Abstract
Socio-demographic factors, such as group size and their effect on predation vulnerability, have, in addition to intrinsic factors, dominated as explanations when attempting to understand animal vigilance behaviour. It is generally assumed that animals evaluate these external factors visually; however, many socially foraging species adopt a foraging technique that directly compromises the visual system. In these instances, such species may instead rely more on the acoustical medium to assess their relative risk and guide their subsequent anti-predator behaviour. We addressed this question in the socially foraging meerkat (Suricata suricatta). Meerkats forage with their head down, but at the same time frequently produce close calls (‘Foraging’ close calls). Close calls are also produced just after an individual has briefly scanned the surrounding environment for predators (‘Guarding’ close calls). Here, we firstly show that these Guarding and Foraging close call variants are in fact acoustically distinct and secondly subjects are less vigilant (in terms of frequency and time) when exposed to Guarding close call playbacks than when they hear Foraging close calls. We argue that this is the first evidence for socially foraging animals using the information encoded within calls, the main adaptive function of which is unrelated to immediate predator encounters, to coordinate their vigilance behaviour. In addition, these results provide new insights into the potential cognitive mechanisms underlying anti-predator behaviour and suggest meerkats may be capable of signalling to group members the ‘absence’ of predatory threat. If we are to fully understand the complexities underlying the coordination of animal anti-predator behaviour, we encourage future studies to take these additional auditory and cognitive dimensions into account.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding when and why socially foraging animals invest in anti-predator behaviour has been a major focus of evolutionary biology research over the last 30 years. This wave of interest was initially prompted by the suggestion that whilst being vigilant improves chances of detecting predators, it also brings with it a cost to foraging success (Pulliam 1973). Animals might therefore be expected to vary their vigilance behaviour with their relative probability of risk in order to reduce the costs associated with this trade-off. Follow-up studies have since shown that a number of key variables do indeed influence animal vigilance behaviours, such as group size (Pulliam 1973; Carter et al. 2009), predation pressure (Hunter and Skinner 1998), spacing within groups (Jennings and Evans 1980; Blumstein et al. 2001), proximity to other group individuals (Krause and Ruxton 2002; Radford and Ridley 2007) or even the behaviour of surrounding conspecifics (Fernandez-Juricic et al. 2004).
Such factors important in guiding vigilance behaviours are generally assumed to be assessed visually by individuals (Fernandez-Juricic et al. 2004; Radford and Ridley 2007). However, for species whose vision is compromised during foraging, such ‘assessment’ would either be inaccurate or conflict directly with foraging success (Fernandez-Juricic et al. 2004). In these instances, it is plausible that individuals may instead exploit the acoustical medium, using the occurrence of conspecific’s vocalisations to guide individual vigilance levels and its coordination with other group members (Sullivan 1984; Uster and Zuberbuhler 2001). Most research exploring the role of vocalisations on vigilance coordination has focused on species partaking in sentinel duty: where individuals perch themselves above the rest of the group, scan for predators and signal this continuously by emitting quiet ‘surveillance’ vocalisations [meerkats (Suricata suricatta)—Manser 1999; dwarf mongoose (Helogale parvula)—Rasa 1986; babblers—Wickler 1985; Florida scrub jays (Aphelocoma coerulescens)—Bednekoff et al. 2008]. Combined observational and experimental evidence has shown that foraging individuals also attend to these vocalisations and the potential information encoded within them, reducing their own anti-predator behaviours accordingly (Manser 1999; Hollen et al. 2008; Bell et al. 2009). However, sentinel guarding systems in animal societies are generally rare (Clutton-Brock et al. 1999) and those that do exhibit them often spend the majority of time foraging in the absence of a sentinel (Clutton-Brock et al. 1999; Hollen et al. 2008). This therefore begs the question: how visually compromised species coordinate vigilance in the absence of a designated guard?
To date, only a single study has systematically attempted to understand what role additional vocalisations play in governing animal vigilance behaviour. Radford and Ridley (2007) showed that pied babblers (Turdoides bicolor) can use close calls, quiet calls produced during foraging, as a proxy measure of how many individuals are present in their group and their relative location. Such demographic features are known to affect animal vigilance levels and playback experiments of close calls at different frequencies and positions, simulating the presence of more individuals, in different constellations, induced a change in vigilance behaviour (Radford and Ridley 2007). From these results, the authors suggest that close calls may therefore be useful in helping babblers assess their relative risk, information which can then be used to efficiently coordinate their vigilance behaviour at times when they cannot rely on their visual medium.
Whilst it is clear how processing close call production could be advantageous in coordinating vigilance behaviour, the system remains relatively rudimentary as it only indicates the likelihood of shared vigilance through presence of conspecifics and provides no direct information regarding other’s vigilance behaviour. Given the unpredictability posed by predators (Lima and Bednekoff 1999; Bell et al. 2009), it would be more beneficial if susceptible foraging individuals could directly keep up-to-date with surrounding vigilance behaviour performed by the other group members, without having to forego time invested into foraging (Fernandez-Juricic et al. 2004).
We addressed this question in meerkats, socially foraging mongooses that live in the Kalahari Desert, South Africa. Meerkats employ a foraging technique that makes them very susceptible to predation, where they search for food by digging in the sand, keeping their head down and hence compromising their visual system. As a consequence of this, meerkats have evolved a sophisticated vocal communication system, with a repertoire of over 30 different call types (Manser 1998) and an integrated referential and urgency-based alarm call system (Manser 2001; Manser et al. 2001).
Similarly to other cohesively foraging mammals (Palombit et al. 1999) and bird species (Radford 2004), meerkats also exhibit close calls; quiet, medium frequency vocalisations that probably play a more general role in maintaining group cohesion (Manser 1998). Whilst the majority of close calls are produced during social foraging (Manser 1998), we noticed that meerkats also produce single close calls just as they are terminating guarding (GA) and returning to social foraging. In this context, guarding behaviour is defined as when an individual briefly interrupts foraging, stands on its hind legs, scans the surrounding environment for predators for typically only a few seconds (although this can sometimes extend to a few minutes) and then returns to normal foraging behaviour. Meerkat guarding behaviour differs from sentinel behaviour, as during sentinel duty individuals interrupt foraging completely, adopting raised positions on, for example, shrubs, dead trees or large mounds, in order to scan for predators for extended periods of time (Clutton-Brock et al. 1999). Moreover, meerkats continuously signal this behaviour with contextually specific ‘sentinel calls’ which are very different in their acoustic structure in comparison with Guarding close calls (Manser 1999; Townsend and Manser, unpublished data, see Supplementary material). Typically, if a meerkat detects a predator when on guard or indeed sentinel, it will alert the rest of the group with an alarm call appropriate to both the predator type and urgency level (Manser et al. 2001), and receivers respond as if they have seen that specific predator class, at that specific distance, themselves. With respect to the Guarding close call, however, an individual has returned to foraging after being on guard and no predator has been identified; hence, it is possible that these calls transfer contextual information concerning this.
For a vocalisation to encode ‘information’ regarding a given context or event, it must vary consistently in its acoustic properties between such contexts (Hauser 1996). This is a common occurrence in the animal kingdom, with a range of taxa, from primates to birds, showing context-specific vocalisations (Seyfarth et al. 1980; Evans et al. 1993; Zuberbuhler 2000; Bugnyar et al. 2001; Slocombe et al. 2009). We therefore firstly investigated whether Guarding close calls differ consistently in their discrete acoustic structure when compared with the more common very similar Foraging close calls. However, just because acoustic variation exists does not mean that it is used or is meaningful to receivers at any level (Schibler and Manser 2007; Townsend et al. 2010). In light of this, using a playback experiment, we additionally tested whether receivers subsequently attend to any acoustic differences that exist between the close calls given in two different contexts and modify their anti-predator behaviour accordingly. Specifically, we predicted, that if Guarding close calls provide an acoustic indicator to conspecifics of recent vigilance behaviour, we should see a reduction in overall alertness-related behaviours when exposed to such calls. To our knowledge, this would provide the first evidence for a vocalisation used in a predominantly social foraging context also conferring direct information regarding the vigilance behaviour of others; information which receivers may subsequently use to coordinate their own anti-predator behaviour.
Methods
Study population
Audio recordings and playback experiments were conducted on a wild but habituated population of meerkats at the Kalahari Meerkat Project, located in the Kuruman River Reserve (KRR), 30 km east of Van Zylsrus (Clutton-Brock et al. 1998), between August and December 2009. As part of the Kalahari Meerkat Project’s long-term data collection protocol, all animals were tagged with subcutaneous transponders (Clutton-Brock et al. 1998) and marked with dye or hair cuts to facilitate individual identification. All meerkats were sufficiently habituated, allowing recordings to be conducted with 0.5 m and experiments within 1–2 m.
Recording methods
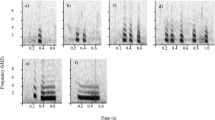
All close calls (see Fig. 1) used in the acoustic analysis and playback experiments were recorded from dominant female meerkats [N = 6, mean call number/individual (±SE) = 13.5 ± 0.42; range = 12−15] at a distance of approximately 1–2 m, using a Sennheiser directional microphone (ME66/K6 and a MZW66 pro windscreen, frequency response 40–20,000 Hz ± 2.5 dB; Sennheiser, Old Lyme, CT, USA) connected to a Marantz PMD-670 solid state recorder (Marantz Japan Inc.). We specifically focused on dominant females as stimuli because we wanted to ensure, for congruency’s sake, that individuals we were playing back were also present in the group at the time. Given that males and subordinate females can periodically leave the group for extended periods to search for mating opportunities or due to social conflict (Clutton-Brock et al. 1999), we considered dominant females as the most reliable option. Furthermore, our primary aim was to test the comprehension of information conveyed in close calls, the most common meerkat vocalisation, and there is no reason to believe that this ability would be confounded by dominance status of the stimulus or playback subject (Cheney and Seyfarth 1990). Calls were transferred digitally onto a PC desktop using Cool Edit Pro 2000 (Syntrillium Software Corporation, Phoenix, AZ, USA; sampling frequency 44.1 kHz, 16 bits accuracy). Only Foraging and Guarding close calls with high signal-to-noise ratio were selected for the acoustic analyses.
Acoustic analysis
To determine if close calls produced during foraging and after being on guard differed in their acoustic structure, we analysed 81 close calls (N Foraging = 41, N Guarding = 40) from six dominant female meerkats belonging to six different meerkat groups. Quantitative call analysis was carried out using PRAAT v.5.1 (www.Praat.org) with the following settings: pitch settings—range: 300–1,000 Hz, view range: 0.00–1,000 Hz; spectrogram window settings—window length: 0.03 s, dynamic range: 70 dB. Nine acoustic parameters were selected that best described the acoustic ‘shape’ of close calls: call duration (s), number of pulses per call, mean fundamental frequency (Hz), maximum frequency (highest frequency in the fundamental band, (Hz)), peak frequency of the call at call beginning, call middle and call end [frequencies at which maximum acoustic energy exists (Hz)], transition onset [frequency of maximum energy in the F0 at call onset minus frequency of maximum energy in the F0 at call middle (Hz)] and transition offset [frequency of maximum energy in the F0 at call middle minus frequency of maximum energy in the F0 at call offset (Hz)]. Fundamental frequency measurements were derived using a custom-built pitch extraction algorithm (Micheal Owren, personal communication). To ensure correct pitch tracking, we compared the time varying numerical representation of the F0 contour with the F0 contour from the spectrograms (Charlton et al. 2010). Measurements of the frequencies at which maximum acoustic energy was present were obtained from creating spectral slices (amplitude plotted against frequency). Collinearity analyses showed that none of the nine acoustic variables suffered from high variance inflation factors and hence could be compared together simultaneously in the same statistical analysis without risking similarity in explained variation (VIFs, >7.0; Allison 1999).
Playback experiments
We investigated the response of 18 subordinate meerkats (>12 months) to a 1-min bout of Guarding close calls (test condition) and Foraging close calls (control condition). To accurately simulate how Guarding close calls occur naturally and to avoid any possible construction biases, for the test condition we randomly embedded five to six Guarding close calls, a rate within the naturally occurring range (range 0–7 calls/min; Townsend and Manser, unpublished data), inside a bout of six Foraging close calls. For the control condition, purely a succession of 12 Foraging close calls was played. All call sequences were from the dominant female belonging to the same group as the playback subjects. For each group, we constructed one playback sequence for both conditions, but randomised the order of calls for each individual tested to avoid habituation effects. Playback sound files were edited with Cool Edit 2000 (Syntrillium Software Corporation). Sound files consisted of uncompressed, high signal-to-noise ratio close calls of dominant adult females that contributed to permutated discriminant function analysis. The rate (mean = 0.2 calls/s) and amplitude [12 dB, measured at 0.3 m in front of the speaker (Voltcraft 329 Sound Level Meter; Conrad Electronic, Hirschau, Germany; accuracy ±2 dB at 94 dB)] of the calls was kept as naturally observed in the different groups (1 call/5 s, range = 0–4 calls/5 s; Manser 1998; Townsend and Manser, unpublished data) simulating the dominant female foraging close by and in the test condition, periodically scanning the sky for predators on her hind legs. While the subject was foraging, a loud speaker (JBL) was attached to the experimenter’s leg at a height equivalent to that of another foraging meerkat. Keeping track of the position of the dominant female (to ensure spatial congruency), we then played back a 1-min bout of Foraging close calls and Guarding–Foraging close calls, from an iPod touch (www.apple.com) at a distance of 2–3 m. A 1-min test period was specifically chosen because playbacks were designed to follow each other (i.e. 2 min total), and we found this duration to be optimal to avoid potential experimental disturbances such as predator alarm calls or the dominant female coming too close into the vicinity of the focal subject. In particular, we ensured the dominant female was out of vocal range of the subject, at a minimum distance of 4 m. In the instances when the dominant female came closer, we paused the playback to avoid presenting subjects with an incongruent social scenario. To control for order effects, we randomised the order that subjects heard test and control conditions, and to reduce habituation to playbacks, we left a break of 5–7 days between playbacks within the same group (range of playbacks per group = 2–4).
Behavioural responses
We analysed videos using The Observer XT 7.0 (Noldus), focusing primarily on the employment of vigilance behaviour during the 1-min playback (experiment duration range = 58–67 s; mean = 60 s). We scored each time we observed the subject to (a) scan the sky or surrounding area for predators whilst remaining stationary and (b) scan the sky or surrounding area for predators whilst raised on its hind legs [standing guard (GA)]. We combined both vigilance categories together to gain an estimate of total vigilance frequency and converted this value into a proportion [frequency/experiment duration (s)]. Similarly, we recorded the duration of each vigilance (stationary vigilance and standing guard) bout scored, combined them together to obtain a total time invested into vigilance behaviours and again converted this value to a proportion [duration/experiment duration (s)].
Finally, to identify the influence of playback type on guarding occurrence, for the instances when subjects exhibited guarding behaviour, we further categorised the data. If a subject employed quantitatively more guarding behaviour during the control than the test playback, it was allocated a 1. If the subject employed more GA behaviour during the test playback than the control, it was allocated a −1, and if there was an equal response, a zero was given. Because the second scenario (more GA behaviour in the test playback) never occurred, we were essentially left with a binary distinction of 1 and 0. To ensure accurate videotape coding, a second observer blind-coded 33% of trials (12 trials). Inter-observer reliability tests showed a high level of agreement for vigilance frequency (Spearman’s rank correlation, R = 0.848, P < 0.01) and vigilance duration (Spearman’s rank correlation, R = 0.962, P < 0.01).
Statistical analyses
To determine if close calls differed in their acoustic structure between behavioural contexts, we first ran linear mixed-effects models (LMMs) on the acoustic parameters measured. In these analyses, we controlled for repeated sampling from the same individual by fitting ‘individual’ as a random factor (Crawley 2002) and avoided potential type I errors by correcting acoustic parameters reaching significance with sequential Bonferroni tests (Rice 1989). To further verify, overall, if calls could be classified by their acoustic structure, we entered the acoustic parameters into a discriminant function analysis (DFA; see Townsend et al. 2010 and Townsend and Manser 2011 for more details). For external validation, we used a leave-one-out cross-validation procedure. Since the data for group signatures were two factorial (context; individual) and contained more than one call exemplar per individual, it has been argued that conventional DFA provides grossly inflated levels of overall significance of discriminability (Mundry and Sommer 2007). To control for this statistical conflict and estimate the significance of the number of correctly cross-validated calls, we subsequently used a crossed permutated DFA (pDFA) (Mundry, personal communication).
To determine if there were differences in vigilance behaviour (frequency and duration) between playback conditions and to control for replications of individuals from the same group, we used LMMs with Group fitted as a random factor. Because the decision to invest in standing guard behaviour was essentially binary, to analyse what role playback type had on this response variable we alternatively used a Monte Carlo generalised linear mixed effects model MCGLMM with Group fitted as a random factor. We first constructed the full model with the explanatory factor (playback type) and tested the overall significance of the full model against a null model which included only the intercept and the random factor, using a likelihood ratio test. Since likelihood ratio tests against a chi-squared distribution can lead to an overestimating of effect size (Faraway 2006), we used parametric bootstrapping with 1,000 Monte Carlo simulations to generate a distribution of likelihood ratios (LR) from the fitted parameter estimates and tested the observed LR against this distribution (Faraway 2006; F. Korner, personal communication). All tests were conducted in SPSS version 16.0 and R version 2.8.1 and were two tailed with alpha values set at 0.05.
Results
Close calls vary acoustically with behavioural context
Linear mixed effects models (with Individual fitted as a random factor) showed that both temporal and spectral acoustic parameters varied significantly between the two contexts (see Table 1): duration (LMM F (1,6) = 57.8, P = 0.001), number of pulses (F (1,6) = 50.1, P = 0.001) and peak frequency at call beginning (F (1,6) = 39.3, P = 0.001). A cross-classified permutated discriminant function analysis (pDFA) showed that, overall, close calls could be correctly classified to the appropriate context based on their acoustic structure (number of correctly cross-classified elements = 16.54/1,000, P = 0.025).
Playback experiments
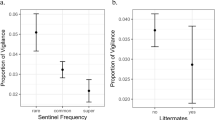
The type of close calls in playbacks had a significant effect on meerkat vigilance behaviour. Meerkats were generally less frequently vigilant and vigilant for shorter periods when exposed to Guarding close calls (test) than to the Foraging close call (control) playback condition [vigilance frequency/s (mean ± SD)—Guarding = 0.031 ± 0.020, Foraging = 0.067 ± 0.034, LMM (with Group fitted as a random factor) F (1,18) = 77.9, P < 0.001, see Fig. 2; proportion of time spent vigilant (s)—Guarding = 0.032 ± 0.049, Foraging = 0.082 ± 0.065, LMM, F (1,18) = 7.08, P = 0.043, see Fig. 2].
Out of the 18 individuals, overall six employed standing guard (GA) behaviour during the playbacks. From these six instances, we found that standing guard was less frequent during the test Guarding condition (mean GA/min ± SD = 0.114 ± 0.3) than the control Foraging condition (0.378 ± 0.54, LR = 12.1, df = 1, P = 0.0019).
Discussion
Given the inherent foraging costs associated with anti-predator behaviour, it is crucial that animals living in risky environments make efficient decisions regarding how much time to invest in vigilance behaviour (Lima and Bednekoff 1999; Valone 2007; Hollen et al. 2008; Bell et al. 2009). For animals whose foraging technique compromises their ability to assess relative risk through visual measures known to be important in predation probability, such as group size or spatial position, this trade-off becomes even more skewed. In these species, it is highly likely that alternative sensory mediums may be exploited in order to gain information regarding their relative predation risk and use this ‘information’ to subsequently coordinate vigilance behaviours (Radford and Ridley 2007).
Our results here directly corroborate and elaborate on this assumption. Firstly, from our acoustic analyses, we show that close calls produced after briefly being on guard differ in their fine acoustic structure, when compared to control close calls given while foraging. Secondly, we demonstrate that foraging meerkats also attend to this contextual information encoded within the acoustic structure of close calls. When exposed to the test Guarding close calls, individuals were generally less vigilant in terms of both frequency and duration than when being played Foraging close calls at the same calling rate.
Interestingly, only six of the 18 individuals we tested invested in actual guarding behaviour during the playbacks, which might suggest that these vocalisations are not important in helping the group to coordinate this specific vigilance system. However, when looking in more detail at these six instances, subjects were significantly more likely to employ guarding behaviour when exposed to the control foraging condition then when Guarding close calls were played back. It therefore appears that the decision of meerkats to go on guard is, at some level, mediated by information encoded in Guarding close calls. A potential reason for the infrequent occurrence of this more extreme vigilance behaviour is that, due to methodological constraints (see ‘Methods’), playback experiments were conducted for just 1 min, which may not have been long enough to cover the probability of an individual employing guarding behaviour (which occurs 10% of total foraging time/individual, under natural conditions; Clutton-Brock et al. 1999). Future experiments with varying lengths of playbacks and intensities of Guarding close calls will help to clarify this issue.
Previous studies investigating acoustic coordination of anti-predator behaviour have generally focused more on how the pure exposure to vocalisations, their production rate or the information content of sentinel-based calls affect general vigilance levels (Sullivan 1984; Manser 1999; Radford and Ridley 2007; Hollen et al. 2008). For example, meerkats adjust their vigilance or probability to go on guard depending on whether sentinel calls are emitted or not (Manser 1999). Pied babblers also modify their contribution to anti-predator behaviour based on the production rate of ‘sentinel calls’ or indeed foraging ‘close calls’ (Radford and Ridley 2007; Bell et al. 2010). Furthermore, recent work has suggested that the information encoded within pied babbler ‘surveillance calls’ can be used by receivers as a possible indication of predator risk (Bell et al. 2009). However, our work shows for the first time that the actual information content of calls that are generally unrelated to immediate predator risk or detection (unlike sentinel or alarm calls) appears to be processed by foraging individuals and guides subsequent vigilance decisions. These results are particularly interesting from a cognitive standpoint as it inevitably raises the question regarding what level of abstraction is occurring when meerkats hear such subtle close call variants. The most parsimonious explanation would be that receivers have acquired the contingent relationship between the differing acoustic structure of the close call types and the consistent behavioural context in which they are produced (Hauser 1996; Seyfarth et al. 2010). Simply, meerkats have learned that Guarding close calls are produced after an individual has been actively vigilant and returned to social foraging. Because meerkats are very adept at discovering predators over considerable distances (up to 2–3 km; see Manser 1998), it is unlikely that after hearing a Guarding close call another predator would be able to get within a proximity that poses a significant risk to foragers. Therefore, such calls may work as a signal of safety, and reassure receivers, allowing them to be less vigilant themselves. It has been previously suggested that socially foraging bird species may use peripheral vision to assess the anti-predator behaviour of others, reducing the need to interrupt foraging (Fernandez-Juricic et al. 2004). Our results suggest that animals may also be capable of assessing conspecific’s vigilance behaviour acoustically, complementing visual evaluation or even bypassing it altogether. Such direct information regarding the recent vigilance behaviour of a group member is likely to be far more useful in guiding an individual’s vigilance and potentially coordinating it with other group members than just relying on indirect measures of predation risk through, for example, shared vigilance (Radford and Ridley 2007).
In addition, the consistent variation in close calls (Guarding and Foraging) could potentially inform receivers about the current state of their external world, a topic that has received considerable debate, particularly in the field of animal communication (Seyfarth et al. 1980; Gouzoules et al. 1984; Macedonia and Evans 1993; Bugnyar et al. 2001). Typically, if an individual invests in guarding behaviour and spots a predator, it will warn the rest of the group with an alarm call—information that receivers can then use to execute the correct behavioural response (Manser 2001; Manser et al. 2001). However, when a Guarding close call is emitted, no predator has been detected in the surrounding environment. It could therefore be that meerkats have made an extra processing step whereby not only do receivers know someone has been on guard but such close calls have also reduced the receiver’s uncertainty (see Shannon 1948; Weiner 1961; Seyfarth et al. 2010) with respect to predation threat in its external environment. In many instances where visually compromised animals are subjected to heavy predation risk, predator-specific alarm call systems are often exhibited (Zuberbuhler 2000; Manser 2001; Schel et al. 2010). However, here we suggest that meerkats may also be able to use an additional call variant, the main adaptive function of which is probably unrelated to immediate predator encounters, to gain up-to-date information regarding the likelihood of attack.
Whether or not meerkats have a nominalised representation of their external world (Gallistel 1990; Evans and Evans 2007) or indeed a ‘concept’ of the absence of a predator is something our results do not, as yet, allow us to address, though further ‘manipulation of experience’ (Evans and Evans 2007) experiments will help us begin to pull apart the alternative explanations regarding what Guarding close calls ‘mean’ to receivers.
Our findings show that meerkat close calls encode contextual information regarding the anti-predator behaviour of signallers and therefore potentially also the risk of immediate attack. Playback experiments indicate receivers attend to this information, reducing their own vigilance behaviour. For animals whose visual medium is compromised by their foraging technique, such acoustic information may be crucial if individuals are to efficiently balance the trade-off between foraging and investment in anti-predator behaviour. In species that show labour division, such as in cooperative societies where individuals participate in group-wide ‘helping’ behaviours (Ridley and Raihani 2008), the need for vocal coordination may be even more exaggerated, aiding efficient transitions between behavioural states (Snowdon and Elowson 2001; Burkart and Van Schaik 2010). We hope our results will encourage future work focusing on coordination of individual and group behaviours to also take into account the acoustic medium and particularly the potential wealth of information hidden within it.
References
Allison PD (1999) Multiple regression: a primer. Pine Forge, Thousand Oaks
Bednekoff PA, Bowman R, Woolfenden GE (2008) Do conversational gutturals help Florida scrub-jays coordinate their sentinel behavior? Ethology 114:313–317
Bell MBV, Radford AN, Rose R, Wade H, Ridley AR (2009) The value of constant surveillance in a risky environment. Proc R Soc B 276:2997–3005
Bell MBV, Radford AN, Smith RA, Thompson AM, Ridley AR (2010) Bargaining babblers: vocal negotiation of cooperative behaviour in a social bird. Proc R Soc B 277:3223–3228
Blumstein DT, Daniel JC, McLean IG (2001) Group size effects in quokkas. Aust J Zool 49:641–649
Bugnyar T, Kijne M, Kotrschal K (2001) Food calling in ravens: are yells referential signals? Anim Behav 61:949–958
Burkart JM, van Schaik CP (2010) Cognitive consequences of cooperative breeding in primates? Anim Cogn 13(1):1–19
Carter AJ, Pays O, Goldizen AW (2009) Individual variation in the relationship between vigilance and group size in eastern grey kangaroos. Behav Ecol Sociobiol 64:237–245
Charlton BD, Zhang Z, Snyder RJ (2010) Giant pandas perceive and attend to formant frequency variation in male bleats. Anim Behav 79:1221–1227
Cheney DL, Seyfarth RM (1990) How monkeys see the world. University of Chicago Press, Chicago
Clutton-Brock TH, Gaynor D, Kansky R, MacColl ADC, McIlrath G, Chadwick P, Brotherton PNM, O’Riain JM, Manser M, Skinner JD (1998) Costs of cooperative behaviour in suricates (Suricata suricatta). Proc R Soc B 265:185–190
Clutton-Brock TH, O’Riain MJ, Brotherton PNM, Gaynor D, Kansky R, Griffin AS, Manser M (1999) Selfish sentinels in cooperative mammals. Science 284:1640–1644
Crawley MJ (2002) Statistical computing: an introduction to data analysis using S-Plus. Wiley, Chichester
Evans CS, Evans L (2007) Representational signaling in birds. Biol Lett 2007(3):8–11
Evans CS, Evans L, Marler P (1993) On the meaning of alarm calls: functional reference in an avian vocal system. Anim Behav 46:23–38
Faraway JJ (2006) Linear models with R. Chapman & Hall/CRC, Boca Raton
Fernandez-Juricic E, Erichsen JT, Kacelnik A (2004) Visual perception and social foraging in birds. Trends Ecol Evol 19:25–31
Gallistel CR (1990) The organization of learning. MIT Press, Cambridge
Gouzoules S, Gouzoules H, Marler P (1984) Rhesus monkey (Macaca mulatta) screams: representational signalling in the recruitment of agonistic aid. Anim Behav 32:182–193
Hauser MD (1996) The evolution of communication. MIT Press, Cambridge
Hollen LI, Bell MBV, Radford AN (2008) Cooperative sentinel calling? Foragers gain increased biomass intake. Curr Biol 18:576–579
Hunter TB, Skinner JD (1998) Vigilance behavior in African ungulates: the role of predation pressure. Behaviour 135:195–211
Jennings T, Evans SM (1980) Influence of position in the flock and flock size on vigilance in the starling, Sturnus vulgaris. Anim Behav 28:634–635
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Lima SL, Bednekoff PA (1999) Back to basics of antipredatory vigilance: can nonvigilant animals detect attack? Anim Behav 58:537–543
Macedonia JM, Evans CS (1993) Variation among mammalian alarm call systems and the problem of meaning in animal signals. Ethology 93:177–197
Manser MB (1998) The evolution of auditory communication in suricates (Suricata suricatta). Ph.D. thesis, University of Cambridge
Manser MB (1999) Response of foraging group members to sentinel calls in suricates, Suricata suricatta. Proc R Soc B 266:1013–1019
Manser MB (2001) The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proc R Soc B 268:2315–2324
Manser MB, Bell MB, Fletcher LB (2001) The information that receivers extract from alarm calls in suricates. Proc R Soc B 268:2485–2491
Mundry R, Sommer C (2007) Discriminant function analysis with nonindependent data: consequences and an alternative. Anim Behav 74:965–976
Palombit RA, Cheney DL, Seyfarth RM (1999) Male grunts as mediators of social interaction with females in wild chacma baboons (Papio cynocephalus ursinus). Behaviour 136:221–242
Pulliam HR (1973) On the advantages of flocking. J Theor Biol 38:419–422
Radford AN (2004) Vocal mediation of foraging competition in the cooperatively breeding green woodhoopoe, Phoeniculus purpureus. Behav Ecol Sociobiol 56:279–285
Radford AN, Ridley AR (2007) Individuals in foraging groups may use vocal cues when assessing their need for antipredator vigilance. Biol Lett 3:249–252
Rasa OAE (1986) Coordinated vigilance in dwarf mongoose family groups: the ‘watchman song’ hypothesis and the costs of guarding. Ethology 71:340–344
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Ridley AR, Raihani NJ (2008) Task partitioning increases reproductive output in cooperative bird. Behav Ecol 19:1136–1142
Schel AM, Candiotti A, Zuberbühler K (2010) Predator-deterring alarm call sequences in Guereza colobus monkeys are meaningful to conspecifics. Anim Behav 80:799–808
Schibler F, Manser MB (2007) The irrelevance of individual discrimination in meerkat alarm calls. Anim Behav 74:1259–1268
Seyfarth RM, Cheney DL, Marler P (1980) Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim Behav 28:1070–1094
Seyfarth RM, Cheney DL, Bergman T, Fischer J, Zuberbuhler K, Hammerschmidt K (2010) The central importance of information in studies of animal communication. Anim Behav 80(1):3–8
Shannon C (1948) A mathematical theory of communication. Bell Syst Tech J 27(379–423):623–656
Slocombe KE, Townsend SW, Zuberbuhler K (2009) Wild chimpanzees distinguish between different scream types: evidence from a playback study. Anim Cogn 12(3):441–449
Snowdon CT, Elowson AM (2001) ‘Babbling’ in pygmy marmosets: development after infancy. Behaviour 138:1235–1248
Sullivan KA (1984) Information exploitation by downy woodpeckers in mixed-species flocks. Behaviour 91:294–311
Townsend SW, Manser MB (2011) The function of non-linear phenomena in meerkat alarm calls. Biol Lett 7:47–49
Townsend SW, Hollen LI, Manser MB (2010) Meerkat close calls encode group-specific signatures but receivers fail to discriminate. Anim Behav 80:133–138
Uster D, Zuberbuhler K (2001) The functional significance of Diana monkey ‘clear calls’. Behaviour 138:741–756
Valone TJ (2007) From eavesdropping on performance to copying the behaviour of others: a review of public information use. Behav Ecol Sociobiol 62:1–14
Weiner N (1961) Cybernetics; or control and communication in the animal and the machine. MIT Press, New York
Wickler W (1985) Coordination of vigilance in bird groups: the “watchman’s song” hypothesis. Z Tierpsychol 69:250–253
Zuberbuhler K (2000) Referential labeling in Diana monkeys. Anim Behav 59:917–927
Acknowledgements
We would like to thank Tim Clutton-Brock for his support to work on the meerkat population of the Kalahari Meerkat Project and the owners of farms surrounding the reserve for allowing us to work on their land. We are thankful to Megan Price and Christele Borgeaud, as field managers, and the volunteers of the project for their help over the duration of the study. Thanks to Micheal Owren for Praat scripts, Roger Mundry for statistical support and for providing pDFA scripts, Katie Slocombe, Beke Graw, David Jansen and Manuela Cadilek for discussions and Amanda Ridley, Peter Bednekoff and two anonymous reviewers for helpful comments on the manuscript. The study was carried out under licenses issued by the Northern Cape Conservation Service and ethical committee of Pretoria University, South Africa. This work was funded by the University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Bednekoff
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Time frequency spectrogram and accompanying waveform showing two distinct tonal meerkat sentinel calls. Spectrogram window settings: FFT length = 512, Hamming window, window length = 0.05 s, bandwidth = 112 Hz, frequency resolution = 86 Hz, dynamic range = 70 dB. (DOC 45 kb)
Rights and permissions
About this article
Cite this article
Townsend, S.W., Zöttl, M. & Manser, M.B. All clear? Meerkats attend to contextual information in close calls to coordinate vigilance. Behav Ecol Sociobiol 65, 1927–1934 (2011). https://doi.org/10.1007/s00265-011-1202-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1202-6