Abstract
Analyses of social structures in baleen whales are rare, and so far, they are thought to consist of mostly short and unstable associations. We investigated the association patterns of individual humpback whales from a summer feeding aggregation in the Gulf of St. Lawrence from 1997 to 2005. Photo-identified animals were sexed using genetic methods and were grouped into five categories: juvenile males/females, mature males and lactating/non-lactating females. We calculated half-weight association indices within and between the groups and found that 45% of the observation showed single animals and another 45% small groups (two to three) consisting mainly of mature animals besides lactating females. Using permutation tests, we found evidence for long-term associations between mature males and non-lactating females as well as among non-lactating females. Standardised lagged association rates revealed that these male–female groups disassociated quickly over about 2 weeks, whereas associations increased again towards the beginning of the breeding season. Non-lactating females of similar age engaged in multi-seasonal stable pairs for up to six consecutive feeding seasons; no mature male–female association was observed in consecutive years. The females with the most stable and long-term associations also had the highest reproductive output. While the risk of predation could not explain these long-term bonds, feeding cooperation seemed the most plausible explanation for group forming behaviour during the summer months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sociality of animals is based upon interactions between individuals in a population (Hinde 1976) and affects access to resources and mates (Wilson 1975), individual fitness (Silk et al. 2003; Marcoux et al. 2007), spatial distribution and segregation (Connor et al. 1998; Conradt 1999) as well as gene flow (Altmann et al. 1996; Storz 1999) and information transfer (McComb et al. 2001, 2003). Many behavioural studies of mammals have focussed on the interactions between males and females during the breeding season (Jarman 1974; Tyack and Whitehead 1983; Fabiani et al. 2006) in order to understand the influence of ecology in the evolution of sociality (Rubenstein and Hack 2004). This emphasis on the mating system can mask important aspects of the social structure of animals (Rubenstein and Hack 2004), especially in species with strong and distinct seasonal cycles. Studies outside the mating season have often found same-sex groups (e.g. Clutton-Brock et al. 1982; Conradt 1999; Ruckstuhl and Neuhaus 2000, 2002), which were explained as a by-product of habitat segregation (Clutton-Brock et al. 1982), but Conradt (1999) showed that they are independent.
Several hypotheses on the evolution of sociality outside breeding seasons have been proposed, predation risk and food distribution being the most popular ones (e.g. Wrangham 1980; Boinski 1988; Krause and Ruxton 2002). For example, group size and composition are often regulated by these two factors for ungulates (Rubenstein and Hack 2004; Shi et al. 2005) and primates (Wrangham 1980; van Schaik 1989; Mitchell 1991; Barton et al. 1996). These factors seem to primarily influence female behaviour and distribution, which in turn determine male behaviour and distribution (Wrangham 1979; Kappeler and van Schaik 2002). Female alliances increase infant survival in primates (Sterck et al. 1997; Silk et al. 2003; Silk 2007), in some cetaceans (Whitehead 1996; Connor et al. 1998) and elephants (Wittemyer et al. 2005). Cooperative hunting (e.g. lions Panther leo; Packer et al. 1990) and defending highly productive food patches are further selective forces favouring group living in ungulates and primates (e.g. Wrangham 1980; Mitchell 1991; Barton et al. 1996).
Studies on primates and ungulates dominate the literature on sociality, but the structure of cetacean societies has received growing attention in recent years (see Mann et al. 2000), although it has mostly focussed on toothed whales (Odontoceti). Among the cetaceans, baleen whales (Mysticeti) are notoriously difficult to study; coupled with their primarily offshore habitat, vast annual migrations can result in multiple study sites hindering holistic coverage of life histories. So far, most studies have been descriptive and focussed on group size and composition (Weinrich 1991; Weinrich and Kuhlberg 1991; Clapham 1993; Sears et al. 1999; Bérubé et al. 2001) and in the case of the humpback whale (Megaptera novaeangliae) on the analysis of kinship within the observed groups (Valsecchi et al. 2002; Weinrich et al. 2006). However, most studies concluded that the social organisation in baleen whales is mostly restricted to small unstable groups with few exceptions (Perry et al. 1990; Weinrich 1991).
The humpback whale is a medium-sized balaenopterid whale (Chittleborough 1965), with females being larger than males (Lockyer 1984). Their maximum life expectancy was estimated to be 48 years (Chittleborough 1965), but this is likely an underestimation. They reach sexual maturity at age 5 to 12 (Clapham 1992; Gabriele et al. 2007), and females give birth on average every 2 to 3 years (Clapham and Mayo 1987). Humpback whales spend the summer months foraging at mid- and high-latitude feeding grounds, while mating and calving occur at common low-latitude breeding grounds during the winter months, where prey is virtually absent (Chittleborough 1965; Dawbin 1966; Baker and Herman 1984b; Katona and Beard 1990). Their migration between those sites is one of the longest recorded for mammals (Stone et al. 1990). Humpback whales exhibit strong site fidelity to specific feeding regions, with calves being recruited to these areas during the maternal care period of approximately 11 months (Clapham and Mayo 1987; Katona and Beard 1990). Humpback whales display a polygynous mating system (Baker and Herman 1984b; Cerchio et al. 2005), in which males fight to gain access to sexually mature females (Tyack 1981; Tyack and Whitehead 1983; Baker and Herman 1984b). Associations in the breeding areas are described as brief, where the only stable groups reported are cow and calf pairs (Mobley and Herman 1985; Mattila et al. 1994). On the feeding grounds, the social organisation has been described as consisting of small unstable groups (Clapham 2000), which are non-competitive and, during foraging, perhaps even cooperative (Whitehead 1983; Baker and Herman 1984a; Weinrich 1991; Weinrich and Kuhlberg 1991; Clapham 1993).
In contrast to the breeding grounds and during migration, where a male-biased sex ratio is observed (Brown et al. 1995; Palsbøll et al. 1997) and juveniles are under-represented (Robbins 2007), all demographic classes are found in the Gulf of St. Lawrence (GSL, QC, Canada). Humpback whales in the St. Lawrence occur in a large aggregation with most animals being observed within a relatively small area. Several studies found a segregation of females with calves (Steiger and Calambokidis 2000; Robbins 2007), with juveniles often foraging at the periphery of aggregations (Weinrich et al. 1997). Doniol-Valcroze (2008) found no evidence of sexual segregation among GSL individuals, with males and females selecting areas with identical habitat characteristics.
Here, we present the results of a 9-year study of humpback whales summering in the GSL. We analysed their association patterns to determine social relationships between different age and sex classes, with emphasis on the temporal scale of associations. Using recent developments in tools to analyse social structure (Whitehead 2008), we estimated association indices for different age–sex classes over the study period and tested if animals formed long-term associations. We quantified the duration of these long-term associations not only within single seasons but also for the entire study period. We were able to estimate for the first time the temporal scale of relations during the feeding season and challenge previous results reporting mostly unstable short-term association in this species (Whitehead 1983; Weinrich and Kuhlberg 1991; Clapham 1993).
Methods
Data collection
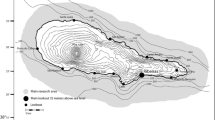
We collected photos of humpback whales during photo-identification surveys between June and October 1997 to 2005 in the Jacques Cartier Passage in the Gulf of St. Lawrence (Fig. 1). The average annual effort over that period was 87 observation days with 580 h of observation covering approximately 10,000 km of track lines (Fig. 1). We photo-identified humpback whales using the ventral pigmentation on the tail and the shape of the dorsal fin (Katona and Whitehead 1981). Surveys were designed to maximise the photo-identification effort, and we did not follow groups or individuals after the identification pictures were taken. We used skin sample biopsies to determine the sex of the identified individuals (Bérubé and Palsbøll 1996). All females seen with a calf were also sexed using biopsy techniques.
Definitions
Group
We defined a group as animals within two body lengths, coordinating their swimming and diving behaviour for at least one surfacing, following previous studies (Baker and Herman 1984a, b; Mattila and Clapham 1989; Weinrich and Kuhlberg 1991; Clapham 1993). The effort to obtain high quality photo-identification pictures varied considerably, depending on size, composition and behaviour of the group. Thus, we did not use the time span during which animals were observed together since it is more a measure of how much time we needed to obtain photos and biopsies rather than an independent measure of temporal association. We counted each unique association/group once per day, regardless of how long they were observed on this particular day. We applied a 1:0 association matrix for every day, in which animals matching the above group definition were assigned a value of 1 and 0 if they did not. This resulted in 245 daily association matrices, marking associated and non-associated individuals sighted for each day. The use of spatial proximity to define associations, as in all other mentioned studies, is likely to be an underestimation of social affiliations.
Age–sex class
We defined an animal as being mature when it was known for at least 5 years and juvenile when it was known for less than 5 years following previous studies (Weinrich and Kuhlberg 1991; Clapham 1993). Our study on humpback whales started in 1982, and therefore, most animals were known prior to 1997. We also regarded females as mature when they were sighted with a calf. Applying these definitions, 11 animals changed from juveniles to adults during the study period, and we adapted the change in the data set.
We further distinguished between lactating and non-lactating females. We defined a lactating female as a large animal accompanied consistently by a smaller one (approximately 1/3 of the larger animal) during the entire season. In one case, a known female was observed several times with a dependent calf until mid-August but then sighted alone in mid-September. This appears to be too early for weaning compared to other studies (Baraff and Weinrich 1993), and we presumed that she had lost the calf. We counted the female as lactating until her last August sighting with the calf and non-lactating for the subsequent sightings without the calf.
The distinction between sex, age and reproductive status resulted in five age–sex classes: juvenile females (JF) and males (JM), mature lactating females (LF), non-lactating females (NF) and mature males (M). Two females were only seen lactating, while 14 mature females were never observed with a calf. Twenty-four females were sighted in both states (see Table 3 for complete sample size).
Cohort groups
We pooled animals to cohorts, to test whether there is any preference to associate with animals of similar age range. We defined a cohort as all animals seen initially in the same year. We did not know whether animals sighted for the first time in the same year were of the same age unless they were sighted as a calf, but we assumed that they were of similar age. We used all sighting histories of the observed animals since 1982.
Analysis
Association index
We calculated the mean and maximum association indices for the entire study period for between and within the age–sex classes and tested for significant differences using a Mantel test. We used the half-weight association index (Cairns and Schwager 1987) since we did not identify every single associate of an individual during all encounters. This index is calculated as follows:
where x equals the number of encounters in which whales a and b were seen together, y ab equals the number of encounters in which whales a and b were seen, but not associated, and y a/y b equals the number of encounters in which whales a or b was seen alone. This index is commonly used in cetacean studies (e.g. Bejder et al. 1998; Gowans et al. 2001; Ottensmeyer and Whitehead 2003).
Testing for preferred/avoided companionship
We used permutations to test for preferred/avoided companions among individuals between sampling periods (days). The observed association data were compared to random (permutated) data following the procedure of Bejder et al. (1998) with the extension of Whitehead (1999) and the correction of Krause et al. (2009). The starting matrix listed the observed data including all sampling periods (days) of the 9 years of data. A sampling interval (day) was randomly chosen and two pairs of individuals (two associations) were arbitrarily selected of that day, so that each individual of a pair was associated with only one individual of the other pair. The four assignments were flipped, while keeping the total number of associations and individuals constant. The procedure was conducted 1,000 times per permutation. The association matrix permutated in this way is not independent, but if repeated sufficient times, the test statistics are equivalent to independent random permutations (Manly 1995). We achieved this by conducting the test after 1,000 permutations and increased the number of permutations (3,000, 5,000) until the p value of the test statistics stabilised (Bejder et al. 1998). Animals associating more often than the average dyad have a higher association index than the mean, while dyads associating rarely have a lower (close to zero) association index. Both deviations inflate the standard deviation (SD) of the mean association index. Thus, if the SD of the association index of the observed data was significantly larger (at the 0.05 level) than the SD of the permutated data, then the null hypothesis of no preferred/avoided companionship between sampling periods was rejected (Whitehead 1999). We tested only for preferred companionship between 1-day sampling periods since sampling intervals should be short, so that movement in and out of the area is unlikely during one sampling interval (Whitehead 1999). To investigate the temporal aspect of such long-term companionships, we conducted a temporal analysis for the significant age–sex class combinations from the permutation tests.
Temporal association patterns
We used lagged association rates (LAR) to estimate the probability that two individuals seen together at any given time were still together at a certain time lag (τ) later (Whitehead 1995). The null association rate showed the expected LAR, given random associations. We standardised both rates by dividing them by the number of associations per occasion since not all associates of an individual could be identified in each sampling period. Jackknife techniques were used to estimate the precision of the standardised lagged association rate (SLAR). We used the results of the permutation tests (preferred/avoided companionship) to plot a SLAR of the permuted (random) data. The comparison between SLAR of the random and SLAR of the observed data basically visualises the test result for preferred companionship, enabling us to determine the significant temporal scale of the companionship (Whitehead 2007). The temporal pattern within a feeding season was analysed by restricting τ to 150 days and over the entire study period leaving τ unlimited.
We applied several models describing various temporal association patterns to the complete and parts of the data set using maximum likelihood techniques (Online Resource 1). Model selection was based on the Quasi-Akaike Information Criterion (QAIC; Burnham and Anderson 2002). We used the programme SOCPROG 2.4 (http://whitelab.biology.dal.ca/index.html) to calculate association indices, conduct the test for preferred/avoided companions and plot the standardised lagged association rates.
Results
Group size and frequency
We identified 130 humpback whales in the study period, including 39 calves. We disregarded the latter ones in their year of birth. Altogether, 26 females were observed with a total of 50 calves (both identified and unidentified). The mean calving interval using consecutive calving intervals was 3.5 years. Twenty-six males and 50 females were sexed, excluding animals seen only as calves. Applying the definition of age and reproductive stage, the data included 17 juvenile females, nine juvenile males, 26 lactating females, 38 non-lactating females and 20 mature males.
The average group size observed was 1.87 (SD 1.03) and the median 2. We observed singles most often, followed by pairs and trios, together accounting for over 90% of the observed groups (Table 1). The larger the group size, the higher was the proportion of cow and calf pairs. The mean group size was the highest in September (Fig. 2), corresponding to the peak of relative abundance of humpback whales in the area (Ramp 2008).
Association indices and preferred companionship
We detected a great variation in the association indices within and between the age and sex classes with the highest association values between NF and NF and between M and NF (Table 2). The mean values were positively correlated with the different number of associations (r = 0.91). Juveniles of both sexes associated little with any other age–sex class. When comparing juvenile (n = 36) and mature (n = 67) animals, including animals of unknown sex, mature animals had a higher mean association index within their class than juvenile animals (0.0159 to 0.0017). LF associated less often with mature animals of either sex, compared to NF. The association indices between and within the sexes varied over the season (Fig. 2). While the association index remained on a similar level among females, the value for males–females and among males increased towards the end of the season. This indicates an increased level of gregariousness of males in fall (Fig. 2), although the SD of the estimates were very large (not shown in Fig. 2 for clarity).
The high (maximum association) values between NF and NF and between M and NF showed that the preferred associate for mature males as well as for mature females was usually another mature female (Table 2). The values between the other age–sex classes were lower and did not differ considerably from each other. The sole exception was the higher association index within JF, indicating that their preferred associate was found among other juvenile females.
The test for preferred companionship between sampling periods within/between juvenile sex classes and between juvenile/mature sex classes could not be computed due to a lack of data (associations). Pooling all juveniles and mature animals, we found no evidence for companionship between juvenile and mature humpback whales (SD observed data 0.022, random data 0.022, p = 0.42). Lactating females did not form any preferred companionship with any other mature sex class. The test within M was also non-significant (SD observed data 0.027, SD random data 0.027, p = 0.64). The null hypothesis of no preferred/avoided companionship was only rejected between M and NF (observed 0.044, random 0.040, p < 0.01, 5,000 permutations) and within NF (observed 0.044, random 0.039, p < 0.01, 5,000 permutations).
Temporal association patterns
Short-term associations
The SLAR between non-lactating mature females (NF–NF) was higher than the null association rate for the entire season. It was characterised by a rapid disassociation during the first 10 days and became stable after approximately 20 days (Fig. 3a). The model ‘casual acquaintances + constant companions’ fitted the SLAR well and had the lowest QAIC. NF exhibited short-term associations lasting days to 3 weeks followed by constant companions over their entire residence time during a season. The intercept of the SLAR (model) with the y-axis is the reciprocal of the number of casual acquaintances. Setting τ to zero, the reciprocal of the intercept was 2.3. The level at which the SLAR stabilised can be seen as the proportion of animals staying with the individual, i.e. the number of constant companions. This was 0.142 or 32% of the intercept, resulting in 0.8 constant companions. Adding the individual itself, we obtained the unit size of constant groups, which was 1.8, corresponding to a pair of humpback whales.
The short-term associations between M and NF showed a different pattern (Fig. 3b). The SLAR declined sharply over the first 10 days and then increased again until the end of the observed time lags. No available model described the SLAR adequately. Most associations between M and NF dissolved rapidly over the first 10 days; only few remained constant over the entire time of the animals’ residency. In addition, re-associations occurred towards the end of the season, corresponding to the increased gregariousness of males in fall (Fig. 2). Without a model, it was difficult to determine group size, but given the approximate level of the intercept of the SLAR, M were usually found in larger groups with several NF.
Long-term associations
The SLAR between NF and NF (Fig. 4a) was higher than the null association rate for a lag of approximately 1,800 days. The SLAR showed disassociations after the first season at around 100 days, although we did not possess any data between seasons (winter months), roughly 120–300 days. The SLAR then stayed relatively constant (with some variation) until another sharp drop at around 1,000 days. Associations became random at around 1,800 days. Thus, some NF engaged in longer-lasting associations exceeding a season. These long-term associations lasted up to 6 years but most disassociated after about 3 years. The model ‘two levels of casual acquaintances’ had most support, but the standard errors of the parameters were enormous, thus giving little credibility to this model and were not shown here (Fig. 4a).
SLAR with 95% jackknife estimates over entire study period with random data SLAR and null association rate. a Within non-lactating females. b Between non-lactating females and mature males with best fitting model: casual acquaintances + constant companions (ca + cc) [a1 0.011446 (SE 0.17), a2 = 0.04289 (SE 0.032), a3 = 0.19007 (SE 0.09)]
The SLAR between M and NF (Fig. 4b) dropped sharply after the first season and almost reached the null association rate after approximately 1 year (∼360 days) and remained at that low level. The best-fitting model was ‘casual acquaintances + constant companions’. The number of casual NF acquaintances of M was 4.3, while the constant unit size was 1.7, but the standard errors of the estimated parameters were disproportionably high. The constant level was very close to the null association rate. In addition, the random data SLAR followed the distribution of the observed data SLAR over the entire time lag, but it became almost identical after 300 days. Thus, the constant companion after the first season could be due to chance and associations between M and NF lasted only from days to weeks within one season.
Cohort effect in the long-term associations between non-lactating females
We observed long-term bonds of NF over several seasons and investigated whether the associations were random among all NF. The individual association matrix of all NF (not shown) showed dyads with higher than average values along the diagonal axis. This indicated a preference towards females known for a similarly long time since individual identification numbers were given in a chronological order. Due to variable and small sample size of the cohorts, we pooled five cohorts to one cohort group (Online Resource 2), resulting four groups: 1982–1986 (N = 7), 1987–1991 (N=11), 1992–1996 (N = 8) and 1997–2001 (N = 10). The last cohort group was disregarded since only two new mature females were identified. The mean association indices varied significantly between and within cohort groups (Mantel test: t = 6.44, p < 0.001), with higher association indices within cohort groups (Table 3). The maximum association was usually within the same cohort group, indicating that a female’s preferred associate was within her cohort group.
Following the above results, we hypothesised that females formed long-term bonds with animals within the same cohort group. We analysed the SLARs between and within cohort groups (Fig. 5). We could not plot SLARs for all cohort group combinations due to small sample sizes, resulting from the subdivision of the data set. The SLARs within the same cohort group were all consistently above their corresponding null association rates for the entire time lag, lasting in the extreme up to 1,800 days (Fig. 5a). The SLARs between different cohort groups were all above the null association rate for the first season and then dropped below it (Fig. 5b). Hence, the long-term companions of NF were other females from the same cohort group. This effect was the strongest for the older cohort groups. The two oldest cohort groups were also the most regular animals, being sighted almost 8 years in the 9-year study period (Table 4). In addition, the oldest cohort group contained the most reproductive active females, with 2.5 calves over study period (Table 4).
Discussion
The results presented in this study provide evidence that the social structure of humpback whales is more complex than previously thought. While many associations between identified individuals were short-term as previously reported (Clapham 2000), we found long-term relationships between mature humpback whales lasting from a few days to several weeks and, in the case of non-lactating females, up to 6 years. To our knowledge, this is the first study to quantify multi-year associations within any baleen whale species.
Although large groups occurred, the most common and stable groups were pairs of individuals, containing either two females or one male and one female. These pairs occurred within large summer aggregations. Due to their vocal capabilities (Payne and Webb 1971), communication likely occurs over distances larger than the spatial proximity used to define groups here. However, the level of communication and the information exchange within the aggregation is unknown, and potential benefits and constraints are difficult to measure. Thus, we focussed here on the relationships between single individuals based on their spatial proximity.
Demographic structure in association patterns
The observed association patterns were different among demographic classes in the population. As observed on the breeding and other feeding grounds, lactating females were less social than non-lactating females and did not associate much with any other age–sex class besides their own calf (Baker and Herman 1984b; Clapham and Mayo 1987; Sardi et al. 2005). Juveniles associated less in general, and male–male associations were only very brief (less than a day), similarly to what is observed in the Gulf of Maine (Weinrich 1991; Clapham 1993). The estimation of SLARs enabled us to show that males engaged more often in associations with females at the end of the feeding season. Non-lactating females of similar age associated over several summers and formed stable bonds, while mature males and mature females formed stable associations only within a single feeding season. Female–male pairs were also the associations most often observed in the Gulf of Maine (Weinrich and Kuhlberg 1991; Clapham 1993). While Weinrich (1991) reported stable female pairs, with few re-associating over more than 1 year, we showed that some females in the Gulf of St. Lawrence had a preferred female partner of similar age with which they associated up to at least 6 years. The average duration of such association was around 1,000 days, in agreement with the observed calving interval of 3.5 years.
Validation of assumptions
We do not know whether non-lactating females stayed together between feeding seasons, but this appears unlikely. The association pattern of humpback whales on their breeding grounds is very different (Tyack and Whitehead 1983; Baker and Herman 1984b; Mattila et al. 1994); female–female associations are extremely rare (Clapham 2000; but see Pomilla and Rosenbaum 2006), and the grouping pattern during migration resembles more that on the breeding grounds (Brown and Corkeron 1995). Thus, it appears that long-term female associations lasted only for the summer months. This would indicate a split in fall and a subsequent re-association in the following feeding season, raising questions about when and where these females pair up again as well as the ability for individual recognition. However, during migration, the animals might not required to swim in close proximity, hence fulfilling the usual definition of group, but might be still associated or in contact over greater distances. These questions are beyond the scope of this analysis, and additional data during migration are required to answer them in the future.
The preferred associate of a female was usually another female known in our study for a similar time period. The assumption that they are of similar age is debatable. Animals could have had a higher chance of being sighted together in the year they were sighted initially since they were both present. This first year could bias the association indices. Since the majority of females were sighted initially many years prior to the study period, we regard this bias as unlikely. Moreover, alternative age clusters (pooling four or six cohorts) revealed similar results (not presented here).
Modelling temporal association
The SLAR enabled us to quantify the durations of associations between and within different age–sex classes for the first time for a baleen whale species. However, the analysis of temporal associations requires a large amount of data, and therefore, it could not be applied to all age–sex or cohort classes/groups. Moreover, if certain classes/groups do not associate often in general, it is apparent that the duration cannot be estimated and no pattern determined. The available models under SOCPROG (Online Resource 1) did not fit the data adequately with one exception (Fig. 3a). Casual acquaintances and constant companions and the combination of the two definitions did not describe the observed association patterns, especially between NF and M. There are likely more factors shaping the temporal association patterns between individuals and classes. In addition, some of our definitions (e.g. the discrimination between mature and juveniles animals) could have been biased and therefore affected the SLAR. More precise data on the age of individuals will help to make such definitions more precise.
Potential benefits of group forming in humpback whales
Group forming in animals occurs only if the benefits outweigh the costs (Alexander 1974) and is often a result of predation, mating, cooperation, competition or kinship (Alexander 1974; Wrangham 1980). Here, we discuss each of these factors as a potential explanation for the association patterns in St. Lawrence humpback whales, focussing on stable associations within and between feeding seasons.
Predation pressure is a significant factor influencing group forming in many animals (Alexander 1974) including cetaceans (Norris and Dohl 1980). Female–female bonds have been shown to increase infant survival in baboons (Papio cynocephalus; Silk et al. 2003), and the protection of calves and juveniles is seen as the predominant reason for the evolution of strong female bonds in sperm whales (Physeter macrocephalus; Whitehead 1996). Killer whales (Orcinus orca) are the only predators of importance to humpback whales and Mysticeti in general (Jefferson et al. 1991). While a considerable proportion of humpback whales (8–22%) bear killer whale teeth marks in the North Atlantic (Mehta et al. 2007), observations of attacks are rare (Whitehead and Glass 1985; Dolphin 1987), despite several long-term studies on both feeding and breeding grounds. In the St. Lawrence, sightings of killer whales were extremely rare (unpublished data). It seems that most attacks happen to calves or juveniles on their migration routes (Clapham 2000; Mehta et al. 2007). Group size on the migration to the feeding grounds is larger than on the reverse lag, suggesting enhanced calf protection (Brown and Corkeron 1995). However, in our study, juveniles and lactating females had the lowest association indices, and thus, we conclude that predation was an improbable cause for the observed association pattern in the feeding ground.
Although behaviour related to mating has been observed mostly on the breeding grounds and during migration, Weinrich (1991) and Clapham (1993) suggest that males might try to extend bonds formed with females in the summer to the breeding season. While mate guarding during the migration to the breeding grounds was proposed (Brown and Corkeron 1995), additional evidence is needed to confirm that males are able to extend these associations. The increased gregariousness of males observed in fall seems to lend some support to this hypothesis.
Food distribution is a major selective force for group living, such as in cooperative hunting species (e.g. lions (Packer et al. 1990) and African wild dogs (Lycaon pictus; Creel and Creel 1995)). Cooperative feeding of mature humpback whales of both sexes has been observed in Alaska and the Gulf of Maine (Baker and Herman 1984a; Perry et al. 1990; Weinrich and Kuhlberg 1991; Clapham 1993). Males might avoid each other on the feeding grounds since cooperative feeding would mean supporting a potential future competitor during the next breeding season (Weinrich and Kuhlberg 1991). The low male–male association indices may also result from their preference to associate with females (Clapham 1993). Inter-annual stable groups involved in cooperative feeding strategies on herring (Clupea spp.) in Alaska (Jurasz and Jurasz 1979; Baker and Herman 1984a; Perry et al. 1990) could be specific to fast-moving prey species (Clapham 2000). Observations of surface feeding were rare in our study area. Fatty acid analyses showed that humpback whales in the Gulf of St. Lawrence are feeding at a low trophic level (Borobia et al. 1995), indicating a large proportion of euphausiids in their diet (Sourisseau et al. 2006). Following Clapham’s theory (2000), foraging on euphausiids should not require cooperative feeding in large groups and might explain why pairs were the most stable social affiliations. Given the duration of associations between mature individuals within a feeding season, it seems probable that associated humpbacks spent some time foraging together. Inter-regional variation in prey composition, density and distribution might require different feeding strategies and thus may result in different levels of cooperation and association patterns between feeding grounds.
Defending resources, especially if food distribution is uneven, is another selective force to form groups (Wrangham 1980) and represents another type of cooperation. Competition for limited resources is often accompanied by intra-group dominance or aggression towards other groups (Isbell 1991). So far, no evidence for any kind of hierarchy exists in humpback whales (Clapham 2000). Most studies have dismissed competition as factor shaping sociality in humpback whales (Clapham 1993, 2000) since resources in the marine environment are patchy and often too spatially and temporally variable (Steele 1976) to be worth defending.
We currently lack the genetic data needed to determine whether associating animals are kin-related. Many studies provide evidence of group living among kin-related individuals, especially females (e.g. Whitehead 1996; Connor et al. 1998; Archie et al. 2006). Humpback whales in the Gulf of Maine, especially females, associate more often with animals of the same mitochondrial DNA (mtDNA) haplotype (Weinrich et al. 2006). The authors hypothesised that individual feeding strategies and prey preferences could be maternally transmitted and could correlate with the haplotypes. However, the authors acknowledge that the use of mtDNA haplotypes as evidence of kin selection is limited and that more genetic markers such as microsatellites are needed to further test this hypothesis.
Although we could not correlate feeding with the observed association patterns, we regard cooperative feeding as the most plausible factor to explain the observed patterns of associations, given the duration of the associations in the feeding areas. The animals meet their energetic requirements for the entire year on the summer feeding grounds; thus, pairs associating the entire season are likely to feed. Weinrich (1991) noted that the majority of stable pairs in the Gulf of Maine are made up of females and suggested that their higher energetic demand, especially when pregnant, makes them more inclined to feed cooperatively. The cohort group with the strongest association was the one observed with most calves, hence more often being pregnant, lending some support to this theory. However, as Clapham (2000) stated, if feeding cooperation truly is beneficial, then whales from other sex and age classes should probably adopt it as well. More data about the underwater behaviour of whale groups are required to address the cooperative feeding hypothesis.
Long-term associations of non-lactating females
The most notable exception to previous studies was the multi-year relationship between mature females of similar age. These females might have the same level of experience since they have known each other for a long time. They may have developed the same feeding style or prey preferences as Weinrich et al. (2006) suggested, whether due to maternal relatedness or not. Jaquet et al. (2007) showed that female right whales (Eubalaena glacialis) are also more gregarious than males and have preferred associates for several years, even in times with low food resources, but the authors were unable to determine the ultimate cause of this gregariousness.
Female pairs might also represent coalitions against male harassment (Clutton-Brock and Parker 1995). However, male–female pairs were common in this and other studies (Weinrich and Kuhlberg 1991; Clapham 1993), and it is questionable whether such behaviour on a feeding ground is necessary, especially since females are larger than males. Nonetheless, while we provided evidence of female long-term bonds, it is still unclear if they represent mere feeding bonds or if there are additional motives for these associations.
It also remains unclear how females ultimately benefit from a more gregarious behaviour. In a mark-recapture study on the same population, females experienced a higher apparent survival than males (Ramp et al. 2010). The cohort group with the strongest long-term bonds was the oldest one and had also the highest reproductive output (Table 4). This sounds contradicting at first since we showed that non-lactating females are building long-term associations and lactating females were in general less social. However, the older females seem to build stronger relations in years without a dependent calf. In ungulates, female reproductive success increases with age, longevity and maternal experience (Clutton-Brock et al. 1988; Gaillard et al. 2000). Choosing a partner of similar age seems beneficial for female humpback whales. The high association index between juvenile females might even suggest that females start selecting their preferred associates in early years of their life. Juvenile ungulates of both sexes also tend to seek the company of same sex peers (Bon and Campan 1996).
The increased sociality was attended by an increase in reproductive output. However, so far, we have analysed only 9 years of data and cannot determine the life-long reproductive rate of a female nor include calf survival to really evaluate reproductive success. Reproductive success has been used as a proxy for fitness and linked to increased sociality in many mammalian species (see review by Silk 2007). Whether the increased sociality of females leads to an increase in fitness in humpback whales, as shown for some species (Silk et al. 2003; Marcoux et al. 2007), remains speculative and awaits further testing (Silk 2007).
Whatever benefit females derived from non-random associating was apparently outweighed when one of them had a calf since females tended to be more solitary when nursing. This is in contrast to other species where female–female bonds are found to increase infant survival (Whitehead 1996; Silk et al. 2003). Female humpback whales involved in stable feeding groups in Alaska (Perry et al. 1990) do not participate in years they had a dependent calf (Gabriele et al. 1995). Calves start to feed on solid prey in addition to being nursed during the summer months (Clapham and Mayo 1987) and are weaned in fall (Baraff and Weinrich 1993). They are most likely feeding on prey that is easier to catch, and the mother might prefer specific habitats with certain prey type and abundance to meet her own but also her offspring’s energetic demands (Sardi et al. 2005). However, that does not explain why lactating females do not form associations as observed in many other species.
Conclusions
The social system of humpback whales resembles a fission–fusion society as previously proposed (Clapham 1993), but long-lasting stable associations are more important than formerly suggested. Female humpback whales are more social than males in the summer and form partnerships spanning several years. The increased sociality is independent of sexual segregation and likely not a result of predation pressure. While feeding cooperation could explain intra- as well as inter-sexual pairs in humpback whales within one season, it remains unanswered why only female–female bonds exceed beyond single feeding seasons and if this increased sociality increases their reproductive success. Further research is needed to determine the relatedness of associated individuals. Focal sampling of individuals and groups (see Clapham 2000) and the use of modern tags (data loggers) to investigate the underwater behaviour of animals associated at the surface should allow testing the cooperative feeding hypothesis. Furthermore, the hypothesis of increased sociality linked with higher fitness (Silk 2007), namely survival and reproductive success, should be tested.
References
Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383
Altmann J, Alberts SC, Haines SA, Dubach J, Muruthi P, Coote T, Geffen E, Cheesman DJ, Mututua RS, Saiyelel SN, Wayne RK, Lacy RC, Bruford MW (1996) Behavior predicts genetic structure in a wild primate group. Proc Natl Acad Sci USA 93:5797–5801
Archie EA, Moss CJ, Alberts SC (2006) The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc R Soc Lond B 273:513–522
Baker CS, Herman LM (1984a) Seasonal contrasts in the social behavior of the North Pacific humpback whale. Cetus 5:14–16
Baker CS, Herman LM (1984b) Aggressive behavior between humpback whales (Megaptera novaeangliae) wintering in Hawaiian waters. Can J Zool 62:1922–1937
Baraff L, Weinrich MT (1993) Separation of humpback whale mothers and calves on a feeding ground in early autumn. Mar Mamm Sci 9:431–434
Barton RA, Byrne RW, Whiten A (1996) Ecology, feeding competition and social structure in baboons. Behav Ecol Sociobiol 38:321–329
Bejder L, Fletcher D, Bräger S (1998) A method for testing association patterns of social animals. Anim Behav 56:719–725
Bérubé M, Palsbøll PJ (1996) Identification of sex in cetaceans by multiplexing with three ZFX and ZFY specific primers. Mol Ecol 5:283–287
Bérubé M, Berchok C, Sears R (2001) Observation of a male-biased sex ratio in the Gulf of St. Lawrence fin whales (Balaenoptera physalus): temporal, geographical, or group structure segregation? Mar Mamm Sci 17:371–381
Boinski S (1988) Sex differences in the foraging behavior of squirrel monkeys in a seasonal habitat. Behav Ecol Sociobiol 23:177–186
Bon R, Campan R (1996) Unexplained sexual segregation in polygynous ungulates: a defense of an ontogenetic approach. Behav Process 38:131–154
Borobia M, Gearing PJ, Simard Y, Gearing JN, Beland P (1995) Blubber fatty-acids of finback and humpback whales from the Gulf of St. Lawrence. Mar Biol 122:341–353
Brown M, Corkeron P (1995) Pod characteristics of migrating humpback whales (Megaptera novaeangliae) off the east Australian coast. Behaviour 132:163–179
Brown MR, Corkeron PJ, Hale PT, Schultz KW, Bryden MM (1995) Evidence for a sex-segregated migration in the humpback whale (Megaptera novaeangliae). Proc R Soc Lond B 259:229–234
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, Berlin
Cairns SJ, Schwager SJ (1987) A comparison of association indices. Anim Behav 35:1454–1469
Cerchio S, Jacobsen JK, Cholewiak DM, Falcone EA, Merriwether DA (2005) Paternity in humpback whales, Megaptera novaeangliae: assessing polygyny and skew in male reproductive success. Anim Behav 70:267–277
Chittleborough SJ (1965) Dynamics of two populations of humpback whale, Megaptera nodosa (Bonnaterre). Aust J Mar Freshw Res 16:33–128
Clapham P (1993) Social organization of humpback whales on a North Atlantic feeding ground. Symp Zool Soc Lond 66:131–145
Clapham PJ (1992) Age at attainment of sexual maturity in humpback whales, Megaptera novaeangliae. Can J Zool 70:1470–1472
Clapham PJ (2000) The humpback whale: seasonal feeding and breeding in a baleen whale. In: Mann J, Connor RC, Tyack P, Whitehead H (eds) Cetacean societies—field studies of dolphins and whales. University of Chicago Press, Chicago, pp 173–196
Clapham PJ, Mayo CA (1987) Reproduction and recruitment of individually identified humpback whales, Megaptera novaeangliae, observed in Massachusetts Bay, 1979–1985. Can J Zool 65:2853–2863
Clutton-Brock TH, Parker GA (1995) Sexual coercion in animal societies. Anim Behav 49:1345–1365
Clutton-Brock TH, Guinness FE, Albon SD (1982) Red deer. The behaviour and ecology of two sexes. Edinburgh University Press, Scotland
Clutton-Brock TH, Albon SD, Guiness FE (1988) Reproductive success in male and female red deer. In: Clutton-Brock TH (ed) Reproductive success. University of Chicago Press, Chicago, pp 325–343
Connor RC, Mann J, Tyack P, Whitehead H (1998) Social evolution in toothed whales. Trends Ecol Evol 13:228–232
Conradt L (1999) Social segregation is not a consequence of habitat segregation in red deer and feral soay sheep. Anim Behav 57:1151–1157
Creel S, Creel NM (1995) Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim Behav 50:1325–1339
Dawbin WH (1966) The seasonal migratory cycle of humpback whales. In: Norris KS (ed) Whales, dolphins and porpoises. University of California Press, Berkeley, pp 145–170
Dolphin WF (1987) Observations of humpback whale, Megaptera novaeangliae—killer whale, Orcinus orca, interactions in Alaska—comparison with terrestrial predator prey relationships. Can Field-Nat 101:70–75
Doniol-Valcroze T (2008) Habitat selection and niche characteristics of rorqual whales in the northern Gulf of St. Lawrence (Canada). Ph.D. thesis, McGill University, Montreal, Canada
Fabiani A, Galimberti F, Sanvito S, Hoelzel AR (2006) Relatedness and site fidelity at the southern elephant seal, Mirounga leonina, breeding colony in the Falkland Islands. Anim Behav 72:617–626
Gabriele CM, Baker C, Perry A, Straley JM (1995) Long-term, repeated associations among humpback whales in Glacier Bay and Icy Strait, Southeastern Alaska. In: Proceedings 11th Biennial Conference on the Biology of Marine Mammals, 14 December–18 December 1995, Orlando, Florida, USA
Gabriele CM, Straley JM, Neilson JL (2007) Age at first calving of female humpback whales in southeastern Alaska. Mar Mamm Sci 23:226–239
Gaillard JM, Festa-Bianchet M, Yoccoz NG, Loison A, Toigo C (2000) Temporal variation in fitness components and population dynamics of large herbivores. Annu Rev Ecol Syst 31:367–393
Gowans S, Whitehead H, Hooker SK (2001) Social organization in northern bottlenose whales, Hyperoodon ampullatus: not driven by deep-water foraging? Anim Behav 62:369–377
Hinde RA (1976) Interactions, relationships and social structure. Man 11:1–17
Isbell LA (1991) Contest and scramble competition: patterns of female aggression and ranging behavior among primates. Behav Ecol 2:143–155
Jaquet N, Browning C, Kibler P, Nilsson P, Risch D, Webster T, Brown MW (2007) Lonely males—gregarious females? Social organization and association patterns of North Atlantic right whales in Cape Cod bay. Abstract. In: Proceedings 17th Biennial conference on the biology of marine mammals, Cape Town, South Africa
Jarman PJ (1974) The social organisation of antelope in relation to their ecology. Behaviour 48:215–267
Jefferson TA, Stacey PJ, Baird RW (1991) A review of killer whale interactions with other marine mammals—predation to coexistence. Mamm Rev 21:151–180
Jurasz CM, Jurasz VP (1979) The feeding modes of the humpback whale, Megaptera novaeangliae, in southeast Alaska. Sci Rep Whales Res Inst 31:69–83
Kappeler PM, van Schaik CP (2002) Evolution of primate social systems. Int J Primatol 23:707–740
Katona S, Whitehead H (1981) Identifying humpback whales using their natural markings. Polar Rec 20:439–444
Katona SK, Beard JA (1990) Population size, migrations and feeding aggregations of the humpback whale (Megaptera novaeangliae) in the western North Atlantic Ocean. Rep Int Whal Comm Spec Issue 12:295–305
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Krause S, Mattner L, James R, Guttridge T, Corcoran MJ, Gruber SH, Krause J (2009) Social network analysis and valid Markov chain Monte Carlo tests of null models. Behav Ecol Sociobiol 63:1089–1096
Lockyer CH (1984) Review of baleen whale (Mysticeti) reproduction and implication for management. Rep Int Whal Comm Spec Issue 6:27–50
Manly BFJ (1995) A note on the analysis of species co-occurrences. Ecology 76:1109–1115
Mann J, Connor RC, Tyack P, Whitehead H (2000) Cetacean societies—field studies of dolphins and whales. University of Chicago Press, Chicago
Marcoux M, Rendell L, Whitehead H (2007) Indications of fitness differences among vocal clans of sperm whales. Behav Ecol Sociobiol 61:1093–1098
Mattila DK, Clapham PJ (1989) Humpback whales, Megaptera novaeangliae, and other cetaceans on Virgin Bank and in the northern Leeward Islands, 1985 and 1986. Can J Zool 67:2201–2211
Mattila DK, Clapham PJ, Vasquez O, Bowman RS (1994) Occurrence, population composition, and habitat use of humpback whales in Samana Bay, Dominican Republic. Can J Zool 72:1898–1907
McComb K, Moss C, Durant SM, Baker L, Sayialel S (2001) Matriachs as repositories of social knowledge in African elephants. Science 292:491–494
McComb K, Reby D, Baker L, Moss C, Sayialel S (2003) Long-distance communication of acoustic cues to social identity in African elephants. Anim Behav 65:317–329
Mehta AV, Allen JM, Constantine R, Garrigue C, Jann B, Jenner C, Marx MK, Matkin CO, Mattila DK, Minton G, Mizroch SA, Olavarria C, Robbins J, Russell KG, Seton RE, Steiger GH, Vikingsson GA, Wade PR, Witteveen BH, Clapham PJ (2007) Baleen whales are not important as prey for killer whales (Orcinus orca) in high-latitude regions. Mar Ecol Prog Ser 348:297–307
Mitchell CL (1991) Competitive regimes and female bonding in two species of squirrel monkeys (Saimiri oerstedi and S. sciureus). Behav Ecol Sociobiol 28:55–60
Mobley JR, Herman LM (1985) Transience of social affiliations among humpback whales (Megaptera novaeangliae) on the Hawaiian wintering grounds. Can J Zool 63:762–772
Norris KS, Dohl TP (1980) Behavior of the Hawaiian spinner dolphin, Stenella longirostris. Fish Bull 77:821–849
Ottensmeyer CA, Whitehead H (2003) Behavioural evidence for social units in long-finned pilot whales. Can J Zool 81:1327–1338
Packer C, Scheel D, Pusey AE (1990) Why lions form groups: food is not enough. Am Nat 136:1–19
Palsbøll P, Allen J, Bérubé M, Clapham PJ, Feddersen TP, Hammond PS, Hudson RR, Jorgensen H, Katona S, Larsen AH, Larsen F, Lien J, Mattila DK, Sigurjónsson J, Sears R, Smith T, Sponer R, Stevick P, Øien N (1997) Genetic tagging of humpback whales. Nature 388:767–769
Payne R, Webb D (1971) Orientation by means of long range acoustic signalling in baleen whales. Ann NY Acad Sci 188:110–141
Perry A, Baker C, Herman LM (1990) Population characteristics of individually identified humpback whales in the central and eastern tropical Pacific: a summary and critique. Rep Int Whal Comm Spec Issue 12:307–317
Pomilla C, Rosenbaum HC (2006) Estimates of relatedness in groups of humpback whales (Megaptera novaeangliae) on two wintering grounds of the southern hemisphere. Mol Ecol 15:2541–2555
Ramp C (2008) Population dynamics and social organisation of humpback whales (Megaptera novaeangliae) in the Gulf of St. Lawrence—a long-term study. Ph.D. thesis, University of Bremen, Germany. http://nbn-resolving.de/urn:nbn:de:gbv:46-diss000111355
Ramp C, Berube M, Palsbøll PJ, Hagen W, Sears R (2010) Sex-specific survival in the humpback whale (Megaptera novaeangliae) in the Gulf of St. Lawrence, Canada. Mar Ecol Prog Ser 400:267–276
Robbins J (2007) Structure and dynamics of the Gulf of Maine humpback whale population. Ph.D. thesis, University of St. Andrews, Great Britain
Rubenstein DI, Hack M (2004) Natural and sexual selection and the evolution of multi-level societies: insights from zebras with comparison to primates. In: Kappeler PM, van Schaik CP (eds) Sexual selection in primates: new and comparative perspectives. Cambridge University Press, Cambridge, pp 266–279
Ruckstuhl KE, Neuhaus P (2000) Sexual segregation in ungulates: a new approach. Behaviour 137:361–377
Ruckstuhl KE, Neuhaus P (2002) Sexual segregation in ungulates: a comparative test of three hypotheses. Biol Rev 77:77–96
Sardi KA, Weinrich MT, Connor RC (2005) Social interactions of humpback whale (Megaptera novaeangliae) mother/calf pairs on a North Atlantic feeding ground. Behaviour 142:731–750
Sears R, Berchok C, Palsbøll PJ, Doniol-Valcroze T, Ramp C (1999) Gender related structure in blue whale (Balaenoptera musculus) pairs from eastern Canadian waters. Abstract. In: Proceedings of the 13th Biennial Conference of Marine Mammals, Maui, Hawaii, USA
Shi J, Dunbar RIM, Buckland D, Miller D (2005) Dynamics of grouping patterns and social segregation in feral goats (Capra hircus) on the Isle of Rum, NW Scotland. Mammalia 69:185–199
Silk JB (2007) The adaptive value of sociality in mammalian groups. Phil Trans Roy Soc Lond 362:539–559
Silk JB, Alberts SC, Altmann J (2003) Social bonds of female baboons enhance infant survival. Science 302:1231–1234
Sourisseau M, Simard Y, Saucier FJ (2006) Krill aggregation in the St. Lawrence system, and supply of krill to the whale feeding grounds in the Estuary from the Gulf. Mar Ecol Prog Ser 314:257–270
Steele JH (1976) Patchiness. In: Cushing DH, Walsh JJ (eds) The ecology of the seas. Blackwell Scientific, Oxford, pp 98–115
Steiger GH, Calambokidis J (2000) Reproductive rates of humpback whales off California. Mar Mamm Sci 16:220–239
Sterck EHM, Watts DP, van Schaik CP (1997) The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol 41:291–309
Stone GS, Florez-Gonzales L, Katona S (1990) Whale migration record. Nature 346:705
Storz JF (1999) Genetic consequences of mammalian social structure. J Mammal 80:553–569
Tyack P (1981) Interaction between singing Hawaiian humpback whales and conspecifics nearby. Behav Ecol Sociobiol 8:105–116
Tyack P, Whitehead H (1983) Male competition in large groups of wintering humpback whales. Behaviour 83:132–154
Valsecchi E, Haie P, Corkeron P, Amos W (2002) Social structure in migrating humpback whales (Megaptera novaeangliae). Mol Ecol 11:507–518
van Schaik CP (1989) The ecology of social relationships among female primates. In: Standen V, Foley RA (eds) Comparative socioecology. Blackwell, Oxford, pp 195–218
Weinrich M, Martin M, Griffiths R, Bove J, Schilling M (1997) A shift in distribution of humpback whales, Megaptera novaeangliae, in response to prey in the southern Gulf of Maine. Fish Bull 95:826–836
Weinrich MT (1991) Stable social associations among humpback whales (Megaptera novaeangliae) in the southern Gulf of Maine. Can J Zool 69:3012–3019
Weinrich MT, Kuhlberg AE (1991) Short-term association patterns of humpback whale (Megaptera novaeangliae) groups on their feeding grounds in the southern Gulf of Maine. Can J Zool 69:3005–3011
Weinrich MT, Rosenbaum H, Baker C, Blackmer AL, Whitehead H (2006) The influence of maternal lineages on social affiliations among humpback whales (Megaptera novaeangliae) on their feeding grounds in the southern Gulf of Maine. J Hered 97:226–234
Whitehead H (1983) Structure and stability of humpback whale groups off Newfoundland. Can J Zool 61:1391–1397
Whitehead H (1995) Investigating structure and temporal scale in social organizations using identified individuals. Behav Ecol 6:199–208
Whitehead H (1996) Babysitting, dive synchrony, and indications of alloparental care in sperm whales. Behav Ecol Sociobiol 38:237–244
Whitehead H (1999) Testing association patterns of social animals. Anim Behav 57:F26–F29
Whitehead H (2007) Program for analyzing social structure. SOCPROG 2.3 (for MATLAB 7.4, release 2007a). Department of Biology, Dalhousie University. (http://whitelab.biology.dal.ca/index.html).73pp
Whitehead H (2008) Analyzing animal societies: quantitative methods for vertebrate social analysis. University of Chicago Press, Chicago
Whitehead H, Glass C (1985) Orcas (killer whales) attack humpback whales. J Mammal 66:183–185
Wilson EO (1975) Sociobiology: the new synthesis. Belknap, Cambridge
Wittemyer G, Douglas-Hamilton I, Getz WM (2005) The socioecology of elephants: analysis of the process creating multitiered social structures. Anim Behav 69:1357–1371
Wrangham RW (1979) On the evolution of ape social systems. Soc Sci Inf 18:335–368
Wrangham RW (1980) An ecological model of female bonded primate groups. Behaviour 75:262–300
Acknowledgements
We would like to thank the team of the Mingan Island Cetacean Study for the data collection, Hal Whitehead for his extensive help with the analysis and SOCPROG details and Thomas Doniol-Valcroze, Brian Kot, Julien Delarue, Nathalie Jaquet, Jooke Robbins, Marianne Marcoux, Petra Reimann and three anonymous reviewers for comments on the draft. An earlier version of this article was submitted in partial fulfilment of a doctoral thesis at the University of Bremen. The study complies with the laws of Canada. All fieldwork was conducted under permits of the Department of Fisheries and Oceans, Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by V. Janik.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Description of models fitted to the SLAR of the different data sets. Time lag (τ) (PDF 17 kb)
ESM 2
Annual sample size of identified humpback whales in the research area over the study period, divided by sex. Females pooled in the cohort groups were sighted as non-lactating mature females in the study period (1997–2005) (PDF 18 kb)
Rights and permissions
About this article
Cite this article
Ramp, C., Hagen, W., Palsbøll, P. et al. Age-related multi-year associations in female humpback whales (Megaptera novaeangliae). Behav Ecol Sociobiol 64, 1563–1576 (2010). https://doi.org/10.1007/s00265-010-0970-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-0970-8