful food: Menzel 1985) and predator avoidance contexts (Ings and Chittka 2008; Ings and Chittka 2009), little is known about their use of search images (NB in our work we refer to ‘learnt’ search images rather than the ‘innate’ search images proposed by Menzel 1985). By inference, it appears that honeybees can use search images as they are able to distinguish camouflaged shapes after training (Zhang and Srinivasan 1994). Field observations also indicate that pollinators are able to recognise specific elements of a spider shape (the raptorial forelegs: Gonçalves-Souza et al. 2008). However, the spider models used in that study were not cryptic on the flowers and only wild pollinators were tested, so it was not possible to determine if avoidance was an innate or learned response.
Therefore, in this study, we test whether bees can form a generalised search image for cryptic predators independent of colour. We utilise the interaction between bumblebees and the predatory crab spider Misumena vatia, which is able to reversibly change its colour between white and yellow and thus camouflage itself on white or yellow flowers respectively (Morse 2007; Insausti and Casas 2008). One hypothesis is that bees only learn to avoid predators of the colour they have been exposed to, or they are only vigilant for predators when they encounter flowers of the same colour as those where they have experienced predation threat. Alternatively, bees might form a generalised, colour-independent search image (Hempel de Ibarra and Giurfa 2003) and will thus avoid spider shapes irrespective of spider or flower colour.
Materials and methods
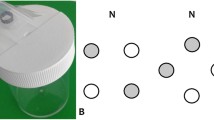
Two colonies of bumblebees (Bombus terrestris) were obtained from Syngenta Bioline Bees, The Netherlands. All bees were reared in a dark environment devoid of visual cues (colour and shape) prior to commencement of the experiments. The experiments were conducted in a wooden flight arena (l = 1 m, w = 0.72 m and h = 0.73 m) with a UV-transmittent Plexiglas® lid and lit by two twin lamps (TMS 24 F with HF-B 236 TLD (4.3 kHz) ballasts, Philips, The Netherlands) fitted with Activa daylight fluorescent tubes (Osram, Germany). The side walls were painted white and the end wall, containing an artificial ‘meadow’ of 16 ‘flowers’ arranged in four evenly spaced rows (“Electronic supplementary material”, Fig. 1a), was painted grey to provide good contrast against the flowers (for further details, see Ings and Chittka (2008)). Each flower consisted of a landing platform (“Electronic supplementary material”, Fig. 1b), where bees could land and extend their proboscises through a hole in the wall to feed on sucrose droplets (50% v/v) being formed at the end of syringe needles (BD MicrolanceTM Drogheda, Ireland, 3 26 G 0.45 × 13 mm, delivered at a rate of 1 μl min−1 by syringe pumps: KD Scientific, KD200, Holliston, MA, USA), and a removable square (7 × 7 cm) floral colour signal (for further details, see Ings and Chittka (2009)).
Experimental design
To determine whether bees are able to form generalised, colour-independent search images of predators, we carried out two sets of experiments (Table 1). Our initial focus (the generalised spider avoidance experiment) was to determine whether bees that had learnt to avoid cryptic predators in one colour context (yellow spiders on yellow flowers) would also be able to avoid cryptic predators in a different colour context (white spiders on yellow flowers). In our second experiment (the shape-specific search image experiment), we specifically tested our hypothesis that the transference of avoidance responses between colour contexts results from bees using a generalised shape-specific search image for crab spiders. The alternative hypothesis was that bees learn to discriminate between simple ‘safe’ flowers with a uniform floral display from more complex ‘dangerous’ flowers with 3D shapes attached to the floral displays.
Generalised spider avoidance experiment
Pre-training
Prior to training, individually marked bees foraged freely in the arena. Once motivated foragers were identified (i.e. they filled their crops and returned to the nest repeatedly), they were individually pre-trained on yellow flowers (a 7 × 7-cm flat yellow floral signal was placed flush with the wall at each feeding position). Pre-training lasted for a minimum of 100 flower visits (134.4 ± 4.4) to ensure that bees had learned to associate yellow flowers with a sucrose reward (Ings and Chittka 2008). Subsequently, bees were allocated into two treatment groups for training (naive and experienced).
Training
During training, both groups of bees foraged individually within the meadow of yellow flowers. Their foraging behaviour was observed and scored into four categories: (1) choices—where bees chose to land on flowers, (2) acceptances—where the bees remained and extended their proboscises to feed, (3) aborts—where bees landed but rapidly left without attempting to feed and (4) rejections—where bees inspected flowers (by entering a defined zone [h = 9 cm, w = 9 cm, d = 7 cm] in front of the floral display; Fig. 1a) but rejected them without landing. In addition, the flight paths of all bees were recorded using a 3D tracking system (Trackit, Biobserve, Germany). Before training ceased, bees were required to make a minimum of 200 flower choices, after which they were allowed to continue foraging and return to the nest under their own volition (thus, the total number of choices varied among bees: naive = 240.4 ± 7.9, experienced = 227.0 ± 8.0). This ensured that experienced bees received sufficient (5.9 ± 1.0) simulated predation attempts to learn about predation risk from camouflaged spiders (Ings and Chittka 2008).
Analysis of turning points. a A dangerous flower and the flower inspection zone (dashed cube) surrounding the flower. The bold line shows the 3D flight path of the bee as it inspects the flower. b This path is translated to show the horizontal (y) displacement relative to the platform against time to detect turning points (changes of direction >5 mm) which are indicated by arrows
For the naive bees group (n = 12), the artificial meadow remained the same as the pre-training phase and was free from predation risk. Bees in the experienced group (n = 12) were exposed to the same meadow of yellow flowers as naive bees, but there was a 25% risk of being attacked by a predator (“Electronic supplementary material”, Fig. 1a). Four randomly selected ‘dangerous’ flowers (out of 16) harboured cryptic ‘predators’. Bees received a simulated predation attempt by a ‘crab spider’ whenever they landed on one of these flowers (for details, see Fig. 1 in Ings and Chittka 2008). Predators consisted of a 3D life-size model of the crab spider M. vatia (placed above the feeding hole) and a trapping mechanism that grasped bees between two foam-coated pincers for 2 s (“Electronic supplementary material”, Fig. 1b). The pincers, which projected from the arena wall to either side of the landing platform, were operated by a remotely controlled solenoid. During training, yellow spider models (painted the same yellow as the floral display) that were cryptic to bumblebees were used. To avoid bees learning the location of the spiders, their positions were randomly redistributed between foraging bouts.
Avoidance assays
Directly after training, bees from both groups were tested in a new ‘meadow’ containing 16 white flowers. Four randomly chosen flowers harboured cryptic spiders (painted the same white as the floral displays), although bees landing on these flowers were not attacked. The behaviour and flight paths of bees in this new meadow were monitored until a minimum of 30 flower choices had been made. The majority of bees (22 out of 24) reached this criterion within their first foraging bout; only two bees, both in the naive group, required two foraging bouts.
Shape-specific search image experiment
All bees were pre-trained using the same procedures as the generalised spider avoidance experiment and subdivided into two treatment groups. The first group (n = 12) of bees (‘spider’) was trained to avoid cryptic yellow spiders in the same way as the experienced bees in the generalised spider avoidance experiment. However, in the avoidance assay, they were exposed to a meadow containing 16 white flowers where four randomly positioned flowers harboured cryptic white spiders and an additional four flowers harboured white circles. These circles were of similar area (323.7 mm2) to the spiders (322.6 ± 6.5 mm2) and protruded from the flower surface (they were made from 1-mm- thick plastic). Thus, the general appearance of the dangerous flowers (flat white with a 3D shape by the feeding hole) remained the same as those bearing spiders. As before, the behaviour of bees was monitored until they had made a minimum of 30 choices. A second group (n = 12) of bees (‘circle’) from the same colony acted as a control group to ensure that bees are able to detect and learn to avoid circles. Therefore, the training and avoidance assays were carried out in the same manner as for the experienced group in the generalised spider avoidance assay, with the exception that the four spiders were replaced with four circles in both training and avoidance assay phases (Table 1).
Analyses
As we were interested in how bees’ past experience of spiders, not current risk (i.e. spiders were present but the trapping mechanisms were inactive during tests), influenced their responses to the presence of cryptic spiders during the avoidance assay, only the behaviour and flight paths associated with the first 30 flower choices were included in the analyses. One-sample t-tests were used to determine if the number of ‘dangerous’ flowers chosen during the avoidance assays differed from chance levels (7.5 flower visits). Where necessary, appropriate transformations were carried out to meet the assumptions of the statistical tests: for rejections of safe flowers and aborts of dangerous flowers the log(x + 1) transformation was used. It was not possible to normalise the proportion of dangerous flowers accepted by transformations so these data were analysed using a generalised linear model (GLM) using a binomial error distribution.
For the analysis of the flight paths, the duration, the distance travelled, the number of turning points and the average speed of inspection flights within the zone in front of the floral displays (Fig. 1a) were compared between treatment groups using t-tests. In the case of acceptances and aborts, the inspection flights were taken as the approach flight prior to a bee landing to feed. However, for rejections, the inspection flight was taken as the total flight path in front of the flower. All inspection flights of less than 0.1-s duration were excluded from the analyses to prevent the inclusion of instances where bees passed through the inspection zone on their way to another flower. To quantify scanning behaviour when bees inspected and rejected flowers harbouring spiders, we plotted their flight paths in the horizontal x–y plane (Fig. 1b). A turning point was counted when the bees’ trajectory changed direction along the y axis (parallel to the flower display) and involved a displacement of at least 5 mm (Fig. 1b). Statistical analyses were carried out in SPSS for Windows 11.5 and using the R statistical platform (R Development Core Team 2004).
Results
Generalised spider avoidance experiment
By the end of training, experienced bees had learnt to avoid yellow flowers harbouring cryptic yellow spiders: visitation rates (0.03 ± 0.01 visits per choice) to dangerous flowers during the last 30 choices were significantly below those expected (0.25) if the bees were choosing flowers at random (one sample t-test: t = 16.455, df = 11, p < 0.001). During the avoidance assay, all bees (n = 24) foraged successfully in the new patch of white flowers. There was no significant difference between groups of bees in the number of flowers chosen during their first foraging bouts (experienced, n = 55.5 ± 4.9; naive = 56.1 ± 5.7; t = −0.078, df = 22, p = 0.939). Although the total number of flowers rejected during the first 30 choices (experienced = 9.1 ± 1.7; naive = 17.0 ± 5.7; t = −1.340, df = 22, p = 0.197) did not differ significantly between experienced and naive bees, naive bees did reject more ‘safe’ (no spiders) flowers (experienced = 2.6 ± 1.0; naive = 11.8 ± 4.8; [log transformed] t = 3.183, df = 22, p = 0.004), but not ‘dangerous’ flowers (experienced = 4.3 ± 0.9; naive = 4.6 ± 1.2; t = −0.168, df = 22, p = 0.868) than experienced bees during this period. The latency to forage was variable among bees, and experienced bees tended to start foraging sooner (experienced = 13.8 ± 3.2 s and naive = 40.8 ± 12.5 s), though not significantly so ([log transformed] t = −2.055, df = 22, p = 0.052) than naive bees.
Bees that had experienced attacks by cryptic yellow spiders (on yellow flowers) chose (landed on) 40% fewer white flowers harbouring cryptic (white) spiders than expected by chance during the avoidance assay (Fig. 2a; one-sample t-test: t = −4.413, df = 11, p = 0.001). This was evident from the first flowers visited and the magnitude of the effect increased gradually as bees visited more flowers (Fig. 2a). In contrast, the total number of dangerous flowers chosen by bees with no prior experience of cryptic spiders did not deviate from that expected by chance (Fig. 2a; one-sample t-test: t = −1.239, df = 11, p = 0.241), although there is a suggestion that it fell as bees visited more flowers. Furthermore, while naive bees accepted almost all of the dangerous flowers they chose to land on (93.1 ± 3.0%), experienced bees aborted many landings and only accepted fewer than two thirds (60.9 ± 12.0%) of the dangerous flowers they chose to land on (Fig. 2b; GLM [binomial error]: F 1, 21 = 9.228, p = 0.006). Thus, experienced bees ultimately fed from only half (2.9 ± 0.8) as many dangerous flowers as naive bees (5.9 ± 0.8).
Cumulative foraging choices made by bees during the first 30 flower choices of the avoidance assay. a The mean (±95% CI) number of dangerous flowers (harbouring cryptic white spiders) chosen by bees that had previously experienced spiders (black circles) and spider naive bees (grey diamonds). b The proportion of dangerous flowers chosen that were subsequently accepted, i.e. the bees continued to feed. The line in a represents the expected number of dangerous flowers chosen if bees showed no avoidance response to spiders
Prior experience of cryptic spiders also influenced bees’ flight behaviour. Experienced bees spent 1.4 times longer inspecting safe flowers than naive bees (Fig. 3a; t = 3.862, df = 19, p = 0.001.) and 1.5 times longer inspecting dangerous flowers (Fig. 3a; t = 2.556, df = 20, p = 0.019) that they rejected. Furthermore, when rejecting flowers, experienced bees spent more time inspecting dangerous flowers than safe flowers (Fig. 3a; paired t-test: t = −4.703, df = 7, p = 0.002), whereas there were no differences for naive bees (Fig. 3a; paired t-test: t = −1.706, df = 10, p = 0.119). Bees also altered the distance they travelled whilst inspecting flowers that they rejected (Fig. 3b). The flight paths of experienced bees were longer than those of naive bees when they were rejecting both safe (2.9 ± 1.3 cm longer; t = 2.279, df = 19, p = 0.034) and dangerous (5.0 ± 0.2 cm longer; t = 2.258, df = 20, p = 0.020) flowers. Experienced bees also increased the length of their inspection flights for dangerous flowers relative to safe flowers (Fig. 3b; 4.7 ± 1.2 cm longer; paired t-test: t = −4.007, df = 7, p = 0.005), but no change was observed for naive bees (Fig. 3b; paired t-test: t = −1.201, df = 10, p = 0.258). There were no differences in the length of inspection flights between treatment groups or flower types for flowers that were accepted (Fig. 3b).
The differences in the duration and length of inspection flights for rejected flowers corresponded to changes in the scanning behaviour of bees (Fig. 4). Experienced bees doubled the number of side-to-side scans (Figs. 4 and 5) when they inspected and rejected dangerous flowers (paired t-test: t = −7.029, df = 7, p < 0.001), whereas the number of scans by naive bees showed a slight, but non-significant, increase when they rejected dangerous flowers (paired t-test: t = −1.884, df = 10, p = 0.089).
An example of side-to-side scanning behaviour in front of a flower for a typical bee from the experienced treatment group: inspecting and rejecting a a safe flower and b a dangerous flower. Panels to the left show the 3D flight path within the flower zone relative to the base of the landing platforms (0, 0, 0). The bold lines are the actual trajectories and the grey lines are projections of the trajectories onto the three horizontal and vertical planes. Panels to the right show the turning points (direction changes of >5 mm) of the horizontal component of the bees’ inspection flight for safe (a) and dangerous (b) flowers
Shape-specific search image experiment
By the end of training, bees exposed to dangerous flowers harbouring yellow cryptic spiders (expected probability = 0.25, observed = 0.14 ± 0.03, one-sampled t-test: t = −4.238, df = 11, p = 0.001) or yellow cryptic circles (expected probability = 0.25, observed = 0.12 ± 0.02 , one-sampled t-test: t = −6.434, df = 11, p < 0.001) chose significantly fewer dangerous flowers than expected by chance, i.e. they had learnt to avoid cryptic yellow spiders and circles. When the colour context changed (to white flowers and white spiders/circles), bees in the spider group chose less than half the number of spider-harbouring flowers than expected if they were unable to recognise danger (Fig. 6; one-sampled t-test: t = −12.113, df = 11, p < 0.001). However, the same bees were indifferent to the presence of cryptic white circles on flowers: visitation rates to these flowers did not differ from chance levels (Fig. 6; one-sampled t-test: t = −0.238, df = 11, p = 0.816). Even so, bees in the circle group (that had been trained to avoid cryptic yellow circles) also chose fewer flowers bearing cryptic white circles in the avoidance assay than would have been expected if they were unable to recognise danger (expected probability = 0.25, observed = 0.14 ± 0.02, one-sampled t-test: t = −6.240, df = 11, p < 0.001).
The mean (± 95% CI) proportion of flowers harbouring either cryptic white spiders (left circle) or circles (right circle) chosen by bees in the spider group during their first 30 choices in the avoidance assay. The dashed line represents the expected proportion of spider/circle flowers bees would choose if they showed no avoidance response to the shapes. Note that the shades used for the spider and circle flowers depicted (not to scale) were chosen for clarity and do not accurately represent the colours used in the experiments
Discussion
We found that bumblebees formed a colour-independent search image (spider shape) of cryptic predators which subsequently influenced their foraging behaviour when they were exposed to a new patch of flowers containing differently coloured cryptic predators. This ability is particularly important in the context of bumblebee–crab spider interactions where some species of spider, such as M. vatia, are able to reversibly change their colour (Morse 2007; Insausti and Casas 2008). Thus, rather than learning to detect just the cryptic yellow forms of the spiders, the bees appear to be learning complex shape cues that can be generalised (Stach et al. 2004) to other colour forms of the spider.
Previous work has shown that honeybees are able to use prior experience to enhance their ability to detect camouflaged shapes. Zhang and Srinivasan (1994) found that whilst naive bees were unable to detect camouflaged shapes, detection was possible if they had previously been trained to discriminate the shapes in a simpler context—i.e. they had developed a search image for the shapes. We have now tested whether bees’ search image of a predator consists of the shape memorised together with its colour or whether bees are able to recognise the predator’s shape irrespective of its colour, requiring them to disentangle shape from colour features (Skorupski and Chittka 2011). This is a non-trivial task because when both shape and colour cues are present, as is the case with our yellow spiders, bees tend to focus more on colour cues (Hempel de Ibarra and Giurfa 2003; Lehrer and Campan 2004).
Many animals possess innate avoidance responses to major predators (e.g. birds, molluscs and fish: Veen et al. 2000; Turner et al. 2006; Dixson et al. 2010), but the possibility that bees possess an innate avoidance response to spiders has not been tested to date, although it is often alluded to (Dukas 2001; Reader et al. 2006; Gonçalves-Souza et al. 2008). In the current study, naive bees did not avoid flowers harbouring cryptic white spiders (Fig. 2a), which supports our previous observations (Ings and Chittka 2008) that bumblebees do not appear to have a strong innate avoidance response to spider shapes. However, bees that had experienced attacks by cryptic yellow crab spiders, and learned to avoid such spiders, also avoided cryptic white crab spiders in a new patch of white flowers. They chose (landed on) 40% fewer spider-harbouring flowers than expected by chance. Avoidance of flowers with spiders was evident right from the first few flower choices (Fig. 2a) and strengthened with increased exposure to more spider-harbouring flowers. Furthermore, while naive bees accepted nearly all of the dangerous flowers that they initially chose to land on, experienced bees aborted landings on many dangerous flowers without feeding (Fig. 2b). This suggests that bees only recognised ‘danger’ once they had briefly landed in front of the spider model—an effect already demonstrated for encounters with cryptic spiders (Ings and Chittka 2008). Clearly, experiencing predation attempts by cryptic spiders influences the foraging behaviour of bees in a new patch of flowers, but are bees using colour-independent search images of predators?
One potential explanation for the apparent avoidance of flowers harbouring cryptic white spiders by experienced bees is that they were generally more ‘cautious’ as a result of being attacked during training (e.g. increased vigilance with higher predation risk: Lendrem 1983; Hunter and Skinner 1998; Winnie and Creel 2007). However, evidence from our experiments rules out indiscriminate ‘cautiousness’. The appearance of both flowers and predators was different in the new patch and the predators were highly cryptic. Therefore, if experienced bees were more cautious overall than naive bees, as a result of experiencing simulated spider attacks, we would have expected them to take longer to start foraging in the patch with new flowers and also to reject more safe flowers (Ings and Chittka 2008). Yet there was no clear evidence for an overall change in behaviour of experienced bees compared to naive bees. In particular, there was no difference in the total number of flowers both groups of bees chose to land on during the avoidance assay and naive bees rejected more safe flowers than experienced bees. Furthermore, bees that had experienced attacks from camouflaged spiders on yellow flowers, if anything, started foraging on the new white flowers sooner (though not significantly so) than bees that had no experience of spiders. This behaviour does suggest that, having been attacked on yellow flowers, experienced bees find white flowers more attractive (e.g. see Ings and Chittka 2009) than naive bees. However, overall, we argue that the reluctance of experienced bees to feed on flowers with cryptic white spiders is not a general response to being attacked by spiders but is a specific response to their recognising the shape of the spiders which they associate with danger.
A potentially simpler explanation for the behaviour of experienced bees during the avoidance assay is that they were responding to the general appearance of the dangerous flowers relative to safe flowers rather than spider shapes specifically. In other words, even though the colour of the flowers changed between training and the avoidance assay, bees may have associated flowers that had a 3D object attached to the floral display with danger. However, evidence from the shape-specific search image experiment rules out this possibility. If bees were indeed learning to avoid flowers that differed in general appearance to those that were safe in the training phase, they should have avoided both flowers bearing spiders and those bearing circles. Yet having been trained to avoid cryptic yellow spiders, they only avoided cryptic white spiders and were indifferent to white circles in the avoidance assay (Fig. 6). We can also rule out the possibility that indifference to cryptic circles occurred because bees were unable to detect them because bees in the circle group readily learnt to avoid cryptic yellow circles during training and also avoided cryptic white circles during the avoidance assay. Therefore, we argue that bees in the experienced group of the generalised spider avoidance experiment and the spider group of the shape-specific search image experiment had developed a search image for crab spider shapes.
Further support for the use of colour-independent spider search images by experienced bees in the generalised spider avoidance experiment is provided by the analysis of their 3D flight paths. Experienced bees spent more time inspecting flowers (both with and without spiders) that they rejected than naive bees (Fig. 3a). More importantly, in the context of predator search images, experienced bees spent 30% more time inspecting the dangerous flowers that they rejected when compared to safe flowers. As neither group of bees had previously encountered white flowers before, these differences cannot be attributed to experienced bees associating white flowers with danger. A plausible explanation is that experienced bees invested more time into predator detection than naive bees in response to their recent exposure to high predation risk (Lima and Bednekoff 1999). However, there was no evidence for an overall increase in vigilance as experienced bees did not spend more time inspecting flowers that they chose to accept (Fig. 3a). This suggests that bees modulate vigilance, and potentially employ predator search-images, in response to a high ‘perceived’ predation threat over short time scales (Lima and Bednekoff 1999)—even between flower visits. As bees moved rapidly from flower to flower in the meadow (mean interflower time was only 1.6 ± 0.3 s and the mean approach speed was 0.22 ± 0.01 m s−1), the probability of detection errors (not perceiving a potential predator) is likely to be relatively high (Ings and Chittka 2008). We therefore argue that experienced bees only shift their attention towards predator detection (i.e. used their predator search image) when they detected flowers whose appearance subtly differed (the presence of the 3D cryptic spider) from safe flowers or after they had recently detected a threat on a nearby flower.
Evidence for this switch to predator detection upon perceiving a potential threat is provided by closer scrutiny of the bees’ flight paths. Experienced bees also travelled further when they were inspecting dangerous flowers (Fig. 3b). More importantly, the greater distance travelled was a result of increased side-to-side scanning of the flowers (Figs. 4 and 5). Although we were not able to track the relative position of bumblebees’ heads and thoraxes, their scanning movements were similar to the peering flight manoeuvres recently described in honeybees (Boeddeker and Hemmi 2010). These repeated side-to-side movements would improve edge detection by amplifying the weak spider shape signal through integration over time. For example, scanning may allow bees to use relative motion cues (Zhang and Srinivasan 1994), i.e. enhanced long-receptor contrast (Hempel de Ibarra and Giurfa 2003) of shadows cast by the 3D spider models, to facilitate shape detection.
Our results demonstrate that search images are important in the context of predator avoidance, when prey have to be vigilant for cryptic predators. More importantly, we found that bees are able to develop search images that do not tightly link colour and shape. Rather than search for ‘yellow spiders’, bees were able to extract a generalised search image for ‘spider shapes’. This ability to respond to shape irrespective of colour has only recently been recognised in hymenoptera (Hempel de Ibarra and Giurfa 2003; Lehrer and Campan 2004; Lehrer and Campan 2005). Here we have shown how this ability to disentangle shape from colour can enhance detection of colour-changing cryptic predators.
References
Avarguès-Weber A, Deisig N, Giurfa M (2011) Visual cognition in social insects. Annu Rev Entomol 56:423–443
Berejikian BA, Tezak EP, LaRae AL (2003) Innate and enhanced predator recognition in hatchery-reared chinook salmon. Environ Biol Fishes 67:241–251
Boeddeker N, Hemmi JM (2010) Visual gaze control during peering flight manoeuvres in honeybees. Proc R Soc B 277:1209–1217.
Brown GE (2003) Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish 4:227–234
Chittka L, Niven J (2009) Are bigger brains better? Curr Biol 19:R995–R1008
Cooper WE (2009) Rapid covering by shadow as a cue to predation risk in three lizard species. Behaviour 146:1217–1234
Dixson DL, Munday PL, Jones GP (2010) Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett 13:68–75
Dukas R (2001) Effects of perceived danger on flower choice by bees. Ecol Lett 4:327–333
Gonçalves-Souza T, Omena PM, Souza JC, Romero GQ (2008) Trait-mediated effects on flowers: artificial spiders deceive pollinators and decrease plant fitness. Ecology 89:2407–2413
Hempel de Ibarra N, Giurfa M (2003) Discrimination of closed coloured shapes requires only contrast to the long wavelength receptor. Anim Behav 66:903–910
Hershberger W (1970) Attached-shadow orientation perceived as depth by chickens reared in an environment illuminated from below. J Comp Physiol Psychol 73:407–411
Hunter LTB, Skinner JD (1998) Vigilance behaviour in African ungulates: the role of predation pressure. Behaviour 135:195–211
Ings TC, Chittka L (2008) Speed accuracy tradeoffs and false alarms in bee responses to cryptic predators. Curr Biol 18:1520–1524
Ings TC, Chittka L (2009) Predator crypsis enhances behaviourally-mediated indirect effects on plants by altering bumblebee foraging preferences. Proc R Soc B 276:2031–2036
Insausti TC, Casas J (2008) The functional morphology of color changing in a spider: development of ommochrome pigment granules. J Exp Biol 211:780–789
Kelley JL, Magurran AE (2003) Learned predator recognition and antipredator responses in fishes. Fish Fisheries 4:216–226
Lehrer M, Campan R (2004) Shape discrimination by wasps (Paravespula germanica) at the food source: generalization among various types of contrast. J Comp Physiol A-Neuroethol Sens Neural Behav Physiol 190:651–663
Lehrer M, Campan R (2005) Generalization of convex shapes by bees: what are shapes made of? J Exp Biol 208:3233–3247
Lendrem DW (1983) Predation risk and vigilance in the blue tit (Parus caeruleus). Behav Ecol Sociobiol 14:9–13
Lima SL (1985) Maximizing feeding efficiency and minimizing time exposed to predators: a trade-off in the black-capped chickadee. Oecologia 66:60–67
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation—a review and prospectus. Canadian J Zool-Revue Canadienne De Zoologie 68:619–640
Menzel R (1985) Learning in honey bees in an ecological and behavioral context. In: Hölldobler B, Lindauer M (eds) Experimental behavioral ecology, vol 31. Fischer, Stuttgart, pp 55–74
Morse DH (2007) Predator upon a flower: life history and fitness in a crab spider. Harvard University Press, Cambridge
Oliva D, Medan V, Tomsic D (2007) Escape behavior and neuronal responses to looming stimuli in the crab Chasmagnathus granulatus (Decapoda: Grapsidae). J Exp Biol 210:865–880
R Development Core Team (2004) R: a language and environment for statistical computing. In: R Foundation for Statistical Computing, Vienna
Ramachandran VS (1988) Perception of shape from shading. Nature 331:163–166
Reader T, Higginson AD, Barnard CJ, Gilbert FS (2006) The effects of predation risk from crab spiders on bee foraging behavior. Behav Ecol 17:933–939
Ruxton GD, Sherratt TN, Speed MP (2004) Avoiding attack. The evolutionary ecology of crypsis, warning signals and mimicry. Oxford University Press, Oxford
Skorupski P, Chittka L (2011) Is colour cognitive? Optic Laser Technol 43:251–260
Stach S, Benard J, Giurfa M (2004) Local-feature assembling in visual pattern recognition and generalization in honeybees. Nature 429:758–761
Tinbergen N (1960) The natural control of insects in pine woods: vol. I. Factors influencing the intensity of predation by songbirds. Archives Neelandaises de Zoologie 13:265–343
Troscianko T, Benton CP, Lovell PG, Tolhurst DJ, Pizlo Z (2009) Camouflage and visual perception. Phil Trans R Soc B 364:449–461
Turner AM, Turner SE, Lappi HM (2006) Learning, memory and predator avoidance by freshwater snails: effects of experience on predator recognition and defensive strategy. Anim Behav 72:1443–1450
Veen T, Richardson DS, Blaakmeer K, Komdeur J (2000) Experimental evidence for innate predator recognition in the Seychelles warbler. Proc R Soc B 267:2253–2258
Winnie J, Creel S (2007) Sex-specific behavioural responses of elk to spatial and temporal variation in the threat of wolf predation. Anim Behav 72:215–225
Wisenden BD (2000) Olfactory assessment of predation risk in the aquatic environment. Phil Trans R Soc B 355:1205–1208
Zhang SW, Srinivasan MV (1994) Prior experience enhances pattern discrimination in insect vision. Nature 368:330–332
Acknowledgements
We thank Oscar Ramos Rodríguez for technical support, Syngenta Bioline Bees for providing bumblebees and Dr. Peter Skorupski for commenting on an earlier version of this manuscript. TI and LC were supported by the Natural Environment Research Council (Grant NE/D012813/1) and MYW by the Overseas Research Students Awards Scheme and the Ministry of Education and National Science Council Taiwan Studying Abroad Scholarship. The experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Traniello
TC Ings and M-Y Wang authors contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. 1
Experimental set up. Panel a shows a schematic of the flight arena housing the artificial meadow (dimensions are given in the text) and the axes used in the 3D flight path analyses (bold arrows). A close up of a ‘dangerous’ flower is given in panel b showing how the sponge coated pincers trap any bees which attempt to feed from the flower (PDF 756 kb)
Rights and permissions
About this article
Cite this article
Ings, T.C., Wang, MY. & Chittka, L. Colour-independent shape recognition of cryptic predators by bumblebees. Behav Ecol Sociobiol 66, 487–496 (2012). https://doi.org/10.1007/s00265-011-1295-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1295-y