Abstract
Sexual cannibalism particularly before mating is costly for the male victim but also for the female aggressor if she risks remaining unmated. The aggressive spillover hypothesis explains the persistence of this behavior as a maladaptive side effect of positive selection on aggressiveness in a foraging context. The hypothesis predicts that the occurrence of sexual cannibalism is explained by female aggressiveness but is not related to male phenotype or behavioral type. An alternative hypothesis invokes sexual selection and makes the opposite prediction namely that sexual cannibalism is an expression of female choice and should hence mainly target males of low quality. We tested the above hypotheses on a sexually dimorphic nephilid spider Nephilengys livida, known for male monopolization of females via genital damage, female genital plugging, and mate guarding, by staging mating trials during which we recorded mating behaviors and occurrences of pre- and postcopulatory cannibalism. We did not restrict assessment of aggressiveness to the mating and foraging context but also included aggression against same sex conspecifics. To assess female personalities, i.e., consistent individual differences in behavior including aggressiveness, we repeatedly tested them for intra-sex aggression, voracity towards prey, locomotory activity, and boldness. Females exhibited consistent differences in intra-sex aggressiveness, latency to attack prey, and boldness. Aggressive females had shorter latencies to attack prey and were more active than non-aggressive ones. In contrast to the predictions of the aggressive spillover hypothesis, females that were aggressive towards prey and towards other females were not more likely to attack a male than non-aggressive females. In support of the mate choice hypothesis, less aggressive males were more likely attacked and cannibalized than more aggressive ones. This hints at sexual selection for aggressiveness in males and raises the question of mechanisms that maintain variation in male aggressiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Females attack, kill, and consume males during, after, or even before copulation in a wide range of species, but this is most prevalent in praying mantids and spiders (Elgar 1992; Barry et al. 2008). This phenomenon, referred to as sexual cannibalism, is often viewed as an extreme form of sexual conflict especially if females consume males prior to mating (Elgar 1992; Elgar and Schneider 2004; Wilder et al. 2009). Costs and benefits of sexual cannibalism for both sexes depend on the timing of sexual cannibalism (Elgar and Schneider 2004). While females may profit from precopulatory sexual cannibalism through weight gain and increased fecundity (Birkhead et al. 1988; Elgar and Nash 1988; Elgar 1998; Johnson 2001; Moya-Larano et al. 2003; Barry et al. 2008), postcopulatory cannibalism may be advantageous for males if they benefit through parental investment and reduced sperm competition (Andrade 1996; Prokop and Vaclav 2005). Precopulatory sexual cannibalism may be a result of any of the following mechanisms: failing recognition of a prospective mate by voracious females (Gould 1984); spillover of female aggressiveness towards prey (Arnqvist and Henriksson 1997; Johnson and Sih 2005); mate choice, when females reject unwanted mates (Elgar and Nash 1988; Elgar 1992; Persons and Uetz 2005; Prenter et al. 2006); or hunger, when females benefit from the energy and nutrient intake by devouring the male (adaptive foraging hypothesis) (Newman and Elgar 1991; Snyder et al. 2000 but see Wilder and Rypstra 2010). Frequency and occurrence of sexual cannibalism often positively correlate to low food and perhaps mate availability, as well as high sexual size dimorphism (reviewed in Wilder and Rypstra 2008; Wilder et al. 2009; Roggenbuck et al. 2011). Females may cannibalize males in the context of mating due to any combination of these factors, as the various hypotheses are not mutually exclusive. Here, we test predictions of the aggressive spillover and the mate choice hypothesis.
The aggressive spillover hypothesis predicts that sexual cannibalism represents a spillover of aggression from the juvenile foraging context, where high levels of aggression might be selectively favorable, to the adult mating context (Johnson and Sih 2005). The proposed mechanism is that genetic constraints limit behavioral plasticity (Sih et al. 2004). In the view of personalities, i.e., consistent individual differences in behavior (Bell et al. 2009), positive behavioral correlations for aggression levels across ontogenetic stages (juvenile, adult), and across contexts (foraging, mating), are predicted (Johnson and Sih 2005). Indeed, aggressive carryover across multiple contexts was found in several spider species, e.g., Dolomedes fimbriatus, Dolomedes triton, Agelenopsis aperta, and Anelosimus studiosus (Hedrick and Riechert 1989; Riechert and Hedrick 1993; Arnqvist and Henriksson 1997; Riechert and Johns 2003; Johnson and Sih 2007; Pruitt et al. 2008). Aggression positively correlates to other personality traits, such as boldness in A. aperta and D. triton and activity in A. aperta (Hedrick and Riechert 1989; Riechert and Johns 2003; Johnson and Sih 2007). However, no study tested if female high voracity towards prey and aggression towards same sex conspecifics are also correlated to postcopulatory sexual cannibalism, when females do not compromise their reproductive future. Aggressiveness in the context of same sex interaction could be adaptive through winning competition or maladaptive, for example through unnecessary wasting of energy.
Sexual cannibalism may be a radical form of mate choice if females preferably cannibalize inferior males or if inferior males are less capable of escaping female aggressiveness (Elgar and Nash 1988; Elgar 1992; Persons and Uetz 2005; Prenter et al. 2006). Sexual cannibalism during mating allows females to control copulation duration, and if females mate repeatedly, they may thereby exert control over relative paternity of males (Elgar et al. 2000; Schneider et al. 2006; Herberstein et al. 2011). Sexual cannibalism caused by female choice may be particularly adaptive in species where females are monopolized by males. In several nephilid spiders, males plug female genitalia during copulation, thereby reducing their chance of remating with other males (Fromhage and Schneider 2006; Nessler et al. 2007; Kuntner et al. 2009b; Uhl et al. 2009). Males that survive copulation guard their mates, additionally reducing female polyandry (Kuntner et al. 2009a, b; Kralj-Fišer et al. 2011). We propose that in species where males plug female genitals and then guard them, females may consume the mate to avoid being monopolized, in particular after the first mating encounter (Schneider et al. 2006; Kralj-Fišer et al. 2011). On the other hand, if the (guarding) male is of superior quality (e.g., large, aggressive), females may not oppose monopolization due to heritable advantages of sired offspring in foraging and mating contexts (Elgar 1998; Persons and Uetz 2005; Kralj-Fišer et al. 2011).
Sexual selection can act directly through female aggression targeting undesired suitors or indirectly if females are indiscriminately aggressive towards mates, but only high-quality males survive attacks. If the occurrence of sexual cannibalism depends on the female's physical strength relative to the male's ability to defend his life (Wilder and Rypstra 2008), size assortative mating can result. Studies in wolf spiders Hogna helluo and Schizocosa ocreata as well as in orb-web spider Araneus diadematus showed that size difference between mating partners strongly affected the success of female attacks (Persons and Uetz 2005; Wilder and Rypstra 2008; Roggenbuck et al. 2011). These species are sexually size monomorphic, while in extremely sexually size-dimorphic spiders, small males have little chance to resist an attack by their large female mates, and thus this mechanism is unlikely to hold in, e.g., nephilids. However, if female voracity towards prey correlates to female aggression towards mates, cannibalistic females might be heavier and larger and thus more fecund.
We studied the mating biology, in particular the occurrence of precopulatory and postcopulatory cannibalism in relation to female personalities and male behavior through male–male contests in Nephilengys livida (Araneae: Nephilidae), notorious for extreme sexual size dimorphism, genital plugging, eunuchs (males with broken genitals—pedipalps), sexual cannibalism, and postcopulatory mate guarding (Kuntner 2007; Kuntner et al. 2009a). We tested (1) if female voracity towards prey positively correlates to pre- and postcopulatory cannibalism (which would be in support of aggressive spillover), (2) whether females cannibalize males according to their behavior in the web (supporting direct mate choice), and (3) if female size correlates to their propensity to cannibalize their mates (supporting indirect mate choice). In addition, we investigated (4) if male behavior predicts their mating success and (5) if female voracity towards prey correlates with other behavioral traits such as aggressiveness towards same sex conspecifics, activity, and boldness. To test whether female behaviors can be referred to as personality traits, we estimated temporal consistency of their behaviors.
While the aggressive spillover hypothesis proposes that female-inherited personality predicts occurrences of sexual cannibalism independently of male quality, the mate choice and the mate size dimorphism hypotheses are not mutually exclusive. Male size, important male quality characteristic that we failed to measure, might be a significant factor in mate size dimorphism predictions. Yet, N. livida females are cca four times bigger than males (mean body length, Kuntner and Coddington 2009); hence, female body sizes generate the most of the mate size dimorphism variance. The latter also implies that the male body sizes do not make a difference in triggering female foraging behavior.
Materials and methods
Study animals
We collected N. livida spiders in Andasibe-Mantadia (Toamasina Province) and Ranomafana (Fianarantsoa Province) national parks in Madagascar, between 24 February 2010 and 4 April 2010. To examine personalities in individuals with the same mating history, we collected subadults and reared them to adulthood in the laboratory (females = 25, males = 23). We housed females in glass frames (50 × 50 × 10 cm) and males in 250-ml plastic cups. We watered all spiders daily and fed them Drosophila flies, crickets, and mealworms twice per week.
Mating trials and personality tests
In trials, we observed male–male aggressiveness, occurrence of mating, and pre- and postcopulatory sexual cannibalism. In nine male–male contest trials (9 females, 15 males), we gently placed two virgin males on the web of a virgin female. We scored male–male antagonism as frequencies of being stationary (score = 0), orienting, or walking towards conspecific (score = 1), shaking web (score = 1), chasing (score = 2), attacking (score = 2), and biting (score = 3). We estimated aggressiveness intensity levels as the sum of scores given in the brackets (lowest to highest) (e.g., Kralj-Fišer et al. 2011). Additionally, we recorded locomotory activity, scored as frequency of moves, and frequency of web plucking (courtship behavior). We also observed males' frequency of touching the female and recorded male–female distances every 5 min. The former we used as a measure of risk taking as females are highly cannibalistic (Kralj-Fišer et al. unpublished). Contest trials were terminated after a copulation occurred (N = 8) or after 60 min. Due to the small number of males, we additionally observed occurrences of copulation and female sexual cannibalism in further nine mating trials (nine females), where only one male was introduced into the female web. We used six virgin males from the above contests and three males, which were previously not tested. Hence, we had a total of 18 females that mated once either with or without competition. At the end of the tests, ten of the above (once mated) females were introduced to one male (previously mated or virgin) for the second mating opportunity. We observed spiders for 60 min to allow enough time for copulation.
We observed 12 of the 18 previously used females (from the above experiment) and an additional 8 unmated females (N = 20) in a series of standardized tests for personality characterization, i.e., contest (aggressiveness towards a conspecific of the same sex, locomotor activity), feeding (aggressiveness towards prey), and predatory test (antipredator behavior). To test for behavioral repeatability, we examined each individual twice in each test situation, with 5–10 days between repeats.
We observed the females' aggressiveness in two different contexts: (1) we gently placed a female conspecific on the web 5 cm from the observed female for 30 min, and 2) we introduced prey on the web 10 cm from the observed individual. Female–female aggression was scored as explained above for males. We measured aggressiveness towards prey as the latency to first reaction and latency to bite prey. In predator tests, we touched the female's abdomen with a paintbrush and scored boldness as follows: spider feints death (score = 0), spider runs away (score = 1), spider does nothing (score = 2), spider shakes the web up to 65 s (score = 3), spider shakes the web more than 65 s (score = 4), and spider “attacks” a predator (score = 5). We chose a limit of 65 s, because duration of shaking varied highly among individuals, and 65 s was the median.
At the end of the experiments, we weighed the females (N = 20) and measured their first patella + tibia and carapace lengths (N = 16). As many males were cannibalized during copulation, we could not take their body measures.
Statistical analyses
Personality measures in both males (waving legs, touching female, exploring, walking towards female, distance to female) and females (boldness, latency to first reaction, latency to first bite, and locomotor activity) and morphological traits in females (mass, patella + tibia I length, carapace length) were subjected separately to Principal Component Analysis. The scores along the first axis were extracted and used as explanatory variables. The effect of these trait variables and the intra-sex aggressiveness on the probability of cannibalism and mating success was studied using Generalized Linear Models with binomial error structure (GLM-b) or Generalized Estimating Equations with binomial error structure (GEE-b) if males were used repeatedly. The correlation structure within GEE was exchangeable (Hardin and Hilbe 2003). The analyses were performed in R (R Development Core Team 2010) using the geepack package (Yan and Fine 2004) and in PASW version 18. We analyzed the repeatability of behaviors using the parametric repeatability test (Falconer 1996). We transformed (log (× + 1)) not normally distributed data, thus rendering them suitable for parametric statistics. Using data from the first series of tests, we analyzed behavioral correlations using Spearman's correlations.
Results
Mating success, precopulatory and postcopulatory sexual cannibalism
Seventeen out of 18 virgin females copulated (94.44%). In 8 out of 17 copulations (47.06%), females cannibalized their mates during the first copulation, but never prior to mating. Six out of ten females that had mated in one copulatory opening (CO) copulated in the other CO with another male. During second copulations, sexual cannibalism occurred in 83.33% of the cases (five of six copulations) precluding further analysis.
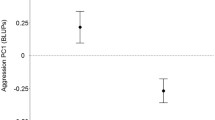
The probability of cannibalism during first copulation decreased significantly with increasing male aggressiveness (GLM-b, X 21 = 5.9, P = 0.002, Fig. 1a, Table 1) and was independent of other male personality traits (GLM-b, X 21 < 0.1, P = 0.98). Probability of mating success increased significantly with increasing male aggressiveness (GEE-b, X 21 = 10.4, P = 0.0012, Fig. 1b) and was independent of other male personality traits (GEE-b, X 21 = 3.2, P = 0.08). Female attacks always resulted in cannibalism.
Consistency of individual behavioral differences and behavioral correlates
Females exhibited consistent individual differences in aggressiveness towards prey (Table 2, latency to first reaction: r = 0.771, N = 18, P = 0.002; latency to first bite: r = 0.59, N = 18, P = 0.042) and consistent differences in antipredator behavior (boldness) (r = 0.661, N = 20, P = 0.011). Females showed consistent individual differences in their aggressiveness towards same sex conspecifics, though these were not significant (repeatability: r = 0.52, N = 19, P = 0.064), whereas female locomotory activity was inconsistent between the repeats (r = −0.467, N = 19, P = 0.788).
Female voracity towards prey correlated with their aggressiveness towards same sex conspecifics: we found the latency to first bite to be negatively correlated with aggression towards same sex conspecifics (r = −0.487, N = 18, P = 0.04). Aggressive females were locomotively more active (r = 0.457, N = 19, P = 0.049). The probability of sexual cannibalism was independent of female personality traits (GLM-b, X 21 = 0.8, P = 0.31), aggressiveness towards conspecifics (GLM-b, X 21 = 1.5, P = 0.22), and morphological traits (GLM-b, X 21 = 0.3, P = 0.57). In males, aggression frequency correlated positively with boldness while approaching a female (distance to the female: r = −0.54, N = 15, P = 0.037).
Discussion
Our study does not support the aggressive spillover hypothesis as an explanation for post-copulatory sexual cannibalism in the nephilid spider N. livida. Forty-seven percent of females cannibalized their males during the first copulation, and female personality did not affect sexual cannibalism, while the male behavior in the male–male contest in the female web was important. Aggressive males were less likely to be attacked and killed after their first copulation than were less aggressive males. Our results suggest that sexual (postcopulatory) cannibalism in N. livida probably results from sexual selection rather than from low plasticity in the female aggressive personality type (aggressive spillover). The direct mate choice model fits the data better than the indirect model as females did not attack every male.
Even though aggressive spillover was proposed to explain sexual cannibalism in several spider species (Arnqvist and Henriksson 1997; Johnson and Sih 2005), this mechanism is not applicable for N. livida, where sexual cannibalism is independent of general aggressiveness. However, we showed that aggression towards the same sex conspecifics, voracity towards prey, and boldness are repeatable and correlated and thus part of female N. livida personality. This is in accordance with other studies suggesting that these traits are inherited components of spider personalities (Riechert and Hedrick 1993; Arnqvist and Henriksson 1997; Johnson and Sih 2005, 2007; Pruitt et al. 2008). According to the above studies, intra-sex aggressiveness positively correlates to voracity towards prey and activity (Riechert and Hedrick 1993; Arnqvist and Henriksson 1997; Johnson and Sih 2005, 2007; Pruitt et al. 2008). Thus, individual aggressiveness carries over from territory defense to foraging context (probably sharing the same proximate mechanism), whereas individual aggressiveness does not spillover into the mating context.
The discrepancy between these studies may be explained by different species' mating biology. Aggressive spillover explains sexual cannibalism in species with a polygamous mating system and moderate sexual size dimorphism (D. fimbriatus, D. triton, A. aperta, and A. studiosus; Hedrick and Riechert 1989; Riechert and Hedrick 1993; Arnqvist and Henriksson 1997; Riechert and Johns 2003; Johnson and Sih 2007; Pruitt et al. 2008). In extremely sexually size-dimorphic N. livida, males obligatorily damage their palps during copulation and thereby plug female copulatory openings (CO, Kuntner et al. 2009a). Thus, males are monogamous or bigamous (two palps) by default. In nephilids where males emasculate their palps during mating, the plugs prevent remating into the used female CO by the rival males in approximately 70% of the cases (Kuntner et al. 2009b; Kralj-Fišer et al. 2011). Mate plugging will thus limit female mating to a single male if both of her genital openings get plugged. Females, however, may benefit from polyandry and may control the number of their mates through sexual cannibalism during or after copulation (Schneider et al. 2006). Under a high risk of monopolization, selection may favor females that are selective and only allow high-quality males two copulations. If male quality is reflected in high aggressiveness (and boldness), this can explain why female N. livida more readily cannibalize less aggressive (and shy) males and prevent them from inseminating both of her spermathecae (Schneider et al. 2006), while aggressive (and bold) males are allowed to monopolize both of them. Females might prefer monopolization by an aggressive (and bold) male if they can gain genetic benefits. Thus, sexual cannibalism in N. livida may be a female mechanism to select her offspring's behavioral phenotypes (Riechert and Johns 2003); her offspring may benefit from aggressiveness (and boldness) in the foraging and mating contexts. Our explanation assumes that high male aggressiveness equals high quality, but in reality, more features may define male quality. For example, in a study on a wolf spider, sexual cannibalism depended on male size and relative size of their secondary sexual ornaments (Prenter et al. 2006). Nevertheless, aggressiveness often correlates with other traits, e.g., body size, boldness, mating and foraging success, which reflect mate quality (this study, Riechert and Tracy 1975; Riechert and Johns 2003).
However, despite selection seemingly favoring aggressive and bold males in N. livida, males of this species are not overly aggressive and are rather cautious when approaching a female (e.g., Kralj-Fišer et al. 2011). Theoretically, males with intact palps are expected to avoid contests and potential injuries prior to mating, thus not risking their future mating opportunities (Fromhage and Schneider 2005). A relatively low aggressiveness in males may relate to their residual reproductive value. Thus, the low levels of precopulatory cannibalism in nephilids may also be the result of male behavioral adaptations, i.e., cautious behavior in a female web (Uhl and Vollrath 1998). Nevertheless, more aggressive males had higher chances to achieve copulation in N. livida.
Alternative to the mate choice hypothesis, aggressive males may have better chances to survive copulation due to their greater ability to evade female attacks. However, this explanation seems unlikely as we never observed successful escapes from cannibalism in this species, while escapes seem possible in other nephilids, but in very few cases (Kralj-Fišer et al. 2011). Apparently, the mating position in N. livida allows females to fully control male survival as is the case in the brown widow Latrodectus hasselti (Andrade 1998). In other words, female aggressive attempts in N. livida appear to be always successful regardless of her or male size. The extreme sexual size dimorphism in N. livida (as Nephilengys borbonica in Kuntner and Coddington 2009) also speaks against the adaptive foraging explanation for sexual cannibalism because small males are a poorly nutritious meal compared with their usual prey (e.g., Barry et al. 2008; Wilder and Rypstra 2010).
To conclude, our study shows that female general aggressiveness, measures of body size, and rates of sexual cannibalism are independent and thus provide no support for the aggressive spillover hypothesis in N. livida. We showed that the mate choice hypothesis with a mechanism of direct choice best explained N. livida sexual cannibalism, where females preferentially cannibalize non-aggressive (and shy) males.
References
Andrade MCB (1996) Sexual selection for male sacrifice in the Australian redback spider. Science 271:70–72
Andrade MCB (1998) Female hunger can explain variation in cannibalistic behavior despite male sacrifice in redback spiders. Behav Ecol 9:33–42
Arnqvist G, Henriksson S (1997) Sexual cannibalism in the fishing spider and a model for the evolution of sexual cannibalism based on genetic constraints. Evol Ecol 11:255–273
Barry KL, Holwell GI, Herberstein ME (2008) Female praying mantids use sexual cannibalism as a foraging strategy to increase fecundity. Behav Ecol 19:710–715
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783
Birkhead TR, Lee KE, Young P (1988) Sexual cannibalism in the praying mantis Hierodula membranacea. Behaviour 106:112–118
R Development Core Team (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://www.R-project.org Accessed 15 May 2011
Elgar MA (1998) Sperm competition and sexual selection in spiders and other arachnids. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic, San Diego, pp 307–339
Elgar MA (1992) Sexual cannibalism in spiders and other invertebrates. In: Elgar MA, Crespi BJ (eds) Cannibalism: ecology and evolution amongst diverse taxa, 1st edn. Oxford University Press, Oxford, pp 128–155
Elgar MA, Nash DR (1988) Sexual cannibalism in the garden spider Araneus diadematus. Anim Behav 36:1511–1517
Elgar MA, Schneider JM (2004) Evolutionary significance of sexual cannibalism. Adv Stud Behav 34:135–163
Elgar MA, Schneider JM, Herberstein ME (2000) Female control of paternity in the sexually cannibalistic spider Argiope keyserlingi. P Roy Soc Lond B Biol Sci 267:2439–2443
Falconer DS (1996) Introduction to quantitative genetics, 3rd edn. Longman, New York
Fromhage L, Schneider JM (2005) Virgin doves and mated hawks: contest behaviour in a spider. Anim Behav 70:1099–1104
Fromhage L, Schneider JM (2006) Emasculation to plug up females: the significance of pedipalp damage in Nephila fenestrata. Behav Ecol 17:353–357
Gould SJ (1984) Only his wings remained. Natural History 93(9):10–18
Hardin JW, Hilbe JM (2003) Generalized estimating equations. Chapman & Hall/CRC, Boca
Hedrick AV, Riechert SE (1989) Genetically-based variation between 2 spider populations in foraging behavior. Oecologia 80:533–539
Herberstein ME, Schneider JM, Harmer AMT, Gaskett AC, Robinson K, Shaddick K, Soetkamp D, Wilson PD, Pekar S, Elgar MA (2011) Sperm storage and copulation duration in a sexually cannibalistic spider. J Ethol 29:9–15
Johnson JC (2001) Sexual cannibalism in fishing spiders (Dolomedes triton): an evaluation of two explanations for female aggression towards potential mates. Anim Behav 61:905–914
Johnson JC, Sih A (2005) Precopulatory sexual cannibalism in fishing spiders (Dolomedes triton): a role for behavioral syndromes. Behav Ecol Sociobiol 58:390–396
Johnson JC, Sih A (2007) Fear, food, sex and parental care: a syndrome of boldness in the fishing spider, Dolomedes triton. Anim Behav 74:1131–1138
Kralj-Fišer S, Gregorič M, Zhang SC, Li DQ, Kuntner M (2011) Eunuchs are better fighters. Anim Behav 81:933–939
Kuntner M (2007) A monograph of Nephilengys, the pantropical 'hermit spiders' (Araneae, Nephilidae, Nephilinae). Syst Entomol 32:95–135
Kuntner M, Coddington JA (2009) Discovery of the largest orbweaving spider species: the evolution of gigantism in Nephila. PLoS One 4(10):e7516
Kuntner M, Agnarsson I, Gregorič M (2009a) Nephilid spider eunuch phenomenon induced by female or rival male aggressiveness. J Arachnol 37:266–271
Kuntner M, Kralj-Fišer S, Schneider JM, Li D (2009b) Mate plugging via genital mutilation in nephilid spiders: an evolutionary hypothesis. J Zool 277:257–266
Moya-Larano J, Orta-Ocana JM, Barrientos JA, Bach C, Wise DH (2003) Intriguing compensation by adult female spiders for food limitation experienced as juveniles. Oikos 101:539–548
Nessler SH, Uhl G, Schneider JM (2007) Genital damage in the orb-web spider Argiope bruennichi (Araneae: Araneidae) increases paternity success. Behav Ecol 18:174–181
Newman JA, Elgar MA (1991) Sexual cannibalism in orb-weaving spiders-an economic-model. Am Nat 138:1372–1395
Persons MH, Uetz GW (2005) Sexual cannibalism and mate choice decisions in wolf spiders: influence of male size and secondary sexual characters. Anim Behav 69:83–94
Prenter J, MacNeil C, Elwood RW (2006) Sexual cannibalism and mate choice. Anim Behav 71:481–490
Prokop P, Vaclav R (2005) Males respond to the risk of sperm competition in the sexually cannibalistic praying mantis, Mantis religiosa. Ethology 111:836–848
Pruitt JN, Riechert SE, Jones TC (2008) Behavioural syndromes and their fitness consequences in a socially polymorphic spider, Anelosimus studiosus. Anim Behav 76:871–879
Riechert SE, Hedrick AV (1993) A test for correlations among fitness-linked behavioral traits in the spider Agelenopsis aperta (Araneae, Agelenidae). Anim Behav 46:669–675
Riechert SE, Johns PM (2003) Do female spiders select heavier males for the genes for behavioral aggressiveness they offer their offspring? Evolution 57:1367–1373
Riechert SE, Tracy CR (1975) Thermal balance and prey availability–bases for a model relating web-site characteristics to spider reproductive success. Ecology 56:265–284
Roggenbuck H, Pekar S, Schneider JM (2011) Sexual cannibalism in the European garden spider Araneus diadematus: the role of female hunger and mate size dimorphism. Anim Behav 81:749–755
Schneider JM, Gilberg S, Fromhage L, Uhl G (2006) Sexual conflict over copulation duration in a cannibalistic spider. Anim Behav 71:781–788
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Snyder WE, Joseph SB, Preziosi RF, Moore AJ (2000) Nutritional benefits of cannibalism for the lady beetle Harmonia axyridis (Coleoptera: Coccinellidae) when prey quality is poor. Environ Entomol 29:1173–1179
Uhl G, Vollrath F (1998) Little evidence for size-selective sexual cannibalism in two species of Nephila (Araneae). Zool-Anal Complex Sy 101:101–106
Uhl G, Nessler S, Schneider J (2009) Securing paternity in spiders? A review on occurrence and effects of mating plugs and male genital mutilation. Genetica 138:75–104
Wilder SM, Rypstra AL (2008) Sexual size dimorphism mediates the occurrence of state-dependent sexual cannibalism in a wolf spider. Anim Behav 76:447–454
Wilder SM, Rypstra AL (2010) Males make poor meals: a comparison of nutrient extraction during sexual cannibalism and predation. Oecologia 162:617–625
Wilder SM, Rypstra AL, Elgar MA (2009) The importance of ecological and phylogenetic conditions for the occurrence and frequency of sexual cannibalism. Annu Rev Ecol Evol Syst 40:21–39
Yan J, Fine JP (2004) Estimating equations for association structures. Stat Med 23:859–880
Acknowledgments
We thank two anonymous reviewers for their helpful comments. We thank Ingi Agnarsson, Sahondra Lalao Rahanitriniaina, and Honore Rabarison for their help in the field. This work was funded by the Slovenian Research Agency (grant J1-2063 to MK) and the National Geographic Society (grant 8655-09 to I. Agnarsson, M. Kuntner, and T. Blackledge). SKF was supported by a Humboldt fellowship for postdoctoral researchers and a Humboldtian Return Fellowship. SP was supported by grant no. 0021622416 from the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Hauber
Rights and permissions
About this article
Cite this article
Kralj-Fišer, S., Schneider, J.M., Justinek, Ž. et al. Mate quality, not aggressive spillover, explains sexual cannibalism in a size-dimorphic spider. Behav Ecol Sociobiol 66, 145–151 (2012). https://doi.org/10.1007/s00265-011-1262-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1262-7