Abstract
Myrmecophiles, i.e., organisms associated with ants live in a variety of ecological niches in the vicinity or inside ant colonies and employ different strategies to survive ant encounters. Because different niches are characterized by different encounter rates with host ants, strategies used to avoid ant aggressions should depend on these niches. This hypothesis was studied with three rove beetle species of the genus Pella, which are myrmecophiles of the ant Lasius fuliginosus and the non-myrmecophilous relative Drusilla canaliculata. Behavioral tests in the field revealed that Pella species are better adapted to interactions with ants than D. canaliculata, but that they use species-specific strategies in ant interactions. Pella cognata and Pella funesta avoid encounters with ants by swift movements. Chemical analyses of the defensive tergal gland secretions showed that P. cognata has replaced the aggression inducing undecane by the behaviorally neutral tridecane. P. funesta repels the ants by releasing the panic alarm pheromone sulcatone from its tergal gland resulting in an “ant free space” around the beetles. Finally, Pella laticollis uses a specific and unique appeasing behavior. Behavioral and chemical data did not reveal any indication for the mimicry of the ants' cuticular hydrocarbon profiles by any of the beetle species. It is discussed that the employed strategies correlate with the ecological niches of the beetles. P. cognata and P. funesta are living along ant trails with ample space to escape and the employed strategies are probably sufficient to escape from dangerous conflicts. In contrast, P. laticollis lives in refuse areas of ant nests with frequent ant encounters, and its appeasement strategy allows it to stay at the encounter site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Many ant species live in large, long persisting colonies with up to several million members, which are sometimes regarded as “factories in a fortress” (Wilson 1968) or “ecological islands” (Hölldobler and Wilson 1990). The size and the longevity of these colonies, as well as the fact that they provide climatically stable and protected environments, makes them attractive for other organisms so-called myrmecophiles, which live in a variety of ecological niches in the vicinity or inside the colonies. Potential niches consist in trails, refuse dumps, or so called “kitchen middens”, peripheral nest chambers, and brood chambers (Hölldobler and Wilson 1990). It is assumed that the biodiversity of myrmecophiles evolved together with the large insect societies (Hughes et al. 2008).
One of the major challenges for myrmecophiles is to overcome the defense mechanisms used by the ants to protect their nests against intruders. Therefore, many of these species have specific strategies to circumvent nest-mate recognition cues or to deal with their host ants' aggressions (Kistner 1979). These strategies should be associated with the different encounter rates with host ants, which depend on the different levels of integration in the host nest and consequently on the ecological niches of the myrmecophiles. Thus, we hypothesize that different myrmecophiles differ in their strategies to avoid ant attacks and that strategies should correlate with the ecological niches occupied.
Experimental evidence in support of the idea that the defensive strategy correlates with the ecological niche in myrmecophiles is scarce. Recently, Witte et al. (2009) reported that different myrmecophiles of army ants use different strategies to be integrated in the host nest. However, because myrmecophilous species of different taxonomic groups were studied, it is discussed that observed differences are due to constraints of the taxonomic group and not due to ecological niches (Witte et al. 2009). An example of myrmecophilous species within a taxonomic group is provided by Komatsu et al. (2009), who studied two congeneric cricket species sharing the same host ant, which differ in their behavior depending on their degree of host specialization.
One group of insects particularly suitable to study this hypothesis is rove beetles (Staphylinidae) of the tribe Lomechusini. This tribe comprises genera like Lomechusa and Lomechusoides, which are highly integrated in ant colonies (Hölldobler 1967 and 1970), as well as non-myrmecophilous, free-living genera like Drusilla. Intermediate are species of the genus Pella, which occupy different ecological niches in and around colonies of the formicine ant Lasius fuliginosus (Latreille 1798) (Hölldobler et al. 1981; Maruyama 2006). In spring, summer, and autumn, adults of Pella cognata (Maerkel 1842) and Pella funesta (Gravenhorst 1806) are regularly caught alongside ant trails, where they hunt for living ants. In contrast, Pella laticollis (Maerkel 1845) is mostly found in the vicinity of refuse areas in the field. In the laboratory, P. cognata was demonstrated to prey on living ants, whereas P. laticollis hoards dead ants under shelters, a behavior which does not occur in the two other Pella species. (Stoeffler 2008, Stoeffler, unpublished data).
With respect to the mechanisms employed within the genus Pella to avoid attacks by host ants, the mimicry of the host alarm pheromones was suggested for two Japanese Pella species based on olfactory field observations (Kistner and Blum 1971). Hölldobler et al. (1981) described an appeasement gland at the tip of the abdomen of beetles, from which a whitish secretion is released. More recently, Akino (2002) reported that the Japanese species Pella (Zyras) comes (Sharp 1874) mimics the CHC pattern of its host ant to be accepted. Finally, laboratory studies from our group revealed that two of the European Pella species, P. funesta and Pella humeralis (Gravenhorst 1802), release from their defensive tergal gland the panic alarm inducing pheromone sulcatone of their host ants to deter attacking ants (Stoeffler et al. 2007). Field observations on this behavioral interaction are missing. Thus, different strategies have been described, but it is unclear if these strategies are universal for the whole genus Pella or if they are restricted to certain species, depending on their ecological niche.
Therefore, to study the hypothesis that Pella species with different ecological niches also differ in the strategies used to avoid ant aggressions, we observed ant–beetle interactions under natural conditions in the field. Furthermore, we conducted chemical analyses of the tergal gland secretion and of the cuticular hydrocarbons (CHC), and performed behavioral experiments with identified secretion compounds. The species studied were the myrmecophilous species P. cognata, P. funesta, and P. laticollis, as well as Drusilla canaliculata (Fabricius 1787), a free-living member of the Lomechusini.
Methods
Insects
Beetles of the genus Pella and ants were collected at three different colonies of L. fuliginosus in Southwest Germany. Beetles were caught with an aspirator along ant trails or in ant refuse areas. Ants for chemical analysis were collected exclusively from areas, where beetles were caught (e.g., trails and refuse areas). D. canaliculata was collected under stones and in the soil, or originated from a culture of beetles, which was provided by the Department of Ecology of the University of Freiburg (Germany). Beetles were identified to species level using the identification keys by Freude et al. (1974) and Maruyama (2006). The key by Seifert (2007) was used for ant identification.
Behavioral assays in the field
Behavioral observations were performed along ant trails in the field. A glass arena (diameter, 4.5 cm; depth, 2 cm) with a plaster layer (1 mm) was installed directly into ant trails. Ants were able to enter and leave the arena freely, whereas beetles were retained due to the smooth surface of the glass walls. In most cases, the arena was accepted by the ants several minutes after installation and became part of the trail. Beetles were transferred carefully into the arena and the interactions between ants and beetles were videotaped for 5 min. The videotapes were analyzed using “The Observer 5.0” (Noldus, Wageningen). For each individual beetle, only the first ten interactions were used for further analysis. Beetle specimens were tested at their home nest and at a foreign nest to obtain pairs of behavioral observations for each individual beetle.
Classification of behavior and calculation of an aggression index (AI)
All types of behavior performed by the ants and beetles were identified. Depending on its assumed aggressiveness, each behavior was attributed an aggression value between 0 and 4 (Table 1). A complete interaction sequence started with “contact” and ended with “escape”. The first ten complete beetle–ant interactions were used to calculate an aggression index AI using the following formula:
where MtD = total duration (seconds) of “mandible threat”, BiD = total duration (seconds) of “biting”, ChD = total duration (seconds) of “chase”, AsD = total duration (seconds) of “acid spraying”, ApD = total duration (seconds) of “aggressive presentation”, QdD = total duration (seconds) of “release of tergal gland secretion”, and ToD = total interaction time (seconds).
Species-specific aggression indices were calculated for each beetle specimen based on all interactions and separately for nestmate (NM) and non-nestmate (nNM) interactions. Aggression indices for interactions between NMs and nNMs were compared with the Wilcoxon matched-pairs test. For comparison between species the Kruskall–Wallis test was performed (software package STATISTICA 6.1; Stat Soft, Tulsa, OK, USA). For detailed comparison, we used the non-parametric statistical method provided by the Software package “Primer” (Clarke 1999). All frequencies of behavior were standardized to total of each file and square root transformed. Bray–Curtis distances were calculated and analyzed with a non-metric multidimensional scaling (nMDS). Furthermore, vectors were combined with the nMDS plot to visualize the dominating behavior for each beetle species. To check for differences between the species groups, an ANOSIM (analysis of similarities) was performed.
Chemical analysis of the tergal gland secretion
The volatiles which are released from the tergal glands of the beetles were analyzed as described in Stoeffler et al. (2007). Beetles were teased in a flask with a magnetic stir bar and a magnetic stick, and the volatiles from the headspace were collected using a solid phase micro extraction (SPME) fiber, coated with 65 μm polydimethylsiloxane/divinylbenzene. The volatiles were analyzed using a gas chromatograph (type 6890; Agilent Technologies, HP 5 column; 30 m long, 0.2 mm in diameter, and 0.5 μm film thickness; splitless mode, programmed, 60°C for 3 min, 60–300°C at 3°C/min and then constant over 30 min at 300°C; carrier gas, helium 1.6 ml/min) coupled to a 5973 Network Mass selective Detector (GC-MS) for identification of the substances. Chromatograms and mass spectra were analyzed with Agilent Technologies software (Enhanced Chemstation MSD Chem Station D 01.02.16, 15. June 2002) using Wiley (Wiley275) and NIST databases (NIST Mass Spectral Library 2002 Version). For the determination of double-bond positions, derivatives of the secretion were produced by reaction with dimethyl disulfide (Buser et al. 1983). The resulting vicinal dimethyl thioderivatives were separated on the HP5-MS capillary column as described, the only difference being that the final temperature of 300°C was held for 60 min.
Behavioral assays in the laboratory on the effect of tergal gland substances
These experiments were performed to study the effect of different compounds from the tergal gland secretion on host ants. The effects of undecane and sulcatone have already been studied (Stoeffler et al. 2007) but were included in the experiments to ensure that data are comparable. Ten ants were placed in a Petri dish containing a filter paper ball in the center treated with different test solutions. The ants' behavior towards the filter paper ball was videotaped for 90 s and analyzed afterwards. A behavior was considered as aggressive when ants touched the filter paper ball with both antennae and open mandibles, or when they were biting into it. The filter paper ball was treated with one of the following test solutions in hexane: (1) undecane (0.75 μg/μl), (2) undecane (0.37 μg/μl) and sulcatone (0.16 μg/μl), (3) n-tridecane (0.06 μg/μl), and (4) dodecanal (0.03 μg/μl). The used concentrations matched the concentrations in the tergal gland secretion of the Pella species, found during beetle–ant interactions in the laboratory using headspace analysis (Table 2 and Stoeffler et al. 2007). Control paper balls were treated with hexane. The results of the behavioral assays were analyzed using the Mann–Whitney U test using the software package STATISTICA 6.1 (Stat Soft, Tulsa, OK, USA).
Chemical analysis of the cuticular hydrocarbons (CHC)
Beetles and ants were freeze-killed and extracted thereafter singly for 30 min in 200 μl hexane containing eicosane (C20; 1.25 ng/μl) as an internal standard. To avoid disturbances of the analysis by polar compounds, these substances were separated with 0.25 g of silica gel (230–400 mesh). The CHC were then eluted with 500 μl hexane. The solvent was evaporated and the residue was resolved in 100 μl hexane. This extract was analyzed by GC-MS as described above (splitless mode). Gas chromatographic conditions were the same as for the analysis of the tergal secretion with the exception of the temperature program (120°C for 3 min, 120–300°C at 5°C/min and then constant over 30 min at 300°C). Individual compounds were identified by their mass spectra and their retention indices using n-alkanes (C20–C30) as references.
Statistical analysis of the CHC data
The grouping of samples was analyzed with the relative peak areas of each gas chromatographic file. We used the non-parametric statistical method provided by the Software package “Primer” (Clarke 1999). All peak areas were transformed (fourth root) and afterwards standardized to the maximum peak of each file. Bray–Curtis distances were calculated, and these files were analyzed with a nMDS to visualize chemical distances between the groups of CHC profiles. Proximities between groups of samples were tested by analysis of similarities (ANOSIM; Clarke 1999).
Results
Behavioral assays
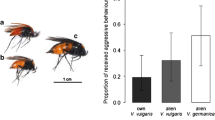
The aggression indices calculated from ant–beetle encounters observed in Petri dishes on ant trails in the field differed significantly depending on the beetle species. The non-myrmecophilous beetle D. canaliculata was treated significantly more aggressive by the ants than the myrmecophilous Pella beetles (Fig. 1). In more than 20% of the experiments with D. canalicula, the beetles were about to be killed and were therefore removed from the arena. This never happened during tests with Pella beetles. Within the genus Pella, encounters of ants with P. laticollis were less aggressive than encounters with P. cognata and P. funesta. No difference was found between the latter two species. The comparison of NM interactions (beetles with ants from the nest, where they were collected) and nNMs interactions (beetles with ants from foreign nests) revealed no significant differences (Fig. 2). Obviously, ants do not discriminate between beetles from their home nests and beetles introduced from a foreign nest.
The overall comparison of the behavioral differences within the Pella species using Bray–Curtis distances combined with nMDS (Fig. 3) and ANOSIM (Table 3) revealed a significant separation of P. laticollis from the other two species, which broadly overlap. An analysis of the different behaviors revealed that this separation of P. laticollis is mainly caused by the appeasing behavior “duck down”, which was almost never observed in the other Pella species \( {\hbox{Kruskal - Wallis test}}:H\left( {df = 2,N = {117}} \right) = 80.84;p \leqslant 0.001 \); see also Suppl. 1). During “duck down”, the beetles stopped and crouched to the ground with the abdomen bent upwards (inset in Fig. 3 and Suppl. 2). The ants' reaction most often consisted in intense antennation of the beetle's abdomen \( \left( {{\hbox{Kruskal}} - {\hbox{Wallis test}}:H\left( {df = 2,N = 117} \right) = 26.45;p \leqslant 0.001} \right) \). In contrast, P. cognata (Suppl. 3) and P. funesta either avoided encounters with ants by changing their direction \( \left( {{\hbox{Kruskal}} - {\hbox{Wallis test}}:H\left( {df = 2,N = 117} \right) = 38.42;p \leqslant 0.001} \right) \) or released tergal gland secretion \( \left( {{\hbox{Kruskal}} - {\hbox{Wallis test}}:H\left( {df = 2,N = 117} \right) = 9.78;p \leqslant 0.007} \right) \). The most pronounced differences between P. cognata and P. funesta consisted in the fact that ants actively avoided P. funesta \( \left( {{\hbox{Kruskal}} - {\hbox{Wallis test}}:H\left( {df = 2,N = 117} \right) = 6.23;p \leqslant 0.044} \right) \), especially after beetles had released defensive secretion from their tergal glands (Suppl. 4). The behaviors “aggressive presentation” by the beetles, i.e., bending the abdominal tip towards the ant and “mandible threat” by the ants were frequently observed in encounters of all Pella species. “Aggressive presentation” was slightly more abundant in encounters with P. cognata \( \left( {{\hbox{Kruskal}} - {\hbox{Wallis test}}:H\left( {df = 2,N = 117} \right) = 9.26;p \leqslant 0.010} \right) \), “mandible threat” was more often shown by ants in encounters with P. laticollis \( \left( {{\hbox{Kruskal}} - {\hbox{Wallis test}}:H\left( {df = 2,N = 117} \right) = 7.82;p \leqslant 0.020} \right) \).
Multidimensional scaling of Bray–Curtis similarities calculated from behavioral frequencies of encounters of Lasius fuliginosus ants with three Pella species. Vectors show the contribution of those behaviors, which were significantly different between species using Kruskal–Wallis test. Inset: Appeasing behavior “duck down” that is more often shown by P. laticollis as compared to the other species during encounters with L. fuliginosus (Drawing by B. Schmid, University of Hohenheim)
Chemical analysis of the tergal gland secretion
The composition of the tergal secretion studied via head-space analysis of teased beetles with SPME remarkably differed between the four beetle species. D. canaliculata showed a composition similar to other free-living Aleocharinae (Steidle and Dettner 1993), with quinones and undecane dominating (Suppl. 5). This agrees with the results of an earlier study on the tergal gland secretion of this species, obtained by GC-MS analysis of the complete dissected tergal glands (Steidle and Dettner 1993) and demonstrates the validity of the SPME method used here. The secretion in P. cognata contained less undecane, whereas 6-tridecene, 5-tridecene, tridecane, and 7-pentadecene were higher in concentration as compared to the free-living D. canaliculata. Sulcatone was present only in trace amounts (Table 2). In the secretion of P. funesta, sulcatone is present in high concentrations in addition to undecane, which confirms the results by Stoeffler et al. (2007). Finally, P. laticollis has a composition similar to that of D. canaliculata, with the exception of dodecanal, which was present in higher concentrations as compared to the other species. For a more detailed comparison of the data obtained with SPME, we calculated the relative proportions between the species for each substance in accordance with Anonymous (2009). Major differences are the presence of sulcatone in P. funesta, as well as the smaller amount of undecane and the dominance of the tridecenes, tridecane, and 7-pentadecene in P. cognata as compared to the other species.
Experiments on the effect of substances in the tergal gland secretion
Undecane and dodecanal provoked significantly more aggressive behavior in L. fuliginosus ants than the control hexane or the other substances and mixtures tested. When undecane was mixed with sulcatone, the aggressive behavior was reduced to a level comparable to the control. This is in agreement with earlier results, which showed that not only undecane, but also quinones induce aggressions, and that this effect can be blocked by sulcatone (Stoeffler et al. 2007). Tridecane did not provoke more aggression than the control (Fig. 4).
Aggressive acts of Lasius fuliginosus (median ± quartile) in a laboratory experiment towards a filter paper ball treated with different substances, which are present in the tergal gland secretion of Pella rove beetles. Bars with different lower case letters are significantly different at p ≤ 0.05 (Mann–Whitney U test; control, N = 30; substances, N = 20)
Chemical analysis of the cuticular hydrocarbons
GC/MS-analyses of extracts of single beetle specimens from three different colonies revealed the presence of up to 30 different hydrocarbons in different relative mean quantities (Suppl. 6). To study similarities in the CHC profiles between ants and beetle species on the nest level a nMDS using the Bray–Curtis similarities was performed. The resulting plot shows more or less distinct clusters of species, but not of nest sites (Fig. 5). The analysis of similarities (ANOSIM) for L. fuliginosus specimens from the three colonies (B, G and K) resulted in R values of 0.488 (nest B vs. nest K), 0.546 (nest B vs. nest G), and 0.638 (nest G vs. nest K; p ≤ 0.001). Most of the R values for beetles tested with their host ants profiles resulted in much higher R values, indicating a higher dissimilarity between the ants and their respective beetle (Table 4). Only specimens of P. cognata from nest B were in the range of the L. fuliginosus cluster but R values for other P. cognata beetles were highly different from their respective host ants at the other nest sites.
Multidimensional scaling of Bray–Curtis similarity calculated from CHC profiles of workers of Lasius fuliginosus from three different ant nests, myrmecophilous rove beetles of the genus Pella collected in the vicinity of these nests, and specimen of the non-myrmecophilous rove beetle Drusilla canaliculata
Discussion
In behavioral experiments under semi-natural conditions in the field, the interactions between the tested beetle species and ants significantly differed in their level of aggression. The free-living species D. canaliculata was treated far more aggressive than the myrmecophilous Pella beetles and among the latter, interactions with P. cognata and P. funesta were more aggressive than with P. laticollis. Furthermore, within one beetle species, levels of aggressiveness did not differ between interactions with ants from the home nest and from foreign nests. This indicates that
-
(1)
Pella species are better adapted to interactions with ants than the free-living D. canaliculata,
-
(2)
Pella species differ in their strategies and adaptation to ant interactions, and
-
(3)
nest-specific cues are not involved.
A thorough analysis of the ant–beetle interactions revealed that different levels of aggressiveness in ant interactions between Pella species are determined by species-specific behaviors in ant encounters. Most remarkable is the behavior shown by P. laticollis. When encountering ants, this beetle ducks down and presents its abdominal tip to the ants. This prompts the ants to antennate the abdominal tip and allows the beetle to escape, making the encounters between P. laticollis and L. fuliginosus less aggressive as compared to encounters in the other species. In contrast, P. cognata and P. funesta avoided closer contact with ants by swift escape movements upon ant contacts and released tergal gland secretion more often than P. laticollis. Remarkably, P. funesta was avoided by the ants, probably after release of tergal gland secretion, resulting in an “ant free space” around the beetles.
Thus, whereas in P. cognata and P. funesta, no indication for an appeasement behavior by the beetles was observed, the behavior “duck down” with the presentation of the abdominal tip to the ants by P. laticollis agrees with the observations by Hölldobler et al. (1981). It is likely that P. laticollis is one of the species which were described to use an abdominal appeasement gland, different to the defensive tergal gland (Hölldobler et al. 1981). In contrast to this description, however, no release of a whitish secretion was observed. According to Hölldobler, this appeasement gland consists of numerous glandular openings at the tip of the abdomen. Alternatively, it could be identical with a hitherto undescribed anal gland in the Aleocharinae (Dettner, personal communication) that also occurs in Pella species. Further studies are necessary to identify the exact location of the appeasement glands and to identify its chemical content.
The chemical analyses revealed that the composition of the tergal gland secretion differs considerably between species in the amount and the composition of substances. Four different secretion types were identified, all of which agree in the presence of toxic and irritating quinones, but differ in the compounds, which are used as solvents for the quinones. The first type is found in D. canaliculata and contains large amounts of undecane as solvents. This type is similar to the tergal gland secretion of other free-living species of the Aleocharinae (Steidle and Dettner 1993) and therefore has to be considered as plesiomorphic within the Lomechusini. Another type of secretion occurs in P. funesta (this study) and P. humeralis (Stoeffler et al. 2007). In addition to undecane, it contains large amounts of sulcatone. The third type is found in P. cognata. In this species, undecane occurs only in small amounts, whereas other solvents like tridecane and pentadecene are present in larger quantities. Both substances are lacking in the other species. The fourth type is found in P. laticollis, where undecane is dominating and the only notable difference to the first type is the presence of a higher amount of dodecanal.
In laboratory bioassays on the effect of the secretion compounds, undecane and quinones stimulated a high amount of aggression in L. fuliginosus ants (Stoeffler et al. 2007). We believe that this effect causes the highly aggressive interactions in the field between ants and D. canaliculata beetles, which release these compounds from their tergal gland secretion. Sulcatone, a major compound found in the secretion of P. funesta reduces the aggressions caused by undecane and was found to have a repellent effect on the ants (Stoeffler et al. 2007). This explains the field observations in which ants avoided contact with P. funesta after the release of tergal gland secretion. Taken together the behavioral and chemical data from D. canaliculata and P. funesta strongly support the idea that the presence of sulcatone represents an adaptation in myrmecophilous Lomechusini, which prevents the aggression-inducing effect of the plesiomorphic solvent undecane (Stoeffler et al. 2007). Tridecane, found as a solvent in the secretion of P. cognata, did not increase aggressive behavior in the ants. Its presence might be an alternative form of adaptation to avoid the aggression-inducing effect of undecane by the exchange with tridecane. Finally, dodecanal from the fourth type (P. laticollis) did result in higher aggressions. It is very likely that this substance serves more as an irritant agent like the quinones and that its presence is not an adaptation to myrmecophily.
In the chemical analyses of the CHC of ants and beetles, we identified 30 substances from C23 up to C31. All these substances are regularly discussed to be involved in NM recognition in social insects (Howard and Blomquist 2005) and could be used by the beetles to mimic their host ants. However, nMDS with Bray–Curtis similarities of relative peak areas showed that the CHC profiles of the beetles did not match the host ants' profiles and that specimen did cluster according to species and not according to nest sites. Likewise, the ANOSIM showed that profiles from ants and beetles were clearly different. Thus, no indication for chemical mimicry based on CHC was found. Possibly, this mimicry is not necessary for the Pella beetles because the adults are less strongly integrated in the social system of their host ants as compared to other myrmecophiles. As mentioned above, they live in the vicinity of the nests, which offers them sufficient food and shelter. Therefore, they do not have to acquire nest-specific CHC profiles, which can be either dangerous (Akino et al. 1996) or costly and which could restrict them to single L. fuliginosus nests.
In conclusion, the results from the present study support the hypothesis, that to overcome the defensive system of their host ants, Pella species have species-specific strategies, which are associated with different ecological niches. P. cognata and P. funesta avoid encounters with ants by swift movements. In addition, the composition of their defensive tergal gland secretion is altered, most likely to overcome the effect of the plesiomorphic, aggression inducing undecane as solvent. In P. cognata, undecane is partially replaced by the neutral solvent tridecane. P. funesta contains sulcatone in its secretion, which inhibits the effect of undecane and repels the ants, resulting in an “ant free space” around the beetles. Both species are still inflicted in aggressive interactions with their host ants, but we assume that the employed strategies are sufficient to prevent dangerous conflicts in areas with relatively few ant encounters and ample space to escape, i.e., along ant trails where both species hunt for living ants. In contrast, the adaptation in P. laticollis consists in a specific appeasing behavior associated with the presence of appeasement glands. This strategy makes encounters with ants much less aggressive and allows P. laticollis to stay at the encounter site, which is advantageous under situations of frequent ant encounters as they are expected in refuse areas in close vicinity to the ant nests. Therefore, adaptations of the tergal gland secretion as observed in P. cognata and P. funesta are probably not required in P. laticollis. This appeasement strategy might be the first step of a communication between ant and beetle that is more sophisticated than “run away” or “chase them away”. We hypothesize that this strategy could be a preadaptation for the close association with host ants within the Lomechusini, with the appeasement behavior and the hairy glands in Lomechusa and Atemeles representing the peak of this evolution. To address these questions on the evolution of myrmecophily in the Lomechusini, further studies comprising more chemical, behavioral, and molecular studies are required.
References
Akino T (2002) Chemical camouflage by myrmecophilus beetles Zyras comes (Coleoptera: Staphylinidae) and Diaritiger fossulatus (Coleoptera: Pselaphidae) to be integrated into the nest of Lasius fuliginosus (Hymenoptera: Formicidae). Chemoecology 12:83–89
Akino T, Mochizuki R, Morimoti M, Yamaoka R (1996) Chemical camouflage of myrmecophilous cricket Myrmecophilus sp. to be integrated with several ant species. Japn J Appl Entomol 40:39–46
Anonymus (2009) New SPME guidelines. J Chem Ecol 35:1383
Buser H-R, Arn H, Guerin P, Rauscher S (1983) Determination of double bond position in monounsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal Chem 55:818–822
Clarke KR (1999) Nonmetric multivariate analysis in community-level ecotoxicology. Environ Toxicol Chem 18:118–127
Freude H, Harde KH, Lohse GA (1974) Die Käfer Mitteleuropas Band 5. Goecke und Evers Verlag, Krefeld
Hölldobler B (1967) Zur Physiologie der Gast-Wirt-Beziehungen (Myrmecophilie) bei Ameisen - I. Das Gastverhältnis der Atemeles- und Lomechusa-Larven (Col. Staphylinidae) zu Formica (Hym. Formicidae). Z Vgl Physiol 56:1–21
Hölldobler B (1970) Zur Physiologie der Gast-Wirt-Beziehungen (Myrmecophilie) bei Ameisen - II. Das Gastverhältnis des imaginalen Atemeles pubicollis Bris. (Col. Staphylinidae) zu Formica (Hym. Formicidae). Z Vgl Physiol 66:215–250
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Hölldobler B, Möglich M, Maschwitz U (1981) Myrmecophilic relationship of Pella (Coleoptera: Staphylinidae) to Lasius fuliginosus (Hymenoptera: Formicidae). Psyche 88:347–374
Howard RW, Blomquist GJ (2005) Ecological, behavioral and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393
Hughes DP, Pierce NE, Boomsma JJ (2008) Social insect symbionts: evolution in homeostatic fortresses. Trends Ecol Evol 23:672–677
Kistner DH (1979) Social and evolutionary significance of social insect symbionts. In: Hermann HR (ed) Social insects vol 1. Academic, New York, pp 339–413
Kistner DH, Blum MS (1971) Alarm pheromone of Lasius (Dendrolasius) spathebus (Hymenoptera: Formicidae) and its possible mimicry by two species of Pella (Coleoptera: Staphylinidae). Ann Entomol Soc Am 64:589–594
Komatsu T, Maruyama M, Itino T (2009) Behavioral differences between two ant cricket species in Nansei Islands: host-specialist versus host-generalist. Insect Soc 56:389–396
Maruyama M (2006) Revision of the Palearctic species of the myrmecophilous genus Pella (Coleoptera, Staphylinidae, Aleocharinae). National Science Museum Monographs:32
Seifert B (2007) Die Ameisen Mittel- und Nordeuropas. Lutra- Verlags- und Vertriebsgesellschaft, Tauer
Steidle JLM, Dettner K (1993) Chemistry and morphology of the tergal gland of free-living adult Aleocharinae (Coleoptera: Staphylinidae) and its phylogenetic significance. Syst Entomol 18:149–168
Stoeffler M (2008) Zur Biologie myrmekophiler Kurzflügelkäfer der Gattung Pella (Coleoptera: Staphylinidae) in Baden-Württemberg unter besonderer Berücksichtigung von chemischer Verteidigung und Mimikry. Jahreshefte der Gesellschaft fur Naturkunde in Wurttemberg 164:171–195
Stoeffler M, Maier T, Tolasch T, Steidle JLM (2007) Foreign-language skills in rove-beetles? Evidence for chemical mimicry of ant alarm pheromones in myrmecophilous Pella beetles (Coleoptera: Staphylinidae). J Chem Ecol 33:1382–1392
Wilson EO (1968) The ergonomics of caste in the social insects. Am Nat 102:41–66
Witte V, Foitzik S, Hashim R, Maschwitz U, Schulz S (2009) Fine tuning of social integration by two myrmecophiles of the Ponerine army ant, Leptogenys distinguenda. J Chem Ecol 25:355–367
Acknowledgments
We like to thank Robert Pfeifle and Konrad Schwarz who accurately performed behavioral experiments. Many thanks to H. Burger, J. Gögler, and A. M. Rottler from the Institute of Experimental Ecology at the University of Ulm in Germany who helped in the statistics on cuticular hydrocarbons. Thanks to M. Spelleken and K. Peschke (University of Freiburg, Germany) for specimen as a starter for our Drusilla canaliculata rearing. The manuscript was considerably improved thanks to helpful comments of J. Collatz and very valuable remarks of unknown reviewers. All experiments performed for this study were in compliance with the current German laws.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Choe
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Suppl. 1
Frequency of different behaviors in encounters between Pella species and workers of Lasius fuliginosus (PDF 80 kb)
Movie of behavioral interactions between Pella laticollis and Lasius fuliginosus. P. laticollis shows the appeasing behavior “duck down”, which is almost never observed in the other two Pella species described here. During “duck down”, the beetle stops and crouches to the ground with the abdomen bent upwards. The ants' reaction most often consists in intense antennation of the beetle's abdomen. After this, the beetle or the ant leaves the place. This is shown several times in the video (MPG 9305 kb)
Movie of behavioral interactions between Pella cognata and Lasius fuliginosus. P. cognata actively avoids encounters with ants. Nearly, each ant contact is prompted by immediate and swift changes of the beetle's direction (MPG 7173 kb)
Movie of behavioral interactions between Pella funesta and Lasius fuliginosus. P. funesta avoids aggressive encounters with ants by releasing tergal gland secretion. In this video, the beetle releases tergal gland secretion when it is molested by a large number of ants. The secretion contains sulcatone, a panic-alarm-inducing pheromone. As a consequence, ants actively avoid P. funesta (MPG 6558 kb)
Suppl. 5
Total ion chromatograms of volatiles released by Drusilla canaliculata and three Pella species from the tergal gland. Numbers in chromatograms refer to the numbers in Table 2. GC, 30 m HP 5; 60°C/3 min; 60–300°C/3°C/min; hold 30 min/300°C (PDF 111 kb)
Suppl. 6
Relative peak areas of different cuticular hydrocarbons (mean ± SD), which were found in extracts of single specimens of Lasius fuliginosus, Drusilla canaliculata, Pella cognata, P. funesta, and P. laticollis (PDF 31 kb)
Rights and permissions
About this article
Cite this article
Stoeffler, M., Tolasch, T. & Steidle, J.L.M. Three beetles—three concepts. Different defensive strategies of congeneric myrmecophilous beetles. Behav Ecol Sociobiol 65, 1605–1613 (2011). https://doi.org/10.1007/s00265-011-1171-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1171-9