Abstract
The extent to which active female mating preferences influence male reproductive success in mammals is unclear, particularly for promiscuously breeding species like chimpanzees (Pan troglodytes). Previous studies from multiple long-term study sites have shown that female chimpanzees mate more restrictively around ovulation, and this has been taken as evidence for female choice. However, none of these studies rigorously evaluated the alternative hypothesis that restrictive mating results not from unconstrained choice, but in response to coercive mate guarding, in which males use punishment and intimidation to reduce female promiscuity and promote their own mating interests. Nor did they consider evidence for the potential genetic or phenotypic benefits that females might be choosing. Using 11 years of data from the Kanyawara community in Kibale National Park, Uganda, we previously demonstrated that males achieve elevated mating success with those females toward whom they direct high levels of aggression. Here we extend those findings to show that even female copulatory approaches, which have previously been attributed to female choice, are correlated with male aggression. Specifically, individual females at our site initiated periovulatory copulations most frequently with the males who were most aggressive toward them throughout their cycles. Those males showed high rates of aggression toward females throughout estrus, despite achieving high copulation rates, demonstrating a continuing conflict of interest over the exclusivity of mating access. Because sexual coercion is potentially widespread in primates and other mammals, our results stress the importance of considering the influence of male aggression in studies of female choice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Females in a broad range of species, especially birds, are known to choose mates that offer genetic or phenotypic benefits (Andersson 1994; Hill 2006; Mays et al. 2008). Female choice is less well understood in mammals (Clutton-Brock and McAuliffe 2009). Because most mammals are polygynous and rarely provide paternal care, male–male competition for access to females is relatively intense. Consequently, males that are successful at mating tend to be of high quality, which reduces the benefits of active female choice (Clutton-Brock and McAuliffe 2009). Moreover, intense male mating competition can select for both armaments and large body size, which males of some species employ to constrain female mating behavior (Smuts and Smuts 1993; Clutton-Brock and Parker 1995; Muller and Wrangham 2009).
Primates present additional difficulties for the assessment of female choice. Because female primates have slow life histories and produce relatively few offspring, mate selectivity is expected to be particularly important in this order (Kappeler and van Schaik 2004). Yet in many non-human primates, females mate promiscuously by actively soliciting copulations from multiple partners (Dixson 1998; Hrdy 1981; Nunn 1999; Zinner et al. 2004; Clarke et al. 2009). Multi-male mating appears to benefit females primarily by confusing paternity, and thus reducing the risk of male infanticide (Hrdy 1979; van Noordwijk and van Schaik 2000; Paul 2002; van Schaik et al. 2004).
Given these problems, the extent and nature of female choice in promiscuously mating primates is uncertain. However, there has been considerable interest in the idea that a promiscuous strategy predominates only in the early follicular phase, when conception is unlikely to occur, and that females exert a preference for particular males around the time of ovulation, subtly attempting to concentrate paternity in those individuals (Nunn 1999; van Schaik et al. 2004; Clarke et al. 2009). Female choice of high-ranking males has been predicted in this context on the premise that those males would provide the best subsequent defense against infanticide (Nunn 1999; van Schaik and Janson 2000; van Schaik et al. 2004; Clarke et al. 2009). Genetic benefits are also possible (Paul 2002; Byers and Waits 2006).
The prediction of biased mating during the periovulatory period (POP) has been supported by the only empirical tests to date, from data on wild chimpanzees (Pan troglodytes) (Matsumoto-Oda 1999; Stumpf and Boesch 2005, 2006; Pieta 2008). Chimpanzees offer a relevant test because females copulate more than 500 times per conception, normally mating with all of the adult males in their community (Wrangham 2002). Moreover, infanticide by adult males within the social group is an important risk for females (Nishida and Kawanaka 1985; Arcadi and Wrangham 1999; Murray et al. 2007).
In the first study, at Mahale (Tanzania), Matsumoto-Oda (1999) found that the proportion of a female’s copulations with high-ranking males increased significantly during the POP. She inferred from this result that females preferred to mate with high-ranking males when they were likely to conceive. However, her data did not discriminate between the hypothesis of female choice and the alternative hypothesis of female constraint, i.e., that high-ranking males guard females more intensely during the POP and thereby restrict female options. In support of the female-constraint hypothesis, solicitations by adolescent males (who were low ranking) were more likely to succeed when higher-ranking males were absent (34/48 attempts, i.e., 70.8% success) than when they were present (6/23, i.e., 26.1%; Table 5 in Matsumoto-Oda 1999).
In more detailed studies, Stumpf and Boesch (2005, 2006) examined mating patterns in two communities of wild chimpanzees living in Taï National Park (Ivory Coast), quantifying female “preferences” by establishing rates of proceptivity (female-initiated sexual behavior) and resistance (avoidance of male solicitations) across male–female dyads. They reported that males whose sexual advances were generally resisted by a particular female were resisted by that female at higher rates, and solicited at lower rates, during the POP. No such difference was evident for males who were generally approached by a particular female for copulations.
A similar study by Pieta (2008) at our site in Kanyawara, Kibale National Park (Uganda), showed a somewhat different pattern. As at Taï, males whose sexual advances were generally resisted by a particular female were solicited by that female at lower rates during the POP. However, no significant difference was found between rates of resistance in the POP and non-POP. Additionally, and distinct from Taï, males at Kanyawara who were generally approached by a particular female for copulations, were approached by that female at higher rates, and resisted at lower rates, during the POP.
Both Stumpf and Boesch (2005, 2006) and Pieta (2008) construed their findings as strong evidence for female choice in chimpanzees. However, neither study rigorously tested the alternative hypothesis that the distribution of female copulatory approaches during the POP (when females were most attractive) was constrained by male aggression (Muller et al. 2009a). For example, even if a female’s objective were to solicit all of the males in a group equally, she might be thwarted by the efforts of a coercive male interested in monopolizing her. Measures of female resistance were similarly difficult to interpret. Although chimpanzee females might avoid a male’s advances owing to negative preference, they might also do so because mating could invite punishment from a higher-ranked suitor (Muller et al. 2009a). This is particularly problematic because both studies defined resistance according to a female’s initial response to the male solicitation (including “ignoring the solicitation, avoiding the male, screaming, or leaving”, Stumpf and Boesch, 2005). Thus, a female who waited for a high-ranking male to turn his attention elsewhere before mating furtively with a soliciting male would have been classified as “resistant”. Problems inherent in these assumptions of preference are illustrated by the fact that in both Stumpf (2004: Appendix B) and Pieta’s (2008: Table 1) studies, some females simultaneously “preferred” males based on measures of proceptive behavior whom they “eschewed” based on measures of resistant behavior.
Controlling for the potential effects of male behavior on female mating decisions is critical, because much evidence suggests that chimpanzee males use aggression as a coercive mating tactic, making some females more likely to mate with them and less likely to mate with rivals (Muller et al. 2007). For example, we have previously shown that Kanyawara females experience increased rates of male aggression during periods of maximal swelling (i.e., during estrus, Muller et al. 2007), when conception is most likely to occur (Emery Thompson 2005). Parous females, who are more attractive to males (Tutin 1979; Wrangham 2002; Muller et al. 2006), receive higher rates of male aggression during maximal swelling than do less attractive nulliparous females (Muller et al. 2007). Finally, individual males exhibit increased copulation rates with the parous females toward whom they are most aggressive (Muller et al. 2007). This correlation could in theory result partly from females being compelled to copulate more frequently with their aggressors (“direct coercion”). However there is little evidence of direct coercion in chimpanzees (Goodall 1986; Muller et al. 2009a). A more likely explanation is that aggressive males are able to inhibit females from mating with other males (“indirect coercion”) (Muller et al. 2009a).
We have also established that male aggression imposes significant costs on Kanyawara females. Physical injury, including severe wounding, is a regular outcome of the prolonged attacks that are sometimes directed at females (Muller et al. 2009a). Furthermore, levels of stress hormones (cortisol) in females show large increases during periods of cycling and maximal swelling (Muller et al. 2007), a pattern that we have recently shown is driven by an increase in male aggression during these periods (Emery Thompson et al. 2010).
To date, attempts to control for the possible influence of male coercion on female choice have focused on the immediate context of mating. Stumpf and Boesch (2005), for example, reported no significant correlation between rates of male aggression toward females and rates of female proceptivity during the POP. While their result fits the fact that chimpanzee males rarely use force directly in the act of copulation (Goodall 1986; Stumpf and Boesch 2006), it did not address the critical question of whether a female’s willingness to copulate is affected by aggression that she had received in previous periods.
The question is important because a growing body of evidence suggests that sexual coercion in primates is often a long-term strategy that achieves its goal by manipulating the future, rather than simply the immediate behavior of the victim (Wrangham and Muller 2009). Male punishment of both female mating resistance (Clutton-Brock and Parker 1995) and female promiscuity (Clarke et al. 2009) can be effective strategies if females modify their behavior in response to the actions of known males. The development of this response is evident when female hamadryas baboons (Papio hamadryas hamadryas) are first incorporated into a one-male unit (Swedell and Schreier 2009). Male hamadryas employ aggression to enforce female proximity, promoting their long-term social bond. Once a bond is established, and females learn to follow a male, and to avoid rival males, rates of aggression drop (Swedell and Schreier 2009). Such a resolution may not occur, however, if male and female mating interests are in conflict, or if threats to male mating exclusivity emerge. For example, in mountain gorillas, encounters with strange males often provoke aggression by resident silverbacks against their mates (Sicotte 1993).
Because chimpanzees, like hamadryas baboons, live in stable social networks and exhibit cognitive abilities such as individual recognition, memory of specific events, and sophisticated learning (Goodall 1986), male aggression might in theory affect female behavior over the long-term. We have previously shown that males in Kanyawara who direct high levels of aggression toward individual females show increased rates of copulation with those females compared to other males. We have also shown that the majority of POP copulations are initiated by males rather than females (Emery Thompson and Wrangham 2008). However, we have not previously investigated whether the copulations initiated by females represent a free expression of preference, as Stumpf and Boesch (2005, 2006) speculate. Here we employ 11 years of data from Kanyawara to test whether patterns of female-initiated copulation during the POP (when conception is most likely) reflect primarily male coercion or female attempts to bias paternity toward specific males. Because females might choose males based on a range of criteria, we consider predictions for phenotypic and genetic benefits separately (See Table 1).
In theory, females might practice unfettered promiscuity to gain protection from infanticide, giving all males a more or less equal probability of conception. However, previous work at Kanyawara suggests that this does not occur. For example, high-ranking males at Kanyawara show higher rates of copulation with females during the POP, and females show increased copulation rates with aggressors relative to non-aggressors (Emery Thompson and Wrangham 2008; Muller et al. 2007). It is thus necessary to consider alternative hypotheses to explain these biased mating patterns.
If females are actively concentrating paternity in the most socially powerful males to gain protection from infanticide (van Schaik et al. 2000), then all females are expected to initiate periovulatory copulations most frequently with the alpha male (van Schaik et al. 2004). Consequently, the alpha is expected to show decreased rates of male–female aggression during the POP, since his interest in sequestering females from competitors is aligned with the female goal of concentrating paternity (Muller et al. 2009a, b).
If female mate choice is directed toward maximizing genetic quality, then predictions differ depending on whether females are choosing males with “good genes” or males with “compatible genes” (Mays and Hill 2004; Neff and Pitcher 2005). In “good genes” models, females choose mates based on a particular combination of alleles, and females within a community are expected to bias paternity toward the same male or males (as in pronghorn, Antilocapra americana: Byers and Waits 2006). In contrast to the predictions of the infanticide-avoidance hypothesis, this male need not be the alpha (e.g., female preference for brightly colored males in mandrills, Mandrillus sphinx: Setchell 2005). If females are choosing males based on genetic compatibility (i.e., dissimilarity), then females are not expected to bias paternity toward the same males (e.g., potential cryptic choice in the gray mouse lemur, Microcebus murinus: Schwensow et al. 2008). In both models, males who are the targets of paternity concentration are expected to show decreased rates of male–female aggression during the POP, since their interests are aligned with those of the females.
If biases in female sexual initiations reflect constraints imposed by male aggression, then females should solicit periovulatory copulations most frequently from the males who are most aggressive toward them (Muller et al. 2007, 2009a). Such skew could reflect direct coercion (a male increasing his absolute mating success with a female), indirect coercion (a male restricting a female’s ability to solicit other males), or both. Furthermore, if biases in female copulatory initiations result from male constraints on female promiscuity, and not female interest in paternity concentration, then conflict of interest between males and females is expected to continue during the POP, as females continue attempting to mate with males other than the aggressor. Consequently, rates of male aggression against females are expected to remain steady or intensify around ovulation. Finally, if biases in female copulatory initiations reflect primarily male mate guarding (indirect coercion), then females are expected to show increased solicitation rates toward males in the absence of the males who are most aggressive toward them.
Methods
Study population and long-term data
The subjects of the study were members of the Kanyawara community in Kibale National Park, Uganda, a chimpanzee population that has been studied continuously since 1987. This study incorporates data from 29,488 observation hours from January 1996 to December 2006. The community consisted of 47 chimpanzees at the beginning of the study (including 11 adult males and 17 adult females) and 52 individuals at the end of the study (including 10 adult males and 16 adult females).
Behavior was recorded by a team of observers, which normally consisted of two to three long-term Ugandan field assistants and one to two university-based researchers (graduate students, postdoctoral researchers, or one of the authors). Confidence in the accuracy of long-term behavioral data comes from tests documenting close agreement between focal data collected by researchers and all-occurrence sampling data collected independently by field assistants (Muller et al. 2007), together with routine measures of inter-observer reliability (Kibale Chimpanzee Project, unpublished data).
Chimpanzees were located by following their tracks, listening for calls, or waiting near fruiting trees. Whenever possible, observers followed chimpanzees from the time that they woke in the morning until they constructed their night nests. Observers identified all individuals present in a focal party at 15-min intervals throughout the day. A party was defined as all chimpanzees within 50 continuous meters of each other. Observers also detailed the behavior of individual party members during 10 min focal sessions. Focal targets consisted of all age-sex classes, and were randomly selected throughout the day from observable party members. Observers attempted to record all overt submissive vocalizations (pant-grunts) and behaviors, and any aggression that occurred within the party, including the identities of the actors.
Aggression was defined as any directed charge, chase, or attack (see Muller 2002 for definitions). These types of aggression are accompanied by exaggerated movements and vocalizations (e.g., screams) from victims, rendering them highly conspicuous to observers. Thus, our sampling of aggression is equivalent to all-occurrence sampling (Altmann 1974). Nevertheless, the long-term data underestimate true rates of aggression, because some interactions are obscured by vegetation. Muller et al. (2007) compared focal data on intersexual aggression collected by a single observer with long-term data and showed that these underestimates represent an unbiased sample of the behavior.
Dyadic rates of male–female aggression are reported from three different time periods. “POP aggression” was calculated by summing the number of charges, chases, and attacks a male directed at a female across all POP days (see definition below), and dividing by the number of hours the pair were observed together on those days. “Pre-POP aggression” was calculated in the same manner, but for days of maximal swelling prior to the POP. “Cycling aggression” was the same measure again, but calculated over all days in which the female was actively cycling (i.e., not pregnant or experiencing lactational amenorrhea), regardless of whether she was maximally swollen on that day. Thus, all aggression rates controlled for dyadic association times, which are reported in Tables 2 and 3.
Male dominance ranks were assigned based on the direction of submissive vocalizations (pant-grunts) and decided agonistic encounters among male dyads (Muller and Wrangham 2004). Ordinal ranks (r) were assigned to each male on a yearly basis, and these were standardized by the number of adult males in the hierarchy (n M) using the formula: (n M − r)/(n M − 1). Each male was assigned a mean rank over the period of female sexual cycling sampled, based on these standardized yearly ranks.
Ovarian cycle data
Observers used a simple scale to record the degree of tumescence of the sexual swelling for each female in a party. Females with sexual skins that were completely flat received scores of 1. Females with sexual skins that were partially inflated (i.e., soft and/or wrinkled rather than tense and shiny) received scores of 2. Females with sexual skins that were maximally tumescent (i.e., tense and shiny with no drooping) received scores of 3. Estrous females were defined as having maximally tumescent swellings. Nonestrous females were those with partial or flat swellings.
In wild chimpanzees, ovulation occurs within the period of maximal swelling tumescence and, according to independent examinations of ovarian cycle profiles, is most probable (>75%) between 2 and 5 days before the end of swelling, designated D-2 to D-5 if D0 is the first day of detumescence (Deschner et al. 2003; Emery Thompson 2005; Emery Thompson and Wrangham 2008). Because mammalian sperm are predicted to survive in the female reproductive tract for approximately 48–72 h (Johnson and Everitt 1988; Royston 1982; Wilcox et al. 1995), these models also assign a high probability of fertile mating to cycle days D-6 and D-7. Thus, we define the probable periovulatory period as days D-2 to D-7. Days of low fertilization potential (non-POP) included the last day of sexual swelling when ovulation probability is low and female attractiveness drops substantially (Emery Thompson and Wrangham 2008) and up to 10 pre-POP days with maximal swelling tumescence. We excluded both cycles from females known to be pregnant (from hCG testing or other hormonal data), and cycles for which the first day of maximal swelling or the day of detumescence were not observed (Emery Thompson 2005).
We limited our analyses to interactions between adult males (aged 15 years and over) and parous females. Nulliparous female chimpanzees experience both a prolonged period of subfecund cycling following menarche, and relatively high rates of neonatal mortality (Roof et al. 2005; Brewer Marsden et al. 2006). Consequently, males prefer parous females as mates, and compete more intensely for access to them (Muller et al. 2006). Table 4 shows, for each female, the number of cycles sampled in both POP and non-POP periods.
Female proceptivity
Chimpanzee copulations are normally initiated by a clear solicitation from either the male or the female (Goodall 1986). Male courtship behavior includes direct gaze, branch shaking, bipedal swagger, knuckle-rapping, or outstretched arms, all accompanied by penile erection. Female solicitations normally consist of a female approaching a male and crouching with her sexual swelling toward him. Copulation was defined as mounting with intromission and pelvic thrusting (Wrangham 2002). We have omitted cases where observers could not determine who initiated the copulation. This produced a total of 996 copulations in non-pregnant cycles with known POP dates.
Following Stumpf and Boesch (2005) and Pieta (2008), we assigned each male to one of three categories for each female, depending on whether he was generally solicited by that female for copulation. “Approached” males were those whose average solicitation rate by a female, during periods of maximal swelling, exceeded that female’s mean rate of male solicitation by at least 25%. “Non-approached” males were those who fell below a female’s mean solicitation rate by at least 25%. All other males were designated “Neutral.” We favor these terms over Stumpf and Boesch’s “Preferred” and “Non-preferred” males, because they describe behavior without attributing motivation. Proceptivity rates were calculated for each male–female dyad by dividing the number of female-initiated POP copulations by the number of hours the pair were observed together during the POP.
Previous chimpanzee studies have employed rates of “resistance” (defined as ignoring a male solicitation or actively avoiding a copulation attempt) as an additional measure of female choice (Stumpf and Boesch 2005; Pieta 2008). For three reasons we did not incorporate resistance in the present study. First, Pieta’s (2008) data show that, at Kanyawara, female proceptivity rates showed larger and more reliable changes during the POP than did rates of resistance. Second, active female resistance of male copulatory attempts (i.e., screaming at and fleeing from, struggling with, or striking a male) is rare enough to be of questionable significance as an effective mode of female choice (4% at Gombe: Goodall 1986; ~3% overall rate at Kanyawara: Kibale Chimpanzee Project, unpublished data). In the current dataset (which is limited to fully adult males and non-pregnant cycles), fewer than 1.2% of POP copulations were actively resisted by females (n = 5), making it impossible to establish meaningful patterns. Third, the operational definition of resistance is problematic, with rates recorded by different observers varying markedly within sites. At Taï, for example, Boesch and Boesch-Achermann (2000) reported a rate of 8%, whereas Stumpf and Boesch (2005) reported 28%. Similar differences are seen between resistance rates in the long-term data at Kanyawara, and those reported by Pieta (2008). At Kanyawara, the lower rates reflect active resistance by females. The higher rates reported by Pieta can only be generated by classifying females who initially ignore a male’s solicitation as resistant, whether or not they later copulate with him. As discussed previously, this assumption results in behaviors that may have nothing to do with negative preference being classified as resistant (e.g., if a female delays her positive response to a male’s solicitation until the attention of a high-ranking male is directed elsewhere).
Interpreting female resistance is further complicated by the fact that a female may ignore or even flee from a male because she does not want to mate with him, but also out of anxiety—male solicitations frequently include moderately aggressive behaviors such as branch shaking, foot stomping, and piloerection—or from fear of another male nearby. At Kanyawara, we sometimes see females fleeing male copulatory approaches following threats from nearby males. These observations are consistent with the hypothesis that male coercion in this species functions to constrain female mating behavior (Muller et al. 2006). In practice, a female’s motivation can be impossible to determine. This difficulty may explain inconsistencies in prior choice studies, which reported some females having both high proceptivity and high resistance rates to the same male (Stumpf and Boesch 2005; Pieta 2008).
Analyses
Dyadic rates of male–female aggression during cycling and rates of copulation (both overall and female-initiated) during the periovulatory period were compared for eight females and 12 males using the Kr row-wise matrix partial correlation test (Hemelrijk 1990). Because our data span multiple years, it was impossible for some individuals in the dataset to interact (e.g., if a female had died before a male entered adulthood). Consequently, there were missing values in our matrices (25% of 96 cells). To deal with such values, we created a third matrix containing dummy variables—zero for non-missing values, and a constant for missing values (Hemelrijk 1990). The constant was also added to missing values within the aggression and copulation matrices, and the dummy matrix was then partialled out. Statistics were calculated using Matman 1.1 software (Noldus Information Technology, Wageningen, The Netherlands). Significance of the correlation coefficient was estimated with 2,000 permutations.
All other statistical analyses were conducted using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Comparisons between dependent groups employed the Wilcoxon signed-rank test. All correlations report Kendall’s rank correlation coefficient (τ). All statistical tests are two-tailed, and means are reported ±SE.
Results
Proceptivity rates varied across females. Average rates of female proceptivity across the adult males ranged from 0.005 to 0.014 times per hour for all periods of maximal swelling (mean, 0.01 ± 0.002). These figures are comparable to those reported by Stumpf and Boesch (2005). On average, females initiated 28.4% of their copulations with males (female range, 19.6–47.4%; n = 8 parous females; SD = 8.91) whereas male initiation accounted for, on average, 71.6% of copulations.
Patterns of female proceptivity during estrus are summarized in Table 5, which shows approached, non-approached, and neutral males for each female. The alpha male (MS) is a conspicuous outlier in these data, as he was classified as approached for all of the parous females in our sample. For ranks below alpha, there was no consistency among females as to which males received high rates of proceptivity, and which went unsolicited. Every male except the alpha was categorized as non-approached by at least one female, and every male except the lowest-ranking individual was classified as approached by at least one female. Figure 1 shows, for each male, the absolute number of females for whom he was an approached or non-approached male. Neither of these measures correlated with average male rank across the study period (Kendall correlation; approached: τ = 0.287, p = 0.220, n = 12; non-approached: τ = −0.097, p = 0.674, n = 12).
Patterns of female proceptivity by male. White bars indicate, for each male, the number of females whose sexual solicitation rate rose >25% above her average rate across all males. Black bars indicate, for each male, the number of females whose solicitation rate fell >25% below her average solicitation rate across all males. Neither of these measures correlated significantly with mean male rank through the study period (high rank top third, middle rank middle third, low rank bottom third)
It was necessary to use mean male rank for statistical purposes, but it should be noted that the lack of an association between male rank and approach preference cannot be explained by male ranks changing over the study period (e.g., if a male interacted with one estrous female while low ranking and another while high ranking). Our results were similar for the subset of males (n = 6) who remained high (MS and BB), medium (BF), or low ranking (YB, PG, and SY) throughout the study period.
Counter to the predictions of hypotheses based on active female paternity concentration, the alpha male (MS) showed no decrease in aggression toward estrous females during the POP compared to pre-POP days of maximal swelling (Fig. 2). On average, parous females received aggression from the alpha male 0.0183 ± 0.009 times per hour during the POP. This rate was marginally higher than that on non-POP days of maximal swelling (0.0166 ± 0.004 times per hour), but the difference was not statistically significant (Wilcoxon signed-rank test: Z = −0.105, p = 0.917, n = 8 parous females).
Male aggression rates toward parous, estrous females during the periovulatory period (POP) compared to pre-POP days of maximal swelling. Neither the alpha male, approached males (including the alpha), nor non-approached males showed significant decreases in aggression toward parous, estrous females during the 6 days of the POP (black bars) compared to previous days of maximal swelling (white bars)
Similarly, individual approached males showed no difference in rates of aggression, between POP and pre-POP days of maximal swelling, directed toward the females who solicited copulations from them at high rates (Fig. 2). On average, parous females received aggression from their approached males 0.0102 ± 0.003 times per hour during the POP. This was identical to the rate on non-POP days of maximal swelling (0.0102 ± 0.003 times per hour; Wilcoxon signed-rank test: Z = −0.14, p = 0.889, n = 8 parous females).
Consistent with the coercion hypothesis, when females were most likely to conceive (i.e., during the POP), they initiated copulations most frequently with the males who were most aggressive toward them throughout periods of ovarian cycling. A matrix partial correlation procedure (Hemelrijk 1990) revealed a significant positive association between the amount of aggression directed by males at individual parous females during all periods when they were cycling, and the number of times females approached those males for copulation during the POP (τrw; xy.z = 0.37, p < 0.001, n = 8 females, 12 males). There was also a significant positive association between the amount of aggression directed by males at individual cycling females and the number of times females copulated with those males during the POP, regardless of who initiated the copulation (τrw; xy.z = 0.32, p = 0.001, n = 8 females, 12 males). There was no significant correlation between the amount of aggression directed by males at individual cycling females during the POP and the number of times females copulated with those males during the POP (τrw; xy.z = 0.106, p = 0.12, n = 8 females, 12 males).
As an additional test of the relationship between male aggression and female proceptivity, we compared, for each of eight parous females, periovulatory proceptivity rates toward males who were aggressive toward the female at rates above and below the median amount of male aggression received by her (Fig. 3). As expected, individual females solicited periovulatory copulations at significantly higher rates from the males that were more aggressive toward them (0.013 ± 0.002 solicitations per hour), than those who were less aggressive toward them (0.002 ± 0.001 solicitations per hour; Wilcoxon signed-rank test: Z = −2.52, p = 0.012, n = 8 females). Strikingly, half of the females in our sample never solicited a periovulatory copulation from any of the males who directed less than the median amount of aggression toward them.
Females initiated periovulatory copulations most frequently with the males who had previously been the most aggressive toward them. Black bars indicate female-initiated POP copulation rates with males who were aggressive toward the female at rates above (>) her median rate of received male aggression. White bars indicate female-initiated POP copulation rates with males who were aggressive toward the female at rates below (≤) her median rate of received male aggression. Importantly, aggression rates were calculated from all periods when a female was actively cycling (i.e., not pregnant or experiencing lactational amenorrhea), regardless of whether she was maximally swollen. Note that half of the females never initiated a POP copulation with the males who were least aggressive towards them during cycling. All dyads were observed together for at least 50 h during the POP
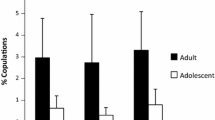
Because the alpha male, MS, was both solicited by, and highly aggressive toward, every female in our sample, we wanted to test whether female proceptive behavior changed in his absence. If female promiscuity is constrained by male aggression, in the form of coercive mate guarding, then females are expected to show higher rates of proceptivity when MS is not in a party. Unfortunately, MS was rarely absent when parous females were observed with full swellings. For the eight females in our sample, the mean of mean dyadic association times with males in parties containing MS was 243 h for periods of maximal swelling. For parties without MS, this figure was only 12 h. Out of 88 potential adult male/estrous female dyads, 45 were never observed in the absence of MS, and 31 were seen together for less than 8 h. The remaining 13 dyads all included either AL or NL (the two females with the largest sample of cycles), who were observed with other males in MS’s absence for an average of 70 h per dyad. Looking at the data from these females, a strong effect of the alpha’s presence on mating behavior is evident, for both male-initiated and female-initiated copulations. Consistent with the male coercion hypothesis, individual males were solicited by AL and NL at significantly higher rates in parties where MS was absent (0.039 ± 0.016 times per hour) than in parties where he was present (0.007 ± 0.002 times per hour; Wilcoxon signed-rank test: Z = −2.366, p = 0.018, n = 8 males; Fig. 4). In turn, males solicited AL and NL at significantly higher rates in parties without MS (0.317 ± 0.126 times per hour) than in parties with him (0.006 ± 0.002 times per hour; Wilcoxon signed-rank test: Z = −2.366, p = 0.018; n = 8 males).
Across the adult males, both female-initiated (a) and male-initiated (b) copulation rates were higher when the alpha male, MS, was absent (black bars) than when he was present (white bars). Includes 13 dyads observed together for at least 25 h during maximal swelling. Females represented are AL and NL. All periods of maximal swelling in non-pregnant cycles are included. Note the different scales on the axes. White bars in (b) are not 0, but indicate (left to right) rates of 0.005, 0.006, 0.015, 0.003, 0.005, 0.002, and 0.006
Discussion
Our study is the first research on female choice in wild primates to rigorously test for the confounding effects of male aggression on female behavior. Using a larger pool of male mating partners than previous studies, and incorporating more female cycles, we found that female copulatory approaches in chimpanzees are not consistent with unfettered female choice, but instead appear constrained by persistent coercive aggression from males. All the females in our sample showed elevated rates of periovulatory proceptivity toward the alpha male (MS), who became dominant in 1997 and maintained his position through the end of this study in 2006. Each female solicited between one and five additional males at high rates during the POP. The identity of solicited males differed by female, and male rank did not appear to be an important criterion for selection. Although universal proceptivity toward the alpha male might ostensibly support a model of female choice for good genes, and the idiosyncratic distribution of proceptivity toward other males could fit with a model of choice for genetic compatibility, additional observations favor the alternative hypothesis that patterns of female proceptivity primarily reflect male sexual coercion (Wrangham and Muller 2009).
First, the males who were most aggressive toward individual females, not only during periods of maximal swelling, but also in contexts not directly related to mating, were the ones most frequently solicited by those females during the POP. This result explains the systematic bias toward the alpha male since, compared to other males, he showed high levels of aggression to all of the females in our sample (Muller et al. 2009a). The present data cannot fully distinguish whether this bias occurs (1) because aggressive males compel females to solicit them more than they would have otherwise, (2) because aggressive males receive a higher relative share of solicitations by reducing the probability that a female will solicit other males, or (3) both. However, the fact that females increased their solicitation rates of all males in the absence of the most aggressive male supports the occurrence of indirect coercion (i.e., coercive mate guarding).
Second, patterns of female-directed aggression by the alpha male and other approached males, during periods of maximal swelling, challenge the notion that females are actively concentrating paternity. If, for example, females are eager to bias conceptions toward the alpha male, and the alpha male is concerned with paternity certainty, then the interests of the pair should be aligned during the POP. Consequently, the alpha male should be less aggressive toward females as ovulation approaches, and the females become more compliant, mating primarily with him. The fact that the alpha male at Kanyawara continued to show high rates of female-directed aggression in periods immediately preceding ovulation indicates a conflict of interest. The existence of such conflict suggests that females were resistant to the alpha’s mate-guarding efforts, and that they were interested in mating with additional males. This interpretation is further supported by the fact that females showed increased rates of male solicitation when the alpha male was absent. A similar argument applies generally to approached males, who also showed a steady rate of aggression throughout the period of maximal swelling toward the females who solicited them most frequently.
Although it is possible that males may simply differ in their overall propensity for aggression, and be incapable of modulating their behavior in response to female compliance or resistance, this seems unlikely for two reasons. First, previous studies from Kanyawara have shown that male aggression is elegantly tailored to context. Males are more aggressive toward attractive, parous females than they are toward subfecund, nulliparous females (Muller et al. 2007). Males aggressively interfere in copulations at higher rates in the POP than the non-POP, and exhibit elevated rates of male–male aggression in conceptive vs. nonconceptive cycles (Emery Thompson and Wrangham 2008). Finally, the alpha male, MS, is less likely to aggressively interfere in copulations involving his male allies, than in those involving non-allies (Duffy et al. 2007). Thus, if females are amenable to being mate-guarded by males, there is no reason to suppose that males should not be capable of tempering their aggression in reply.
Second, with the exception of the alpha, no individual male in the study was generally aggressive toward all parous females. Males showed variable rates of aggression across potential mating partners, clearly singling out individual females for special consideration. Why a male should focus his coercive efforts on a particular female or females is not clear, but the fact that across male–female dyads, total copulation rates during the POP and rates of male aggression during cycling were correlated, suggests that the strategy is a successful one.
Although our data are consistent with the idea that male aggression limits female promiscuity over the long-term, this idea is difficult to test directly. Evidence for such a dynamic in hamadryas baboons is more straightforward, because male–female relationships can be tracked from their inception, and it is clear that male aggression decreases once females reliably maintain proximity and avoid other males (Swedell and Schreier 2009). The current chimpanzee study followed ongoing, long-term relationships, so there was no way to show a direct decrease of female promiscuity in response to male aggression. However, the fact that females showed increased proceptivity in the absence of the most aggressive male suggests a distinct dynamic from that of hamadryas, in which females are resistant to male mate guarding. Future studies will examine the evidence for coercive mate guarding more directly by tracking the development of specific male–female relationships from adolescence.
Why should a female be resistant toward the mate-guarding efforts of a high-ranking male like the alpha? One possibility is that the cost of acquiescence is high, if it invites intense efforts from other males at direct sexual coercion in the form of intimidation and harassment. Another is that the benefits provided by high-ranking males are few. Male chimpanzees provide little or no direct paternal care. And because female chimpanzees frequently travel alone or in small groups, they regularly encounter potentially infanticidal males in the absence of the alpha (Clarke et al. 2009). Consequently, even high-ranking males may not be able to offer reliable protection from infanticide. The most likely potential benefit to females of biasing paternity toward high-ranking males in fission–fusion species is therefore “good genes.” Whether such benefits ever outweigh the risk of infanticide inherent in any attempt to actively concentrate paternity in a single male is an open question.
Furthermore, females could conceivably gain the same genetic benefits under a scenario of passive choice, whereby the “best-male” (Clutton-Brock and Harvey 1976) emerges from the conclusion of male–male competition, mate guarding, and sperm competition. Evidence from our site supports the hypothesis that male chimpanzees both detect and respond to changes in female conception risk without behavioral cues (for Taï see Deschner et al. 2003), and that shifting mating dynamics over the cycle can be accounted for primarily by shifts in the competitive investment and solicitation behavior of high-ranking males (Emery Thompson 2005; Emery Thompson and Wrangham 2008). Even at Taï, where female choice has been argued to be particularly important (Stumpf and Boesch 2005, 2006), long-term paternity data show a precise fit with the predictions of the priority-of-access model, which posits male dominance status as the primary determinant of mating access (Boesch et al. 2006).
The assumption that female chimpanzees should subtly try to realize secret preferences for chosen males makes sense from an anthropocentric perspective, given that women express obvious preferences for certain men over others. However, there is little evidence that chimpanzee females evince this type of mating psychology, or that it would provide a clear evolutionary benefit if they did. If the result of male–male competition for mates, sperm competition, and effective mate guarding were a reliable indicator of male quality in chimpanzees, then attempts by females to thwart these mechanisms via active mate choice would make little sense. And although females might reinforce these mechanisms through active choice, such a strategy would appear not only superfluous, but, in the face of persistent infanticide risk, dangerous. We do not suggest that females are passive players in the chimpanzee mating game. Rather, we acknowledge the possibility that female agency is directed primarily at maximizing offspring survival through a strategy of paternity confusion, and that the evolved mating psychology of female chimpanzees is profoundly different from that of human females.
The Kanyawara data are thus consistent with either of two conclusions: (1) active female choice is absent, and females instead pursue a strategy of unbiased promiscuity to confuse paternity; (2) female mate preferences exist but are constrained by male–male competition and sexual coercion in this male-dominant species. The data do not support a “mixed” strategy in which females attempt to concentrate paternity in preferred males. Although our study was entirely observational, our results are consistent with the one experimental study conducted on a promiscuous primate (Macaca fascicularis) that gave females complete control over access to males, thus reducing the potential for male coercion (Nikitopoulos et al. 2005). In that study, no effect of cycle phase on female preferences was evident, as females apportioned their mating choices to spread copulations evenly across all the males in their social group. In wild studies, female preferences are not so easily isolated from the effects of male aggression. Because such aggression is widespread in primates (Muller and Wrangham 2009) and other mammals (Clutton-Brock and Parker 1995), the potential for male coercion must be taken into account before mating preferences can be inferred from female behavior.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behav 49:227–267
Andersson M (1994) Sexual Selection. Princeton University Press, Princeton
Arcadi AC, Wrangham RW (1999) Infanticide in chimpanzees: review of cases and a new within-group observation from the Kanyawara study group in Kibale National Park. Primates 40:337–351
Boesch C, Boesch-Achermann H (2000) The chimpanzees of the Tai Forest: behavioral ecology and evolution. Oxford University Press, Oxford
Boesch C, Kohou G, Néné H, Vigilant L (2006) Male competition and paternity in wild chimpanzees of the Taï forest. Am J Phys Anthropol 130:103–115
Brewer Marsden S, Marsden D, Emery Thompson M (2006) Demographic and female life history parameters of free-ranging chimpanzees at the Chimpanzee Rehabilitation Project, River Gambia National Park. Int J Primatol 27:321–410
Byers JA, Waits L (2006) Good genes sexual selection in nature. PNAS 103:16343–16345
Clarke P, Pradhan G, van Schaik CP (2009) Intersexual conflict in primates: infanticide, paternity allocation, and the role of coercion. In: Muller MN, Wrangham RW (eds) Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Harvard University Press, Cambridge, pp 42–77
Clutton-Brock T, McAuliffe K (2009) Female mate choice in mammals. Q Rev Biol 84:3–27
Clutton-Brock TH, Harvey PH (1976) Evolutionary rules and primate societies. In: Bateson PP, Hinde RA (eds) Growing points in ethology. Cambridge University Press, Cambridge, pp 195–237
Clutton-Brock TH, Parker GA (1995) Sexual coercion in animal societies. Anim Behav 49:1345–1365
Deschner T, Heistermann M, Hodges K, Boesch C (2003) Timing and probability of ovulation in relation to sex skin swelling in wild West African chimpanzees, Pan troglodytes verus. Anim Behav 66:551–560
Dixson AF (1998) Primate sexuality: comparative studies of the prosimians, monkeys, apes, and human beings. Oxford University Press, New York
Duffy KG, Wrangham RW, Silk J (2007) Male chimpanzees exchange political support for mating opportunities. Curr Biol 17:586–587
Emery Thompson M (2005) Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): methodological considerations and the role of hormones in sex and conception. Am J Primatol 67:137–158
Emery Thompson M, Wrangham RW (2008) Male mating interest varies with female fecundity in chimpanzees. Int J Primatol 29:885–905
Emery Thompson M, Muller MN, Kahlenberg S, Wrangham RW (2010) Social and ecological correlates of stress in wild female chimpanzees. Horm Behav 58:440–449
Goodall J (1986) The chimpanzees of Gombe: patterns of behavior. Harvard University Press, Cambridge
Hemelrijk C (1990) A matrix partial correlation test used in investigations of reciprocity and other social interaction patterns at group level. J Theoret Biol 143:405–420
Hill GE (2006) Female mate choice for ornamental coloration. In: Hill GE, McGraw KJ (eds) Bird coloration: function and evolution. Harvard University Press, Cambridge, pp 137–200
Hrdy SB (1979) Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol Sociobiol 1:13–40
Hrdy SB (1981) The woman that never evolved. Harvard Press, Cambridge
Johnson MH, Everitt BJ (1988) Essential reproduction. Blackwell Scientific Publications, Oxford
Kappeler P, van Schaik CP (2004) Sexual selection in primates: review and selective preview. In: Kappeler P, van Schaik CP (eds) Sexual selection in primates: new and comparative perspectives. Cambridge University Press, Cambridge, pp 3–23
Matsumoto-Oda A (1999) Female choice in the opportunistic mating of wild chimpanzees (Pan troglodytes schweinfurthii) at Mahale. Behav Ecol Sociobiol 46:258–266
Mays HL, Albrecht T, Liu M, Hill GE (2008) Female choice for genetic complementarity in birds: a review. Genetica 134:147–158
Mays HLJ, Hill GE (2004) Choosing mates: good genes versus genes that are a good fit. Trends Ecol Evol 19:554–559
Muller MN (2002) Agonistic relations among Kanyawara chimpanzees. In: Boesch C, Hohmann G, Marchant L (eds) Behavioural diversity in chimpanzees and bonobos. Cambridge University Press, Cambridge, pp 112–124
Muller MN, Emery Thompson M, Wrangham RW (2006) Male chimpanzees prefer mating with old females. Curr Biol 16:2234–2238
Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW (2007) Male coercion and the costs of promiscuous mating for females chimpanzees. Proc Biol Sci 274:1009–1014
Muller MN, Kahlenberg SM, Wrangham RW (2009a) Male aggression against females and sexual coercion in chimpanzees. In: Muller MN, Wrangham RW (eds) Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Harvard University Press, Cambridge, pp 184–217
Muller MN, Kahlenberg SM, Wrangham RW (2009b) Male aggression and sexual coercion of females in primates. In: Muller MN, Wrangham RW (eds) Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Harvard University Press, Cambridge, pp 3–22
Muller MN, Wrangham RW (2004) Dominance, aggression and testosterone in wild chimpanzees: a test of the “Challenge Hypothesis”. Anim Behav 67:113–123
Muller MN, Wrangham RW (2009) Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Harvard University Press, Cambridge
Murray CM, Wroblewski E, Pusey AE (2007) New case of intragroup infanticide in the chimpanzees of Gombe National Park. Int J Primatol 28:23–37
Neff BD, Pitcher TE (2005) Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol Ecol 14:19–38
Nikitopoulos E, Heistermann M, de Vries H, van Hoof JARAM, Sterck EM (2005) A pair choice test to identify female mating pattern relative to ovulation in longtailed macaques, Macaca fascicularis. Anim Behav 70:1283–1296
Nishida T, Kawanaka K (1985) Within-group cannibalism by adult male chimpanzees. Primates 26:274–284
Nunn CL (1999) The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav 58:229–246
Paul A (2002) Sexual selection and mate choice. Int J Primatol 23:877–904
Pieta K (2008) Female mate preferences among Pan troglodytes schweinfurthii of Kanyawara, Kibale National Park, Uganda. Int J Primatol 29:845–864
Roof KA, Hopkins WD, Izard MK, Hook M, Schapiro SJ (2005) Maternal age, parity, and reproductive outcome in captive chimpanzees (Pan troglodytes). Am J Primatol 67:199–207
Royston J (1982) Basal body temperature, ovulation, and the risk of conception, with special reference to the lifetimes of sperm and egg. Biometrics 38:397–406
Schwensow N, Eberle M, Sommer S (2008) Compatibility counts: MHC-associated mate choice in a wild promiscuous primate. Proc Biol Sci 275:555–564
Setchell JM (2005) Do female mandrills prefer brightly colored males. Int J Primatol 26:715–735
Sicotte P (1993) Inter-group encounters and female transfer in mountain gorillas: influence of group composition on male behavior. Am J Primatol 30:21–36
Smuts BB, Smuts RW (1993) Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv Study Behav 22:1–63
Stumpf R (2004) Female Reproductive Strategies in Chimpanzees of the Taï Forest, Côte d’Ivoire. Ph.D. dissertation. Anthropology. Stony Brook University, Stony Brook, NY
Stumpf R, Boesch C (2005) Does promiscuous mating preclude female choice? Female sexual strategies in chimpanzees (Pan troglodytes verus) of the Taï National Park, Cote d'Ivoire. Behav Ecol Sociobiol 57:511–524
Stumpf R, Boesch C (2006) The efficacy of female choice in chimpanzees of the Taï Forest, Côte d’Ivoire. Behav Ecol Sociobiol 60:749–765
Swedell L, Schreier A (2009) Male aggression toward females in hamadryas baboons: conditioning, coercion, and control. In: Muller MN, Wrangham RW (eds) Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Harvard University Press, Cambridge, pp 244–268
Tutin CEG (1979) Mating patterns and reproductive strategies in a community of wild chimpanzees. Behav Ecol Sociobiol 6:39–48
van Noordwijk MA, van Schaik CP (2000) Reproductive patterns in eutherian mammals: adaptations against infanticide? In: van Schaik CP, Janson CH (eds) Infanticide by Males and Its Implications. Cambridge University Press, Cambridge, pp 322–360
van Schaik CP, Hodges JK, Nunn CL (2000) Paternity confusion and the ovarian cycles of female primates. In: van Schaik CP, Janson CH (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge pp 361–387
van Schaik CP, Janson CH (2000) Infanticide by males and its implications. Cambridge University Press, Cambridge, p 569
van Schaik CP, Pradhan GR, van Noordwijk MA (2004) Mating conflict in primates: infanticide, sexual harassment and female sexuality. In: Kappeler P, van Schaik CP (eds) Sexual selection in primates: new and comparative perspectives. Cambridge University Press, Cambridge, pp 131–150
Wilcox AJ, Weinberg CR, Baird DD (1995) Timing of sexual intercourse in relation to ovulation: effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 333:1517–1521
Wrangham RW (2002) The cost of sexual attraction: is there a tradeoff in female Pan between sex appeal and received coercion? In: Boesch C, Hohmann G, Marchant L (eds) Behavioural diversity in chimpanzees and bonobos. Cambridge University Press, Cambridge, pp 204–215
Wrangham RW, Muller MN (2009) Sexual coercion in humans and other primates: the road ahead. In: Muller MN, Wrangham RW (eds) Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Harvard University Press, Cambridge, pp 451–468
Zinner DP, Nunn CL, van Schaik CP, Kappeler PM (2004) Sexual selection and exaggerated sexual swellings of female primates. In: Kappeler P, van Schaik CP (eds) Sexual selection in primates: new and comparative perspectives. Cambridge University Press, Cambridge, pp 71–78
Acknowledgments
Research at Kibale was supported by grants from the US National Science Foundation (awards 9807448 and 0416125), the L.S.B. Leakey Foundation, the National Geographic Society, and the Wenner-Gren Foundation. For sponsoring long-term research in Kibale National Park, we thank the Uganda Wildlife Authority and Makerere University Biological Field Station. We thank Gilbert Isabirye-Basuta, John Kasenene, and Jerry Lwanga for their continuing support, and Emily Otali for research oversight. For assistance in the field, we thank the late John Barwogeza, the late Joseph Basagara, Deo Kateeba, Christopher Katongole, Francis Mugurusi, the late Donor Muhangyi, the late Christopher Muruuli, Solomon Musana, Dennis Sebugwawo, and Peter Tuhairwe. Special thanks to Zarin Machanda for assistance with the KCP database.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Silk
Rights and permissions
About this article
Cite this article
Muller, M.N., Thompson, M.E., Kahlenberg, S.M. et al. Sexual coercion by male chimpanzees shows that female choice may be more apparent than real. Behav Ecol Sociobiol 65, 921–933 (2011). https://doi.org/10.1007/s00265-010-1093-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-1093-y