Abstract
Transfer of maternal hormones to the eggs is a major source of offspring phenotypic variation. The developmental and organizational effects of egg hormones can extend into adulthood and affect behavioral and morphological traits involved in sexual and reproductive behavior, with important consequences for offspring fitness. In this study, we injected testosterone (T) in egg albumen of captive ring-necked pheasant (Phasianus colchicus) eggs. We then assessed the consequences for chick growth, cell-mediated immunity, and multiple male secondary sexual traits at maturity by comparison with a control group. We also compared the covariation between traits in the two experimental groups. We found that control males had redder wattles than males from T-injected eggs, suggesting that attractiveness and reproductive success of the offspring might vary depending on maternal transfer of T to the eggs. T treatment also modified the covariation between cell-mediated immunity and wattle coloration and between the area of the wattle and the expression of another secondary sexual trait, the ear tufts. These effects are likely to translate into fitness differences among the offspring if mate acquisition depends on the simultaneous expression of several traits that are differentially affected by the same maternal contribution. Maternal effects mediated by egg hormones might affect the fitness of the offspring not only by directional modification of phenotypic traits, but also by facilitating or inhibiting their covariation. This suggests the possibility that female choice based on the relative expression of multiple secondary sexual traits exerts a pressure on how maternal transfer of androgens contributes to developmental programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The transfer of maternal hormones to the eggs is an important pathway whereby mothers can affect the phenotype of their progeny (Groothuis et al. 2005; Eising et al. 2006; Gil 2008; Groothuis and Schwabl 2008). Hormones are essential mediators of regulatory signals during developmental processes (Arnold 2002) as they are involved in the translational routes linking genes to development of morphological and behavioral traits, with effects across multiple targets (Groothuis and Schwabl 2008). As a result, phenotypic variation among individuals of the same sex can be due to differences in exposure to maternal steroid hormones (Adkins-Regan et al. 1995; Badyaev 2002; Groothuis et al. 2005; Rubolini et al. 2007; Strasser and Schwabl 2004).
Experimental studies altering the hormonal quality of the eggs have shown that maternal transfer of hormones can entail a wide array of consequences for the offspring (reviews in Groothuis et al. 2005; Gil 2008; Groothuis and Schwabl 2008). The experimental increase in yolk testosterone (T) shortened time to hatching (Eising et al. 2001, 2003) and promoted embryonic development and postnatal growth (Schwabl 1996; Eising et al. 2001). Moreover, exposure to steroids during development participates in sexual differentiation processes and sexually dimorphic growth, through sex-specific temporal patterns of action or tissue targeting (Owens and Short 1995; Badyaev 2002; Carere and Balthazart 2007). Accordingly, long-term effects of yolk T have also been documented on several morphological and behavioral traits, including those involved in sexual and/or reproductive behavior (Uller et al. 2005; Eising et al. 2006; Rubolini et al. 2006, 2007; Partecke and Schwabl 2008; Ruuskanen and Laaksonen 2010; Bonisoli-Alquati et al. 2011). T treatment of the eggs promoted the expression of the nuptial plumage (the black hood) of black-headed gulls (Larus ridibundus, Eising et al. 2006) and increased bib size (a secondary sexual trait) of male house sparrows (Passer domesticus, Strasser and Schwabl 2004). Moreover, T injection increased the frequency of aggressive, dominance, and reproductive behavior in house sparrows, black-headed gulls, and pied flycatchers (Ficedula hypoleuca, Strasser and Schwabl 2004; Eising et al. 2006; Partecke and Schwabl 2008; Ruuskanen and Laaksonen 2010), and interfered with laying behavior in the Chinese quail (Coturnix chinensis) and the ring-necked pheasant (Phasianus colchicus) (Uller et al. 2005; Rubolini et al. 2007). Thus, maternal transfer of hormones can at least partly explain interindividual differences in attractiveness and reproductive performance (Qvarnström and Price 2001). Interestingly, the effects of this maternal transfer might differ in direction between the two sexes, promoting fitness of males (or females) while being detrimental to the other sex (e.g., Bonisoli-Alquati et al. 2011).
Maternal transfer of steroid hormones to the eggs can influence the distribution of resources to different physiological functions of the developing chick (e.g., Navara et al. 2005), with possible consequences on the relative expression of different traits in adulthood. However, the effects of egg hormones on the expression of multiple traits in adulthood as well as on their covariation have seldom been investigated in avian species (but see Rubolini et al. 2007; Müller and Eens 2009). Interestingly, the observation that different traits vary in their sensitivity to the same manipulation of egg steroid levels would not only support the hypothesis that traits can differ in the quality of the information that they convey (the so-called multiple message hypothesis, Møller and Pomiankowski 1993), but also imply that they may signal the quality of the developmental environment.
In this study of captive ring-necked pheasants, we experimentally injected T in the albumen of unincubated eggs and assessed the consequences for offspring growth, cell-mediated immune response (CMI), and development of secondary sexual traits, while establishing a control group of sham-inoculated eggs. We also evaluated whether T injection disrupted the covariation between traits. Ring-necked pheasants have elaborated multiple ornaments with at least partly different roles in intra- and intersexual selection (von Schantz et al. 1989; Mateos and Carranza 1995, 1996, 1997; review in Mateos 1998). While some traits are believed to function either in agonistic encounters or as cues for female mate choice, other traits, such as the wattle (a fleshy, periorbital tissue) and the ear tufts, function in both intra- and intersexual selection processes (review in Mateos 1998). In the ring-necked pheasant, previous studies have shown differences in the hormonal control of different traits (Owens and Short 1995 and references therein). While plumage brightness is influenced by estrogens (Owens and Short 1995), the size of the wattle is T dependent (Briganti et al. 1999). Wattle size thus reflects male androgen levels and might be an honest signal of a male's ability to cope with the immunosuppressive effects of high T levels (Folstad and Karter 1992; Owens and Short 1995; Briganti et al. 1999). However, the immunosuppressive effect of plasma T could be buffered by an increase in circulating levels of carotenoids that is also associated with high plasma T (Blas et al. 2006; McGraw 2006; Álonso-Alvarez et al. 2008). If carotenoids were limiting (but see Hartley and Kennedy 2004), this would imply that buffering the immunosuppressive effect of T could only occur at the expense of their use for other functions, including ornamentation (Álonso-Alvarez et al. 2008). Thus, plasma T may mediate a trade-off between immunity and ornamentation. Consistently with this, the comb of the red-legged partridge (Alectoris rufa, a trait homologous to the pheasant's wattle; Kimball and Braun 2008) has been recently shown to vary in color according to T plasma levels, high levels causing a decreased color expression (Álonso-Alvarez et al. 2008). If egg T levels increase T production later in life or exert a sensitization effect on tissues targeted by T, as it has been shown for yolk T levels (Daisley et al. 2005; Benowitz-Fredericks et al. 2006; Müller et al. 2007), we could expect T injection in the egg to indirectly affect wattle coloration, that it is known to be at least partly carotenoid dependent in this species (Ohlsson et al. 2003). Based on the expected priming effects of egg T as well as on previous findings for both morphology (Rubolini et al. 2006) and behavior of this same species (Bonisoli-Alquati et al. 2011), we expect our treatment to increase the expression of T-dependent traits, including wattle size. We also expect our treatment to strengthen the covariation between T-dependent traits. Conversely, we expect the covariation between traits that are not both T dependent to be disrupted by T injection. Admittedly, however, the developmental control of some of the traits analyzed here is unknown and differences in covariation have never been explicitly assessed in relation to quality of the developmental environment.
Materials and methods
Study organism
The ring-necked pheasant is a large (ca. 1 kg), sexually dimorphic galliform with maternal care of the offspring (Cramp 1998). Males have multiple sexual ornaments and a colorful plumage, while females are cryptic (Cramp 1998). Males defend mating territories, and variance in mating success is high and dependent on the acquisition of a territory (Ridley and Hill 1987). Males possess wattles and ear tufts that are absent in females. Spur length and tail feathers are markedly dimorphic. Defense of territory is attained through agonistic interactions, involving threats, direct attacks, and the wattle display (Ridley and Hill 1987) that is performed through blood irrigation of the wattle and erection of the ear tufts (Mateos and Carranza 1996, 1997; Papeschi et al. 2003). Females also use the wattle display, together with other behavioral traits, as a cue for mate choice (Mateos 1998). The fact that average wattle size decreases after the mating season has been taken as evidence that the costly maintenance of the wattle display is facultative and condition dependent (Zuk et al. 1990), a suggestion further supported by the role of testosterone in wattle development and growth (Owens and Short 1995; Briganti et al. 1999; Papeschi et al. 2000). Interestingly, it has recently been shown that wattle size is associated with the genotype at the major histocompatibility complex, thus suggesting that female pheasants may use the ornament size as a cue to male quality in terms of the capacity to fight off parasites (Baratti et al. 2010).
Hormones assay
Before analyses, six pools each containing the albumen from 10 eggs were formed. Equal amounts of albumen were taken from each egg after homogenization for constituting the pool. Pools of different eggs were used because preliminary analyses showed that T concentration in the albumen was insufficient for the albumen of a single egg to provide enough T for quantification with our procedure. A sample (50 g) was collected from each homogenized pool and added with 2 ng of internal standard ([3,4-13C2]testosterone). After equilibration, the samples were extracted using 450 mL of an acetonitrile/chloroform mixture (1:1 v/v). The extracts were dried using a rotary evaporator (Büchi Rotavapor R-124; Flawil, Switzerland). The dried samples were resuspended in 2.5 mL of ethanol, dried again by means of a slow nitrogen flow, and then left overnight at 4°C. The samples were then resuspended in 150 μL of ethanol. We centrifuged the solution (9 min, 3,000 rpm) and transferred the recovered supernatant on a column where a standard testosterone solution (30 μL, 1 μg/μL) and 100 μL of sample were layered on silica gel. We recovered the analyte from the silica gel using a dichloromethane/methanol mixture (9:1 v/v) and repeated this step twice. To precipitate the silica, we then centrifuged the obtained suspensions. The supernatant was then removed and the solvent was evaporated in a water bath under slow nitrogen flow. The residual was derivatized with 50 μL of heptafluorobutyric acid and 100 μL of benzene, and incubated at 80°C for 20 min. We dried the solution under nitrogen flow and resuspended the extract in 50 μL of benzene. We injected 1 μL of this solution in a gas chromatograph (Trace 2000; Thermo Fisher, MA, USA) coupled with a Polaris Q ion-trap mass spectrometer (Thermo Fisher). The whole system was controlled by the Xcalibur software (Thermo Fisher). The gas chromatograph employed a BPX capillary column (30 m×0.25 mm, 0.25-μm film thickness; SGE Analytical Science, Victoria, Australia). Helium was used as the carrier gas at a constant pressure (100 kPa). We set the injector, transfer line, and ion source temperatures at 50, 300, and 200°C, respectively. The samples were injected (1 μL) into the PTV injector system operating in splitless mode (valve opening time 0.8 min). The initial column temperature was 160°C. After injection of the sample, the temperature was increased to 320°C (at 6°C /min) and then maintained constant for 2 min. Mass spectrometric detection was carried out in selected reaction monitoring mode. We monitored the reactions converting unlabeled testosterone (m/z=680) and [3,4-13C2]testosterone (as internal standard; m/z=682) to their ion derivatives (m/z=665 and 667, respectively). Each sample was processed in duplicate and two procedural blanks (50 g of distilled H2O) were extracted and processed. Recoveries of added testosterone in three samples with varying levels of testosterone were always higher than 85%. The linearity of recovery of added testosterone was R=0.97. Limits of detection were defined as three times the noise level. Mean coefficient of variation for two pools that were assayed twice was 11.5%.
The concentration of testosterone in the albumen of pheasant eggs that we detected was 25.8±7.2 (SE) pg/g (n=6 pools of m=10 eggs each). As all eggs contributed equally to a pool, the standard error of mean T concentration among pools estimates the standard deviation of T concentration in the albumen of individual eggs. We thus estimated the standard deviation of T concentration as SD=(mσ 2)1/2 viz. the square root of the product between the variance in the T concentration in the six pools (σ 2) and the number of eggs in each pool (m, Sokal and Rohlf 1995). This gave an estimated SD of 22.7 pg/g.
Testosterone manipulation procedure
Commercially purchased eggs (L'envol de Retz, Machecoul, France) were maintained at 16°C before injection. We treated all eggs on the same day. We randomly assigned eggs to either of two treatments (T injection or sham inoculation). Injection was performed close to the acute pole with a 250-μL syringe mounting a 25-gauge, 16-mm-long needle. Before injection, we left eggs horizontal for a few minutes to allow yolk migration away from the injection site. Dissection of 10 eggs that were frozen after being injected with a food dye invariably revealed that the dye had been correctly injected in the albumen. The quantity injected was designed to increase the T albumen concentration by two SD of the measured concentration in our samples (see above). An initial solution of 10 mg of T in 5 mL of ethyl alcohol was dissolved in distilled H2O to a final volume of 500 mL. A total of 477 pg of T (dissolved in 30 μL of distilled H2O) were inoculated in each egg, based on an average albumen weight (10.5 g, as estimated on a sample of 582 eggs that we dissected for a previous study; Rubolini et al. 2007). Thus, the injected dose presumably resulted in post-manipulation concentrations within the upper limit of the naturally occurring levels in the vast majority of the eggs. The control eggs were injected with 30 μL of a solution of ethyl alcohol in distilled H2O. After injection we sealed the hole by gluing a piece of eggshell upon it.
The study was carried out with the authorization of the ethical committee of the Department of Biology, University of Milan, Italy.
Measurement of sexual and nonsexual traits
All eggs were incubated in the same professional incubator. We placed eggs belonging to different treatments on different layers and alternated layers according to egg treatment to minimize potential differences in incubation conditions. Of the 250 eggs inoculated for each treatment, 201 control eggs (80.4%) and 205 T-injected eggs (82%) hatched. Egg failure was similar to unmanipulated eggs (15–20%, unpublished data) and the difference in hatching success between the two groups was not significant (χ 21 =0.21, P=0.65). We marked newly hatched chicks at hatching with a numbered plastic band, later replaced by a larger one. All chicks were kept in the same aviary (ca. \( {12} \times {8} \times {3}.{5} {\text{m indoor}} \); ca. \( {2}0 \times {1}0 \times {2}.{5} {\text{m outdoor}} \)) until the age of ca. 200 days, when the two sexes were separated in different aviaries (each of the same size as the first aviary).
We measured a suite of secondary sexual traits and of nonsexual traits in a subsample of 92 males (43 from control and 49 from T-injected eggs, respectively control and T males hereafter) and 108 females (53 from control and 55 from T-injected eggs, respectively control and T females hereafter), randomly chosen from the original pool of hatched chicks (subsampling was necessary because of time and space constraints). We recorded body mass at the age of 20, 45, 90, and 270 days after hatching by using an electronic balance (days 20 and 45) or a spring balance (days 90 and 270). We also measured tarsus length at each age, using a digital caliper (0.01 mm). At the age of 270 days, we measured right spur length (both sexes) and right ear tuft (males) using a digital caliper and a ruler (0.05 mm), respectively. We measured baseline wattle size (i.e., when wattle was not inflated by blood infusion) from digital pictures taken by means of a Canon EOS 30D (Canon Inc., Japan), mounting a 18–55-mm Canon lens set to a focal length of 35 mm. We estimated the area of the red fleshy wattle by measuring the number of pixels in the digital image (“Lasso tool” in Adobe Photoshop© CS2) and appropriately transforming this number to a surface measurement (square centimeters) by reference to a ruler placed in a standard position relative to the wattle and captured in each picture (see Rubolini et al. 2006). Wattle size was highly repeatable, as estimated on a random sample of six males that were photographed twice (F 5,6=25.16, P<0.001; repeatability=0.92). We could not measure tail length because of the wear of tail feathers associated with rearing conditions.
Wattle color in the range 320–700 nm was assessed by means of a portable spectrometer (Avantes AvaSpec 2048, The Netherlands) connected to a dual deuterium-halogen light source (Avantes DH-2000). We calibrated measurements every five individuals using a standard WS-2 reference tablet. We took two measurements, at the upper-anterior and at the lower-anterior end of the wattle (upper wattle and lower wattle respectively, hereafter). We placed the reflection probe in a plastic tube, whose end was cut at 45o to prevent specular reflection. Each measure consisted of the average of 15 scans. We measured each region of the wattle twice. Then, we analyzed color measurements using segment classification (Armenta et al. 2008) by means of ad hoc implemented routines in Microsoft Excel. We used each reflectance measurement to calculate hue, chroma, and brightness according to Endler (1990). Hue, chroma, and brightness data within each region were highly significantly repeatable (adjusted R 2>0.63 in all cases, P always < 0.001), and we thus averaged the two measures of each region. The coloration of the two regions was significantly correlated for all variables included in subsequent analyses (Pearson's correlation coefficient; hue, r=0.62; chroma, r=0.56; brightness, r=0.33; P<0.002 in all cases), and we therefore used the average of the two. We calculated brightness as the amount of light reflected by the sample in the human-visible spectrum (400–700 nm) relative to the amount of light reflected by the white standard. For calculating hue and chroma, we first divided each spectrum (the entire spectrum, the visible spectrum or the UV spectrum) into four regions of equal span. In the visible range, these four regions roughly correspond to the violet/blue (B), green (G), yellow/orange (Y), and red (R) wavelengths. We calculated chroma as the Euclidean distance between the spectrum and the origin of the color space (see Endler's equation 16, \( {\text{chroma}} = {\left[ {{{\left( {R - G} \right)}^{{2}}} + {{\left( {Y - B} \right)}^{{2}}}} \right]^{{{1}/{2}}}} \); Armenta et al. 2008). Chroma values therefore increase, indicating higher color purity, as the difference between segment reflectance increases. We measured hue as the clockwise angle, in the color space, between the spectrum and a hypothetical spectrum with reflectance only in the R segment (see Endler's equation 17, \( {\text{hue}} = { \arcsin }\left[ {\left( {Y - B} \right)/{\text{spectral distance}}} \right] \); Endler 1990). Hue values therefore increase from red to violet or, in the case of spectra including the UV component, from red to UV (Armenta et al. 2008). Sequence information concerning the gene coding for the ultraviolet or violet absorbing opsin in the avian retina suggested that Galliformes have “violet-sensitive” cones, whose maximal spectral sensitivity is around 405–420 nm, and are therefore relatively insensitive to UV wavelength (Ödeen and Håstad 2006). In our analyses, we therefore distinguished between features of wattle color in the visible (400–700 nm) and in the UV range (320–400), expecting information signaled by wattle color to be conveyed mostly in the visible range. The rather narrow interval of hue values in our study individuals implies that all data belonged to the same quadrant of the color space (visible range, \( {\text{mean hue}} = 0.{357} \pm 0.0{34} \); range, 0.257–0.484; UV range, \( {\text{mean hue}} = - 0.{455} \pm 0.0{9}0 \); range, −0.680 to −0.274). We therefore analyzed hue data using linear rather than circular statistics.
We collected the final measures of body mass and tarsus length and the measures of all sexually selected traits at the beginning of March (i.e., when sociosexual activities usually start in this species; Hill and Robertson 1988), when our experimental individuals were approximately 270 days old.
In addition, we also measured CMI by subcutaneously injecting 0.2 mg of phytohemagglutinin (PHA) dissolved in 0.05 mL of phosphate buffered saline (PBS) in the right wing web. As a control, we injected the left wing web with PBS only. We measured the thickness of both wing webs after 24 hrs with a pressure-sensitive micrometer (accuracy 0.01 mm; Teclock, Nagano, Japan), assuming that the difference in thickness between the PHA-injected wing web and the sham-injected wing web represents a measure of the intensity of cell-mediated immune response (Lochmiller et al. 1993).
Statistical analyses
We analyzed body mass and tarsus length data (four measures collected at 20, 45, 90, and 270 days of age, see above) using repeated-measures linear models with egg treatment and sex as fixed effects. The effects of T manipulation on sexual secondary traits, body mass and size, and CMI were analyzed by means of t tests. Males and females were analyzed separately. Homogeneity of variances was checked by means of the Levene's test.
We also studied differences between experimental groups in the covariation between traits by comparing the pairwise correlation coefficients in the two groups. We compared Pearson's correlation coefficients in the two groups through calculation of the test statistic z (Fisher 1921). Each correlation coefficient was first standardized as:
The test statistic z was then computed according to the formula:
where r 1′ and r 2′ are the standardized correlation coefficients and n 1 and n 2 are the sample sizes of the two groups. A P value was then obtained for the computed z values (Fisher 1921).
As we investigated the effects of the experimental manipulation on a number of different traits, as well as on their pairwise covariation, our analyses resulted in a large number of tests. We thus decided to reduce the risk of type I statistical errors (i.e., wrongly rejecting the null hypothesis of no difference between the two experimental groups) by applying false discovery rate (FDR) tests to the results of covariation analyses. The FDR is the rate at which significant tests are truly null (Storey and Tibshirani 2003; Pike 2010). The test provides a statistic, the q value, which is the minimum FDR at which the test may be called significant (Storey 2002). It can be interpreted in analogy to the P value and gives each feature its own individual measure of significance. While, the P value is a measure of significance in terms of the probability of committing type I errors, the q value is a measure in terms of the FDR (Storey and Tibshirani 2003; Pike 2010). Differently from other post hoc tests (e.g., the Bonferroni correction for multiple comparisons), FDR tests have the advantage of keeping both the probability of wrongly accepting null hypotheses and the probability of wrongly rejecting null hypotheses acceptably small (Pike 2010). In our analyses, q values were calculated using a bootstrap technique, rather than through the procedures suggested by Pike (2010) or through arbitrary smoothers (Storey et al. 2004), by means of the QVALUE software package (http://faculty.washington.edu/~jstorey/qvalue/).
To test whether a difference in the correlation between traits, as investigated by comparing the within-group correlation coefficients (see above), implied a difference in the slope of the relationships within each experimental group, we ran separate linear models where any given morphological variable was used as the response variable, while treatment (as a dichotomous factor) and each of the other traits (as a covariate) and the interaction between treatment and the covariate were entered as predictors. A significant interaction would indicate a difference in the slopes of the relationship between the two experimental groups. This can be interpreted as evidence that T injection affected the trade-off between the concomitant expression of two traits. Albeit conceptually different, the tests on the difference between the correlation coefficients and the tests on the slopes of the relationships between pairs of variables are expected to lead to similar results because the within-group correlation coefficients and the regression coefficients are inherently related. Thus, negative covariations between traits were regarded as indicative of trade-offs in the simultaneous expression of those traits.
α-Levels for the tests were set to 0.05, both for P values and for q values. q values were not calculated for females because of the much lower number of comparisons made.
Results
Effects of albumen T on phenotypic variables
We ran repeated-measures analyses of body mass and tarsus length at four ages (see Methods) including treatment and sex as factors, together with their interaction. The tests of within-subject effects showed that the effect of age on body mass and tarsus length strongly depended on sex (Table 1), as expected based on the marked sexual size dimorphism. Neither the three-way interaction between age, sex, and treatment nor the interaction between age and treatment had statistically significant effects in any analysis (Table 1). Thus, the experimental increase in T concentration did not influence body mass or tarsus length (Table 1). When the interaction between sex and treatment was removed from the model, the two groups did not differ in either body mass or tarsus length (details not shown). However, as expected, males attained larger body mass and tarsus length than females (Table 1).
Wattles of T males had a higher hue in the visible range (400–700 nm) than wattles of control males (Table 2). On the visible color wheel, this implies that control males had redder wattles than T males. The difference was highly significant also when the entire spectrum (320–700 nm) was considered or if each region of the wattle was analyzed separately (details not shown). However, the difference between treatments was not significant if we analyzed wattle hue in the UV range only (Table 2).
All other male traits (including the secondary sexual traits, the nonsexual traits and the other color variables) did not differ between the two treatments (Table 2). Similarly, control and T females did not differ in body mass, tarsus length, or spur length (Table 2).
Covariation among traits
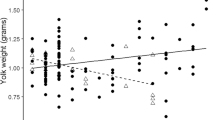
We tested the effect of the experimental increase in T concentration on the covariation between secondary sexual traits and nonsexual traits at maturity by comparing the correlation coefficients between pairs of traits in the two groups, separately for males and females. In these analyses, we did not test for differences in covariation involving wattle hue, chroma, and brightness in the UV range owing to the likely lack of biological significance of these measures (see “Material and Methods”). Control males differed from T males in 7 out of 33 correlation coefficients (Tables 3 and 4). However, only two of these differences remained significant if FDR was applied with a threshold q value of 0.05 (see Table 4; Fig. 1). Control males differed from T males in the covariation between CMI and wattle hue, which was significantly negative in control males and nonsignificantly positive in T males (Tables 3 and 4; Fig. 1a). The length of ear tuft and the wattle area were also positively correlated in both groups, but the relationship was significant among the T males only (Tables 3 and 4 Fig. 1b).
Relationships between a wattle hue and cell-mediated immune response and b length of ear tuft and wattle area in control (open circles) and T (filled circles) male adult pheasants. Simple linear regression lines are indicated (control, dashed line; T, solid line). These two correlations were found to differ between the two experimental groups after the application of FDR procedure (i.e., q<0.05; see “Statistical analyses”). The statistics for the relationships shown are as follows: a control males \( y = - 0.000{27 }\left( {0.0000{7}} \right)x + 0.{36} \), F 1,41=13.15, P<0.001, R 2=0.243; T males \( y = 0.0000{8 }\left( {0.0000{8}} \right)x + 0.{37} \), F 1,47=1.00, P=0.322, R 2=0.021; b control males 0.04 (0.52)x+20.25, F 1,41=0.01, P=0.935, R 2=0.000; T males \( y = {1}.{31}\left( {0.{28}} \right)x + {11}.{5}{1} \), F 1,47=21.45, P<0.001, R 2=0.313
For each pair of traits, we used each trait as response variable in a linear model where we included the treatment (as a two-level factor), the other trait (as a covariate) and the interaction between the two. For each pair of traits, we ran two different models, alternatively holding each trait as the response variable, with the only exception of the covariation between tarsus length and body mass, where we used only this latter trait as the response variable. These models largely confirmed the results of the test for the difference in the correlation coefficients, as the interaction effect was always significant when the correlation coefficients were different between the two groups, thus indicating that the slopes of the relationship between the two traits were significantly different in the two groups (details not shown; see Fig. 1, Tables 3 and 4).
Control females differed from T females in the correlation between tarsus length and body mass (control females: r=0.62; T females: r=0.27; z=2.21, P=0.027). Among females, the difference in correlation between spur length and either body mass or tarsus length was not significant (body mass: control females r=0.38, T females r=0.22; z=0.87, P=0.38; tarsus length: control females r=0.27, T females r=0.17; z=0.57, P=0.70). As for males, a linear model confirmed that the significant difference in the correlation between tarsus length and body mass in the two groups corresponded to a significant difference in the slope of the regression of body mass on tarsus length, as the interaction term between tarsus length and treatment was statistically significant (details not shown).
Discussion
In this study of captive ring-necked pheasants, we manipulated T concentration in the egg and assessed the consequences for chick growth, CMI, and the expression of sexual secondary traits at sexual maturity. We showed that the increase in egg T concentration affected wattle hue and influenced the covariation between wattle hue and CMI, as well as between wattle size and ear tufts.
Control males had redder wattles than T males. Recent studies have demonstrated that high plasma levels of T increased circulating carotenoid levels in different avian species (Blas et al. 2006; McGraw 2006; Álonso-Alvarez et al. 2008). This mobilization of carotenoids would function in buffering the immunosuppressive and/or prooxidant effect of T, while trading off with the carotenoid-dependent coloration of secondary sexual traits (von Schantz et al. 1999; Álonso-Alvarez et al. 2008; Mougeot et al. 2009, but see Mougeot et al. 2007a). Thus, a trade-off in the allocation of carotenoids would ensure the honesty of secondary sexual traits, as only high-quality males can afford to express carotenoid-based ornaments while withstanding the immunosuppressive and/or prooxidant effects of T (von Schantz et al. 1999; Álonso-Alvarez et al. 2008). If exposure to high levels of egg T increases subsequent T secretion or tissue sensitivity to T (Daisley et al. 2005; Müller et al. 2007), increased levels of egg T might have caused a diversion of carotenoids from their use in wattle coloration to supporting the immune system among T males. Carotenoid pigments in the epidermis are presumed to contribute to wattle coloration in our study species, together with the extensive blood irrigation that produces inflation of the wattle during displays (Stettenheim 2000; see also Álonso-Alvarez et al. 2008; Mougeot et al. 2009 for closely related species). In addition, dietary variation in carotenoid availability is known to affect wattle color in pheasants, with males fed an enriched diet achieving redder wattles (Ohlsson et al. 2003, see also Egeland et al. 1993).
This hypothesis could be supported by the difference in the relationship between wattle coloration and immune response in the two groups. Wattle redness and CMI positively covaried among control males, while they were (nonsignificantly) negatively associated among T males (see Fig.1). This result is consistent with previous evidence of a positive relationship between comb color and CMI in the closely related male red grouse Lagopus lagopus scoticus (Mougeot 2008) as well as with the negative relationship between comb color and parasite infestation (Mougeot et al. 2007b). Our T manipulation therefore disrupted this covariation because the likely priming effect of developmental exposure to T on subsequent T production or sensitivity had differential effects depending on individual quality, with only high-quality individual able to allocate carotenoids to ornamentation, rather than to immune function (von Schantz et al. 1999; Blas et al. 2006). Admittedly, this explanation is largely speculative and should be the subject of further tests. First, the premise that carotenoids are limiting has recently been questioned (Hartley and Kennedy 2004; Costantini and Møller 2008). Thus, carotenoid limitation among our experimental individuals should not be uncritically assumed, particularly given that they were raised in captivity and fed ad libitum with high-quality food (but see Ohlsson et al. 2003; see also Egeland et al. 1993). In addition, this hypothesis is based on the assumption of a priming effect of exposure to elevated T while in ovo on subsequent T production or sensitivity. This priming effect is supported by a number of studies in other species (Daisley et al. 2005; Müller et al. 2007; but see Partecke and Schwabl 2008) and is a likely mechanism for the documented behavioral effects of our T treatment that we documented in a companion study (Bonisoli-Alquati et al. 2011). However, here, we did not measure plasma levels of T and therefore suggest that future studies should evaluate the long-term effects of egg T while simultaneously controlling for differences in plasma levels of T and carotenoids as well as in diet quality.
Irrespective of the mechanism, the effect of our manipulation on wattle color may have translated into a reduction in individual reproductive success, if wattle hue functions as a signal of male quality during male–male agonistic encounters and/or female mate choice (Mateos 1998; Mateos and Carranza 1997). However, behavioral observations of a subset of the males included in the present study did not show any influence of wattle coloration on a male's success in obtaining copulations or winning dyadic agonistic encounters (Bonisoli-Alquati et al. 2011). Indeed, none of the traits measured here affected copulation success or social rank in any experimental group. Nonetheless, T males copulated more than control males, particularly with control females (Bonisoli-Alquati et al. 2011). This finding may suggest that our manipulation increased male attractiveness through promotion of T-dependent behavioral displays (e.g., the wattle display), which more reliably reflect male quality in a social context due to social control on deception (Berglund et al. 1996; Mateos and Carranza 1997).
Alternatively, female preference for T males might indicate that females preferred males based on the relative expression of different traits. Mate choice by female ring-necked pheasants is known to rely on a number of male traits (Mateos and Carranza 1995, 1997; review in Mateos 1998). Multiple cues are expected to provide information on different aspects of male quality (“multiple message hypothesis,” Møller and Pomiankowski 1993) and/or to allow a more accurate assessment of male quality (“redundant signal hypothesis,” Møller and Pomiankowski 1993; review in Berglund et al. 1996; Candolin 2003). Our results suggest that females, by evaluating relative expression of different traits, could not only gather more accurate or diverse information concerning male quality, but also exert selection on developmental programs and maternal transfer of androgens.
In fact, different ornamental traits were differentially affected by the same prenatal exposure to egg T. This is consistent with the hypothesis that the development of different secondary sexual traits is differentially sensitive to the same modifiers and to contingent environmental experience and condition (Møller and Pomiankowski 1993; Berglund et al. 1996; Candolin 2003; Müller and Eens 2009). Thus, different secondary sexual traits might also underlie different information concerning the developmental environment. In the ring-necked pheasant, it is known that estrogens, rather than T, control development of the plumage (Owens and Short 1995). Conversely, the development of spurs and wattle is known to depend on T secretion (Owens and Short 1995; but see Briganti et al. 1999) and it has been suggested that the documented negative effect of yolk T on spur length might have occurred through aromatization of the injected T to estradiol (Rubolini et al. 2006). In our study, the increase in egg T caused the wattle area and the length of the ear tufts to positively covary. In this case, the two traits seem thus to share the same developmental program, implying that the information content of the two traits is also similar. This hypothesis is also supported by the participation of both traits in the same behavioral display (i.e., the wattle display, Mateos 1998). Thus, by changing the covariation between traits that are involved in the same display, our treatment might have strengthened the overall effectiveness of the display, with possible consequences on male attractiveness. Yet, the relationship between signal reliability and developmental control of the trait(s) involved in the signal remains to be rigorously tested. In addition, differences in covariation among traits could also represent a maturational effect of T, if the pairwise trait covariation among T males was also associated with male sexual maturity and/or condition. Such maturational effects would imply that exposure to high levels of egg T might have had carry-over effects, rather than organizational effects (sensu Carere and Balthazart 2007).
In our study, we detected and quantified T in the albumen of unincubated eggs. It is unlikely that T detected in the albumen had passively leaked from the yolk, as the leakage of T from the yolk and the corresponding increase in albumen T levels are caused by embryo development and do not take place before the incubation starts (Elf and Fivizzani 2002; Gilbert et al. 2007; von Engelhardt et al. 2006; but see Eising et al. 2003). Direct transfer of T to the albumen could also take place while the eggs are in the oviduct (Johnston and Moore 2005). At present, we are unable to conclude whether albumen T exerts its phenotypic effects on the offspring though a similar or a different physiological pathway than yolk T. Although albumen T is expected to ultimately bind to the same receptors as yolk T, it might be taken up by the embryo at a different stage of development or at a different rate compared to yolk T. If this is the case, the differences with a previous study where yolk T manipulation was found to reduce spur length (Rubolini et al. 2006) could be explained by differences in the physiological destiny of T from the two egg compartments. Alternatively, this difference might be explained by the difference in concentration of T in the albumen compared to the yolk, where its concentration is hundreds of times higher (Rubolini et al. 2006; Carere and Balthazart 2007). However, our procedure likely resulted in early migration of polar T to the yolk, for which T has much higher affinity, thus making meaningless any distinction between T reservoirs in the two egg compartments. In addition, embryo development ultimately leads yolk and albumen to mix (Gilbert et al. 2007; von Engelhardt et al. 2006). Unless specific pathways are discovered for the utilization of albumen T, mixing with the much more abundant yolk T seems its likely fate (but see Paitz and Bowden 2008). Further studies are clearly needed, particularly because of the increasingly recognized importance of albumen as a source of nutrients (Ferrari et al. 2006; Bonisoli-Alquati et al. 2007, 2008, 2010), as well as because of the interesting possibility that avian mothers might be selected for allocating T to the albumen. In fact albumen and yolk are produced at different times during the reproductive cycle and albumen production takes place rapidly compared to yolk deposition (Palmer and Guillette 1991). Thus, transfer of steroids to the albumen could be sensitive to different environmental cues than transfer of steroids to the yolk and be differently related to maternal circulating levels, and possibly allows albumen T levels to be independently regulated for each egg.
References
Adkins-Regan E, Ottinger MA, Park J (1995) Maternal transfer of estradiol to egg yolks alters sexual differentiation of avian offspring. J Exp Zool 271:466–470
Álonso-Alvarez C, Perez-Rodriguez L, Mateo R, Chastel O, Vinuela J (2008) The oxidation handicap hypothesis and the carotenoid allocation trade-off. J Evol Biol 21:1789–1797
Armenta JK, Dunn PO, Whittingham LA (2008) Quantifying avian sexual dichromatism: a comparison of methods. J Exp Biol 211:2423–2430
Arnold AP (2002) Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the twentieth century, with special reference to the brain. In: Pfaff E, Pfaff E et al (eds) Hormones, brain, and behavior, vol 4. Academic, New York, pp 105–135
Badyaev AV (2002) Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol Evol 17:369–378
Baratti M, Ammannati M, Magnelli C, Massolo A, Dessì-Fulgheri F (2010) Are large wattles related to particular MHC genotypes in the male pheasant? Genetica 138:657–665
Benowitz-Fredericks M, Kitaysky A, Meddle S (2006) Effects of elevated yolk steroids on steroid receptor and aromatase mRNA expression in the hatchling quail (Coturnix japonica) brain. In Poster E-bird Conf. Glasgow, November 2006
Berglund A, Bisazza A, Pilastro A (1996) Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol J Linn Soc 58:385–399
Blas J, Perez-Rodriguez L, Bortolotti GR, Vinuela J, Marchant TA (2006) Testosterone increases bioavailability of carotenoids: insights into the honesty of sexual signalling. Proc Natl Acad Sci U S A 103:18633–18637
Bonisoli-Alquati A, Rubolini D, Romano M, Boncoraglio G, Fasola M, Saino N (2007) Effects of egg albumen removal on yellow-legged gull chick phenotype. Funct Ecol 21:310–316
Bonisoli-Alquati A, Martinelli R, Rubolini D, Saino N (2008) Sex-specific effects of albumen removal and nest environment manipulation on barn swallow nestlings. Ecology 89:2315–2324
Bonisoli-Alquati A, Rubolini D, Romano M, Cucco M, Fasola M, Caprioli M, Saino N (2010) Egg antimicrobials, embryo sex and chick phenotype in the yellow-legged gull. Behav Ecol Sociobiol 64:845–865
Bonisoli-Alquati A, Matteo A, Ambrosini R, Rubolini D, Romano M, Caprioli M, Dessì-Fulgheri F, Baratti M, Saino N (2011) Effects of egg testosterone on female mate choice and male sexual behavior in the pheasant. Horm Behav 59:75–82
Briganti F, Papeschi A, Mugnai T, Dessì-Fulgheri F (1999) Effect of testosterone on male traits and behaviour in juvenile pheasants. Ethol Ecol Evol 11:171–178
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev 78:575–595
Carere C, Balthazart J (2007) Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrinol Metab 18:73–80
Costantini D, Møller AP (2008) Carotenoids are minor antioxidants for birds. Funct Ecol 22:367–370
Cramp S (1998) The complete birds of the Western Palearctic on CD-ROM. Oxford University Press, Oxford
Daisley JN, Bromundt V, Möstl E, Kotrschal K (2005) Enhanced yolk testosterone influences behavioral phenotype independent of sex in Japanese quail chicks Coturnix japonica. Horm Behav 47:185–194
Egeland ES, Parker H, Liaaenjensen S (1993) Carotenoids in combs of capercaillie (Tetrao urogallus) fed defined diets. Poult Sci 72:747–751
Eising CM, Eikenaar C, Schwabl H, Groothuis TGG (2001) Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc R Soc Lond B Biol Sci 268:839–846
Eising CM, Müller W, Dijkstra C, Groothuis TGG (2003) Maternal androgens in egg yolks: relation with sex, incubation time and embryonic growth. Gen Comp Endocrinol 32:241–247
Eising CM, Müller W, Groothuis TGG (2006) Avian mothers create different phenotypes by hormone deposition in their eggs. Biol Lett 2:20–22
Elf PK, Fivizzani AJ (2002) Changes in sex steroid levels in yolks of the leghorn chicken, Gallus domesticus, during embryonic development. J Exp Zool 293:594–600
Endler JA (1990) On the measurement and classification of color in studies of animal color patterns. Biol J Linn Soc 41:315–352
Ferrari RP, Martinelli R, Saino N (2006) Differential effects of egg albumen content on barn swallow nestlings in relation to hatch order. J Evol Biol 19:981–993
Fisher RA (1921) On the probable error of a coefficient of correlation deduced from a small sample. Metron 1:3–32
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
Gil D (2008) Hormones in avian eggs: physiology, ecology and behavior. In: Brockmann HJ, Roper TJ, Naguib M, Wynne-Edwards KE, Barnard C, Mitani J (eds) Advances in the study of behavior. Elsevier, Amsterdam, pp 337–398
Gilbert L, Bulmer E, Arnold KE, Graves JA (2007) Yolk androgens and embryo sex: maternal effects or confounding factors? Horm Behav 51:231–238
Groothuis TGG, Schwabl H (2008) Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Phil Trans R Soc B 363:1647–1661
Groothuis TGG, Müller W, Engelhardt NV, Carere C, Eising C (2005) Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev 9:329–352
Hartley RC, Kennedy MW (2004) Are carotenoids a red herring in sexual display? Trends Ecol Evol 19:353–354
Hill DA, Robertson P (1988) The pheasant. Ecology, management and conservation. BSP Professional Books, Oxford
Johnston GIH, Moore MC (2005) Transfer of maternal corticosterone to yolk in preovulatory and postovulatory tree lizard (Urosaurus ornatus) eggs. Integr Comp Biol 45:1021
Kimball RT, Braun EL (2008) A multigene phylogeny of Galliformes supports a single origin of erectile ability in non-feathered facial traits. J Avian Biol 39:438–445
Lochmiller RL, Vestey MR, Boren JC (1993) Relationship between protein nutritional-status and immunocompetence in northern bobwhite chicks. Auk 110:503–510
Mateos C (1998) Sexual selection in the ring-necked pheasant: a review. Ethol Ecol Evol 10:313–332
Mateos C, Carranza J (1995) Female choice for morphological features of male ring-necked pheasants. Anim Behav 49:737–748
Mateos C, Carranza J (1996) On the intersexual selection for spurs in the ring-necked pheasant. Behav Ecol 7:362–369
Mateos C, Carranza J (1997) Signals in intra-sexual competition between ring-necked pheasant males. Anim Behav 53:471–485
McGraw KJ (2006) Sex steroid dependence of carotenoid-based colouration in female zebra finches. Physiol Behav 88:347–352
Møller AP, Pomiankowski A (1993) Why have birds got multiple sexual ornaments? Behav Ecol Sociobiol 32:167–176
Mougeot F (2008) Ornamental comb colour predicts T-cell-mediated immunity in male red grouse Lagopus lagopus scoticus. Naturwissenschaften 95:125–132
Mougeot F, Martínez-Padilla J, Pérez-Rodríguez L, Bortolotti GR (2007a) Carotenoid-based coloration and ultraviolet reflectance of the sexual ornaments of grouse. Behav Ecol Sociobiol 61:741–751
Mougeot F, Pérez-Rodríguez L, Martínez-Padilla J, Leckie F, Redpath FM (2007b) Parasites, testosterone and honest carotenoid-based signalling of health. Funct Ecol 21:886–898
Mougeot F, Martínez-Padilla J, Webster LMI, Blount JD, Pérez-Rodríguez L, Piertney SB (2009) Honest sexual signalling mediated by parasite and testosterone effects on oxidative balance. Proc R Soc Lond B Biol Sci 276:1093–1100
Müller W, Eens M (2009) Elevated yolk androgen levels and the expression of multiple sexually selected male characters. Horm Behav 55:175–181
Müller W, Deptuch K, López-Rull I, Gil D (2007) Elevated yolk androgen levels benefit offspring development in a between clutch context. Behav Ecol 18:929–936
Navara KJ, Hill GE, Mendonça MT (2005) Variable effects of yolk androgens on the growth and immunity in bluebird nestlings. Physiol Biochem Zool 78:570–578
Ödeen A, Håstad O (2006) Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol Biol Evol 20:855–861
Ohlsson T, Smith HG, Råberg L, Hasselquist D (2003) Effects of nutrition on sexual ornaments and humoral immune responsiveness in adult male pheasants. Ethol Ecol Evol 15:31–42
Owens IPF, Short RV (1995) Hormonal basis of sexual dimorphism in birds: implications for new theories of sexual selection. Trends Ecol Evol 10:44–46
Paitz RT, Bowden RM (2008) A proposed role of the sulfotransferase/sulfatase pathway in modulating yolk steroid effects. Integr Comp Biol 48:419–427
Palmer BD, Guillette LJ Jr (1991) Oviductal proteins and their influence on embryonic development in birds and reptiles. In: Ferguson MWJ, Deeming DC (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 29–46
Papeschi A, Briganti F, Dessì-Fulgheri F (2000) Winter androgen levels and wattle size in male common pheasants. Condor 102:193–197
Papeschi A, Carroll JP, Dessì-Fulgheri F (2003) Wattle size is correlated with male territorial rank in juvenile ring-necked pheasants. Condor 105:362–366
Partecke J, Schwabl H (2008) Organizational effects of maternal testosterone on reproductive behavior of adult house sparrows. Dev Neurobiol 68:1538–1548
Pike N (2011) Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol (in press)
Qvarnström A, Price T (2001) Maternal effects, paternal effects, and sexual selection. Trends Ecol Evol 16:95–100
Ridley MW, Hill DA (1987) Social organization in the pheasant (Phasianus colchicus): harem formation, mate selection and the role of mate guarding. J Zool 211:619–630
Rubolini D, Romano M, Martinelli R, Leoni B, Saino N (2006) Effects of prenatal yolk androgens on armaments and ornaments of the ring-necked pheasant. Behav Ecol Sociobiol 59:549–560
Rubolini D, Martinelli R, von Engelhardt N, Romano M, Groothuis TGG, Fasola M, Saino N (2007) Consequences of prenatal androgen exposure for the reproductive performance of female pheasants (Phasianus colchicus). Proc R Soc B Biol Sci 274:137–142
Ruuskanen S, Laaksonen T (2010) Yolk hormones have sex-specific long-term effects on behavior in the pied flycatcher (Ficedula hypoleuca). Horm Behav 57:119–127
Schwabl H (1996) Maternal testosterone in the avian egg enhances postnatal growth. Comp Biochem Physiol 114A:271–276
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, San Francisco
Stettenheim PR (2000) The integumentary morphology of modern birds—an overview. Am Zool 40:461–477
Storey JD (2002) A direct approach to false discovery rate. J R Stat Soc B 64:479–498
Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100:9440–9445
Storey JD, Taylor JE, Siegmund D (2004) Strong control, conservative point estimation, and simultaneous conservative consistency of false discovery rates: a unified approach. J Roy Stat Soc B 66:187–205
Strasser R, Schwabl H (2004) Yolk testosterone organizes behavior and male plumage coloration in house sparrows (Passer domesticus). Behav Ecol Sociobiol 56:491–497
Uller T, Eklöf J, Andersson S (2005) Female egg investment in relation to male sexual traits and the potential for transgenerational effects in sexual selection. Behav Ecol Sociobiol 57:584–590.
von Engelhardt N, Carere C, Dijkstra C, Groothuis TGG (2006) Sex-specific effects of yolk testosterone on survival, begging and growth of zebra finches. Proc R Soc Lond B Biol Sci 273:65–70
von Schantz T, Göransson G, Andersson G, Fröberg I, Grahn M, Helgée A, Wittzell H (1989) Female choice selects for a viability-based trait in pheasants. Nature 337:166–169
von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc Lond B Biol Sci 266:1–12
Zuk M, Thornhill R, Ligon JD, Johnson K, Austad S, Ligon SH, Thornhill NW, Costin C (1990) The role of male ornaments and courtship behavior in female mate choice of red jungle fowl. Am Nat 136:459–473
Acknowledgments
We are sincerely grateful to G.P. Oldani for the helpful technical support and suggestions. We thank R. Martinelli, A. Cavalleri, and E. Venturelli for their precious help during hormone assays. A. Matteo, B. Leoni, U. Oldani, P. Usorini, S. Bocchi, and A.T. Gerevini greatly helped during data collection, and A. Binelli provided useful comments on a previous draft of the paper. All experiments were conducted in compliance with the Italian norms for farming practice. ABA was funded by a PhD grant from the Italian Ministry of Education (MIUR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Brown
Rights and permissions
About this article
Cite this article
Bonisoli-Alquati, A., Rubolini, D., Caprioli, M. et al. Egg testosterone affects wattle color and trait covariation in the ring-necked pheasant. Behav Ecol Sociobiol 65, 1779–1790 (2011). https://doi.org/10.1007/s00265-011-1186-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1186-2