Abstract
Often in colonial seabirds, all colony members are believed to defend against nest predators and experience equal nest predation risk. However, the variation of defense behavior among members and its reproductive consequences are largely unknown. We investigated (1) individual variation in the nest defense of breeding Black-tailed Gulls Larus crassirostris against a natural egg predator, the Jungle Crow Corvus macrorhynchos and (2) how this behavioral variation affects an individual’s own nest predation risk and that of their neighbors. Results were compared between 2 years where crow attack levels were manipulated to average 5 and 22 times normal rates (“low” and “high” predation risk years, respectively) by the placement of varying numbers of artificial nests containing unguarded eggs at the perimeter of the gull colony. In both years, 23–38% of parents, mostly males, showed “aggressive” defense behavior (strikes or chases) against crows and decoys. Other “non-aggressive” gulls showed no defense. In the year of low predation risk, intrusion rates by crows (landing within 0.5 m of an individual gull’s nest) were similar for aggressive and non-aggressive gulls. In the year of high predation risk, however, the rates of intrusion for aggressive gulls (4%) and for non-aggressive gulls with an aggressive neighbor (37%) were significantly lower than for non-aggressive gulls without an aggressive neighbor (76%). These results indicate that aggressive individuals reduce nest predation risk for themselves and conspecific neighbors in a colonially breeding species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reduction of predation risk through direct defense by group members has been suggested to be a significant benefit of group living (reviewed by Krause and Ruxton 2002). In some studies, all group members are assumed to share defense duties equally (Elgar 1989; Caro 2005). However, several studies have shown variation in defense behavior among group members (Arlet and Isbell 2009; Carter et al. 2009). Those individuals with neighbors that vigorously defend against predators may experience decreased predation risk even without participating in defense activities and thus avoid energy costs and injury risk associated with defense efforts (Dugatkin and Godin 1992). Empirical evidence, however, is lacking.
Over 96% of seabird species breed colonially (Coulson 2002). When colonial nesting birds are threatened by predators, group mobbing or attacks reduce nest predation risk by chasing predators away (Montevecchi 1979; Whittam and Leonard 2000). During this direct defense, all members in an area targeted by nest predators are believed to share predation risk and hence all might be expected to participate in nest defense (Burger and Gochfeld 1991). Individual variation in nest defense, however, has been reported in colonial nesting Sabine’s Gulls Xema sabini (Stenhouse et al. 2005).
Black-tailed Gulls Larus crassirostris nest colonially on the slopes of coastal islands where vegetation provides moderate cover. Egg predation by Jungle Crows Corvus macrorhynchos is the dominant factor limiting their breeding success (Kazama 2007). Their parents defend aggressively against crows through intimidation (by opening their bill and spreading wings), body striking, or swooping. We examined whether there was individual variation in their response to crows. Since the proximity and approach speed of predators to nests and weather and wind condition may influence the defense response (Gilchrist et al. 1998), we also exposed individual gulls to crow decoys to evaluate their defense response. We then examined if aggressive gulls decreased the risk of egg predation for themselves and their neighbors.
Methods
Study area and nest census
The study was conducted on Rishiri Island (45°12′N, 141°10′E) in the Sea of Japan/East Sea, 40 km off northern Hokkaido, Japan, from May to July in 2004 and 2005. The island supported >19,000 breeding pairs of Black-tailed Gulls in 2004 (Kosugi et al. 2005). A study site (0.020 ha) was established on the gentle, northwestern slope of the island and was used in both study years (82 nests in 2004 and 76 nests in 2005).
In many ground-nesting larids, nest position within a colony and nest microhabitat affect predation risk from avian nest predators (Brunton 1997; Velando and Márquez 2002; García-Borboroglu and Yorio 2004). In Black-tailed Gulls, peripheral nests (<4 m from edge of the breeding area) and nests located where vegetation is <20 cm high are at higher risk of egg predation (Kazama 2007). Therefore, we established the study site in a small subcolony where all nests were peripheral (Fig. 1). We cut down vegetation around the nests every 1–2 days to keep it shorter than 15 cm. Once the study was completed the vegetation grew quickly and provided sufficient shelter for chicks.
The frequency of nest attacks by Jungle Crows is usually small (0.002 attacks/nest per hour) during the incubation stage in this study area (Kazama 2007). Therefore, to attract crows, we installed four artificial nests (15-cm wooden saucers fixed on 50- to 90-cm-long wooden stakes) around the study site in 2004. To increase the predation risk further, 30 such artificial nests were established in 2005. The artificial nests were placed at even intervals around the study site, 1.5–2.5 m from the edge. One chicken Gallus gallus domesticus egg (similar in size to Black-tailed Gull eggs) was placed on each artificial nest before each observation once a day. The chicken eggs were usually taken by Jungle Crows within several minutes. Thereafter, crows remained nearby and frequently attacked Black-tailed Gull nests in the study site.
All the nests in the study site were mapped and marked with numbered stakes when eggs were laid. Nest contents were checked every 1 or 2 days. In 2005, the length and breadth of the first egg laid in each nest were measured using calipers (D30TN, Mitutoyo Co., Ltd., Kawasaki, Japan), and their volumes were estimated by using the following equation, reported by Harris (1964):
Observations
To identify individuals, 164 and 152 gulls were marked in 2004 and 2005, with black hair dye (Bigen hair color, containing aminophenol and stearic acid as major ingredients; Hoyu Co., Ltd., Nagoya, Japan). During the incubation period, dyed stones or leaves were placed in each nest cup so that the dye would mark the breast or neck of either the male or female parent, whichever returned to incubate the eggs first. The sexes of the marked and non-marked gulls within each pair were distinguished by observation of body size (Chochi et al. 2002) and mating or copulation behavior. None of the parents in the study site abandoned their nests during the study periods. These marks did not appear to have any influence on the gulls’ behavior, their conspicuousness to predators, or the responses of other gulls. We did not attach any permanent tags or rings to individuals, so their identity could not be tracked between years.
Predation by crows and defense behavior by gulls were observed from 17 May to 23 June in 2004 and from 30 May to 27 June in 2005. Observations were made from a blind placed 10 m from the study site for 12–15 h each day (3–5 days/week) between 3:00 and 18:00. Total observation times were 206 h in 2004 and 192 h in 2005. We defined “attack” and “intrusion” based on the distance between crows and gull nests. During observations in 2004, parents started to show defense behavior when crows were within 5.9 m ± 3.52 (standard deviation (SD); N = 163) of the edge of the study site. Therefore, an “attack” by crows was deemed to occur when a crow swooped down and landed or approached by walking within 5 m from the nests. During an attack, 12.0 nests in 2004 (N = 163 attacks) and 17.5 nests in 2005 (N = 646 attacks), on average, were within 5 m of the crow. The nest closest to the crow was considered as the “target nest.” “Intrusion” by a crow was defined as an occasion when a crow was on the ground within 0.5 m of a nest. “Predation” was defined as an occasion when a crow ate or took one or more gull eggs in its bill and flew away from target nests.
During a crow attack, usually a single gull, attending either the target (closest) nest or a neighboring nest, flew toward the crow, and then struck it with its body, jabbed at it with its legs, or dove at it. This “first defender” usually drove the crow away and other gulls rarely (<1% of defense) joined in the nest defense. Therefore, we recorded if each gull was the first defender against the crow as an index of individual response to crows. Occasionally, a crow returned quickly and attacked the same nest repeatedly and the same gull defended. To avoid pseudo-replication, we examined only the attacks that occurred at least 2 min after any previous attack. During each attack, we recorded the following for each gull nest within 5 m of the crow: (1) minimal distance between the crow and the nest, (2) identity of the parent at the nest, (3) if the parent was the first defender (yes or no), (4) intrusion (yes or no), and (5) predation (yes or no).
Susceptibility to nest predation and the strength of nest defense vary with the number of offspring in bird species (Knight and Temple 1986; Wallin 1987). In this study, to maintain the original clutch size, we replenished nests that had been depredated with gull eggs from nests outside of the study site. All gulls whose eggs had been depredated resumed incubation soon after replenishment, and never abandoned their nests.
Decoy experiment
Crow decoys (plastic hunting decoys painted to resemble an American Crow Corvus brachyrhynchos; Carry-Lite Inc., Fort Smith, AR, USA), placed on 1.5-m-long stakes, were deployed at various locations around the study site under sunny and calm conditions from 24 May to 16 June and from 3 to 22 June in 2004 and 2005, respectively. Each decoy was covered with a cloth at first, then presented for 2 min by pulling a line and removing the cloth, before finally the decoy was pulled off from the stake by pulling a second line, thus disappearing from the view of the gulls.
The mean distance between the first defender and a decoy was 10.0 m ± 4.35 (SD; N = 64, in 2004). Thus, any gull within 10 m of a decoy could be the first defender. Distances between the first defender and decoys (10 m) were longer than those between the first defender and live crows (5 m). This indicates that the stimulus created by presenting decoys on stakes (for 2 min) was stronger than attacks by live crows (usually for just a few seconds). However, the modes of first defense behavior exhibited by gulls against a decoy (flew toward it then struck the decoy with its body, jabbed at it with its legs, or dove at it) were similar to those exhibited against live crows. Thus, we believe that it is reasonable to use the response of gulls to decoys as an index of their potential response to live crows.
For each decoy presentation, for all nests within 10 m of the decoy, we recorded (1) the identity of the incubating gull and (2) if they were the first defender (yes or no). Decoys were exposed one to five times per day at minimum intervals of 1 h. In total, we presented decoys 64 times in 2004 and 46 times in 2005. Gulls did not appear to habituate to the decoys.
Statistical analysis
To examine factors affecting individual variation in the probability of gulls being the first defender against live crows in each year, we fitted a generalized linear mixed model (GLMM) with binomial distribution and logit link function using a single binary outcome (the first defender or not) as the dependent variable. Numbers of attacks by crows were 163 and 646 and the sample size of gull-attacks binary outcome data was 1,949 and 11,306 in 2004 and 2005, respectively, since multiple gull nests were associated with each crow attack. Nesting stage, distance to an approaching crow, cumulative frequency of predation attacks that the gulls had previously experienced, egg-laying date (the number of days elapsed since 1 May), clutch size, and sex were used as independent variables. In 2005, egg volume was also included as an independent variable. Nesting stage was categorized as early (from onset of egg laying to completion of clutch), middle (from clutch completion to 15 days of incubation), and late (from 15 days of incubation to first chick hatching). Data collected before the early stage and after the late stage were excluded. Individual identity was included as a random effect to avoid pseudo-replication.
To examine the relationships between response to decoys and to live crows, we employed linear regression analyses. The rate of being the first defender against decoys (the number of occasions each individual was the first defender against decoys divided by the numbers of presentation of decoys within 10 m of each nest) was used as an independent variable. The rate of being the first defender against live crows (the number of occasions each individual was the first defender against live crows divided by the numbers of attacks within 5 m of each nest) was used as a dependent variable.
There was individual variation in the response of gulls to crows. Some became the first defender often (“aggressive” individuals), and others did not (“non-aggressive” individuals; see “Results” section). To examine the effect of nest type (with aggressive or non-aggressive individuals) on the probability of intrusion and predation by crows, we fitted GLMMs with binomial distribution and logit link function to (1) intrusion (for each attack) and (2) predation after intrusion (for each intrusion), both single binary outcomes. Nest types were (1) a nest of an aggressive gull that became the first defender (“aggressive”), (2) a nest of a non-aggressive gull that did not become the first defender but an aggressive gull of a neighboring nest (<1.5 m) did become the first defender (“non-aggressive with an aggressive neighbor”), and (3) a nest of a non-aggressive gull where neither the owner nor neighboring gulls (<1.5 m) became the first defender (“non-aggressive without an aggressive neighbor”). These nest types were used as a categorical independent variable. Nest identity was included as a random effect in order to avoid pseudo-replication. All analyses were performed using R v.2.7.2 (R Developmental Core Team 2005).
Results
Breeding biology
The mean egg-laying date was earlier in 2004 (18.9 ± 4.1 (SD), N = 82; 1 = 1 May) than in 2005 (26.0 ± 5.3 (SD), N = 76; one-way analysis of variance (ANOVA): F 1, 157 = 7.36, P < 0.01). The mean clutch size was slightly larger in 2004 (1.73 ± 0.61, N = 82) than in 2005 (1.57 ± 0.74, N = 76), but the difference was not significant (one-way ANOVA: F 1, 157 = 2.39, P = 0.12).
The number of predatory attacks by live crows was four times greater in 2005 (646 in total or 0.044/nest per hour) than in 2004 (163 in total or 0.010/nest per hour) (Chi-square test: χ 21 = 288.4, P < 0.001).
Factors influencing first defense probability
In both years, males had a greater probability of being the first defender against live crows (the first defender or not) than females (Table 1). In 2004, males with larger clutches had a greater probability of being the first defender than males with smaller clutches, although this was not the case in 2005 (Table 1). Males of nests with eggs laid earlier in the breeding season had a greater probability of being the first defender than males with eggs laid later in 2005, but not in 2004 (Table 1). Neither the cumulative frequency of attacks, distance to attacking crows, nor the volume of the first egg was a factor related to the probability of being the first defenders (Table 1). Nesting stage was also not a significant factor, hence we pooled the data from different nesting stages in the following analyses.
There were no positive relationships in the rates of being the first defender against live crows between males and females within pairs in 2004 (R 2 = 0.019, t = 1.26, df = 81, P = 0.21) and 2005 (R 2 = 0.049, t = 1.41, df = 75, P = 0.16). Therefore, an individual’s probability of being the first defender against live crows was independent of their mate.
Individual variation in first defense probability
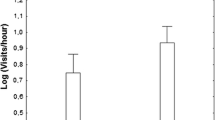
There was considerable variation among individual gulls in the rate of being the first defender against live crows (Fig. 2). The estimates of the variance components of individuals in the GLMM were large in 2004 (9.66 ± 0.24) and 2005 (4.90 ± 0.19). Twenty percent (in 2004) and 37% (in 2005) of individuals became the first defender against live crows, and other individuals never became the first defender when they were attacked (Fig. 2).
Individuals that exhibited a greater rate of being the first defender against live crows also exhibited similar responses to decoys in both years (in 2004, R 2 = 0.37, t = 9.87, df = 163, P < 0.001; in 2005, R 2 = 0.45, t = 10.97, df = 151, P < 0.001, Fig. 3). Of gulls that never became the first defender against live crows, a few became the first defender against decoys: five individuals (4%) in 2004 and two (2%) in 2005.
Relationship between the rates of being the first defender against a decoy and against a Jungle Crow in 2004 and 2005 (64 and 46 experiments in 2004 and 2005). Solid lines are a linear regression (in 2004: \( Y = {1}.{857} \times X + 0.0{22} \); in 2005: \( Y = 0.{527} \times X + 0.0{13} \)). The closed circles represent “aggressive” gulls and open circles represent “non-aggressive” gulls (see text)
Based on these observations, gulls were categorized into those that defended intensely (“aggressive”; the rate of being the first defender against live crows or decoys >0.0, Figs. 2 and 3) and those that never defended against either live crows or decoys (“non-aggressive,” the rate of being the first defender against live crows or decoys = 0.0, Figs. 2 and 3). Thirty-seven individuals (23%, 29 males and eight females) in 2004 and 58 individuals (38%, 40 males and 18 females) in 2005 were considered aggressive.
Reduction of predation risks
In 2004, when predation risk was low on average, nest type did not affect the probability of intrusion (intrusion or not; GLMM, estimate ± SE if non-aggressive without an aggressive neighbor was zero; aggressive, 0.29 ± 1.38, z = 0.21, P = 0.84; non-aggressive with an aggressive neighbor, −0.81 ± 0.83, z = −0.97, P = 0.33, Fig. 4). In 2005, when predation risk was increased, however, nest type did affect the probability of intrusion (GLMM, aggressive, −4.19 ± 0.89, z = −4.71, P < 0.001; non-aggressive with an aggressive neighbor, −1.45 ± 0.61, z = −2.38, P = 0.018, Fig. 4). The rate of intrusion (the number of intrusions divided by the number of attacks) in nests of non-aggressive gulls without an aggressive neighbor was approximately 20 times higher than that of nests of aggressive gulls and two times higher than that of nests of non-aggressive gulls with an aggressive neighbor (Fig. 4).
Intrusion rate (the number of intrusions divided by the numbers of attacks) and predation rate (the number of predation events divided by the numbers of intrusions) among three types of gulls on nests (aggressive, non-aggressive with an aggressive neighbor, and non-aggressive without an aggressive neighbor) in 2004 and 2005. P values obtained with GLMM (see text) are indicated by asterisks: P < 0.001 (single asterisk) and P < 0.05 (double asterisk)
The effects of nest type on the probability of predation after intrusion (predation or not) could not be tested because the frequencies of intrusion were too small in all three nest types in 2004 (one, six, and six times, respectively, Fig. 4). In 2005, the probability of predation after intrusion into nests of non-aggressive gulls without an aggressive neighbor was higher than that into nests of non-aggressive gulls with an aggressive neighbor (GLMM, estimate ± SE of non-aggressive with aggressive if non-aggressive without aggressive was zero, −3.04 ± 0.92, z = −3.31, P < 0.001, Fig. 4). The frequency of intrusion was too small into nests of aggressive gulls (three times, Fig. 4) to examine statistical differences.
Discussion
Factors influencing first defense probability
Birds whose nests are at high predation risk should display high levels of anti-predator behavior (Burger and Gochfeld 1991; Forslund 1993). All nests that we studied were peripheral (i.e., <4 m from the edge of the study site; Fig. 1) and surrounded by short vegetation; hence, all nests were apparently exposed to similar levels of potential egg predation risk. Therefore, the variation we observed in the probability of being the first defender against predators was independent of nest position within the study site or nesting microhabitat.
Sex affected the probability of being the first defender. Male gulls displayed a greater probability of being the first defender against crows than female gulls. In many bird species, males show a higher intensity of nest defense than females (Caro 2005), and at least three explanations have been hypothesized for these differences. First, females are in poorer body condition than males during the incubation period due to their investment in egg production (Reid and Montgomerie 1985). As a result, a potential cost of defense (decreased survival for example) might be lower for males than for females (Montgomerie and Weatherhead 1988). Second, in species with males having a larger body size than females, males might be able to drive off predators more effectively (Montgomerie and Weatherhead 1988). Third, testosterone affects sexual differences in the intensity of territorial defense in Yellow-legged Gull Larus cachinnans (Alonso-Alvarez and Velando 2001). In Black-tailed Gulls, females invest significant energy in egg laying (Y. Niizuma, personal communication) and males are larger than females (Chochi et al. 2002). These are consistent with the first and second hypotheses. Testosterone levels were not measured in this study, so the third hypothesis cannot be evaluated at this time.
Clutch size and timing of egg laying affected the probability of being the first defender but only in a single year. Effects of clutch size were apparent only in 2004, when aggressive males had larger clutches than non-aggressive males. We may have seen differences only in 2004 because the mean clutch size in 2004 was slightly larger than in 2005; only 2% of all nests had three eggs in 2005, compared to 9% in 2004. The intensity of nest defense is possibly decided by the balance between the increase of offspring survival due to predator deterrence versus the risks of injury to the parents (reviewed by Montgomerie and Weatherhead 1988; Caro 2005). Since the benefits of deterring predators should increase with clutch size, parents with larger clutches often defend more vigorously (Knight and Temple 1986; Lambrechts et al. 2000). Although we have no data on the risks associated with defensive behavior, large clutches apparently provide greater benefits and may have prompted males to be more aggressive only in 2004 in this study. In 2005, aggressive males had earlier clutches than non-aggressive males. In seabird species, earlier breeders are generally older, more experienced, or have better body condition than later breeders (Coulson 2002). Aggressive males might be older and/or have better body condition and hence had more time and energy to devote to defense efforts, especially in 2005 when predation risk was high.
Recently, individual differences in behavior have been reported in many bird species (reviewed by Dingemanse et al. 2004). Some of these studies have suggested that there is a dichotomy in behavioral characteristics of individuals that can be interpreted as proactive and reactive personality types (see reviews, Koolhaas et al. 1999; Sih et al. 2004; Groothuis and Carere 2005). Proactive individuals are bold, aggressive in conspecific competitions, and actively explore unfamiliar environments, while reactive individuals are shy, exhibit lower levels of aggression, and pay careful attention to external stimuli (Koolhaas et al. 1999; Sih et al. 2004; Groothuis and Carere 2005). These personalities are moderately heritable (Drent et al. 2003; van Oers et al. 2005) and often correlate with hormonal level (Koolhaas et al. 1997; Cockrem and Silverin 2002; Kralj-Fiser et al. 2007). In our study, the probability of being the first defender against predators seemed to be consistent throughout the egg laying and incubation periods. It is possible that the two types of anti-predator response in Black-tailed Gulls (aggressive/non-aggressive) are the results of proactive or reactive personalities based on certain genetic and/or physiological factors.
Do aggressive males benefit their neighbors?
This study demonstrated that defense behavior by aggressive males reduced the egg predation risk of their own nests and those of their neighbors, but only in the year when predation risk was artificially increased to be 0.04 attacks/nest per hour. This level of risk from avian predators is similar to the highest natural levels observed in this study colony in other years (K. Kazama, personal observation) and within the range of the highest levels reported in other ground-nesting seabirds (0.02–0.17 attacks/nest per hour in Roseate Tern Sterna dougallii, Whittam and Leonard 1999; and 0.01–0.03 in Arctic Tern Sterna paradisaea and Common Tern Sterna hirundo, Whittam and Leonard 2000). Therefore, aggressive males may increase both their own chance of reproductive success and that of their neighbors in natural conditions when potential predation risk is high.
Why do aggressive males defend despite the potential costs? One possible explanation is that the increase of their own chance of reproductive success compensates for the cost of defense. Aggressive males may defend simply for their own benefit, if the cost of defense is low. Under this hypothesis, aggressive males decreased predation risk to the nests of their non-aggressive neighbors only incidentally. A second possible explanation is that aggressive males may mitigate the costs of defense through beneficial interactions with their non-aggressive neighbors, such as chick adoption (Bukaciñski et al. 2000), extra-pair copulation (EPC; Mills 1994; Helfenstein et al. 2004), or food kleptoparasitism (Shealer et al. 2005) and interference behaviors (i.e., egg destruction and chick killing; Pierotti 1980) toward non-aggressive neighbors. In the study area, although spiteful behavior was rarely observed, approximately 15% of nests experienced chick adoption and EPCs between gulls of neighboring nests was observed frequently (Kazama et al. 2009, K. Kazama personal observation). Finally, vigorous nest defense by particular individuals might be explained as altruistic behavior among kin. Neighboring parents are known to comprise small kin groups in colonies of cliff-nesting Thick-billed Murres Uria lomvia (Friesen et al. 1996) and ground-nesting Common Gulls Larus canus (Bukaciñski et al. 2000). A further study on kin relationships would be required to examine this possibility in Black-tailed Gulls.
Our results may have some implications on the costs and benefits of colonial breeding. Sharing of predation risks by all members of a colony is believed to be one of the important selective factors in the evolution of coloniality (Wittenberger and Hunt 1985; Clode 1993; Brown and Brown 2001). Our results, however, highlight different levels of predation risk among nests at small spatial scales, caused by variation in the aggressiveness of adjacent neighbors. These should be incorporated in modeling the balance between costs and benefits of colonial breeding. Furthermore, it is possible that nest-site choice of individuals could be affected by neighbors’ response to predators, and that mate choice in females could be affected by a male’s response to predators, in colonial breeding species.
In conclusion, individual variation in defense against egg predators was apparent in colonially breeding Black-tailed Gulls. Those gulls that defended aggressively against predators reduced predation risks not only of their own nests, but also of neighbors’ nests that did not show any defense behavior.
References
Alonso-Alvarez C, Velando A (2001) Effect of testosterone on the behaviour of Yellow-legged Gulls Larus cachinnans in a high-density colony during the courtship period. Ethol Ecol Evol 13:341–349
Arlet ME, Isbell LA (2009) Variation in behavioral and hormonal responses of adult male gray-cheeked mangabeys (Lophocebus albigena) to crowned eagles (Stephanoaetus coronatus) in Kibale National Park, Uganda. Behav Ecol Sociobiol 63:491–499
Brown CR, Brown MB (2001) Avian coloniality: progress and problem. In: Nolan V Jr, Thompson CF (eds) Current ornithology vol. 16. Plenum Press, New York, pp 1–82
Brunton DH (1997) Impact of predators: center nests are less successful than edge nests in a large nesting colony of Least Terns. Condor 99:372–380
Bukaciñski D, Bukaciñska M, Lubjuhn T (2000) Adoption of chicks and the level of relatedness in common gull, Larus canus, colonies: DNA fingerprinting analyses. Anim Behav 59:289–299
Burger J, Gochfeld M (1991) The common tern. Columbia University Press, New York
Caro T (2005) Antipredator defenses in birds and mammals. The University of Chicago Press, Chicago & London
Carter AJ, Pays O, Goldizen AW (2009) Individual variation in the relationship between vigilance and group size in eastern grey kangaroos. Behav Ecol Sociobiol 64:237–245
Chochi M, Niizuma Y, Takagi M (2002) Sexual differences in the external measurements of Black-tailed Gulls breeding on Rishiri Island, Japan. Ornith Sci 1:163–166
Clode D (1993) Colonially breeding seabirds: predators or prey? Trends Ecol Evol 8:336–338
Cockrem JF, Silverin B (2002) Variation within and between birds in corticosterone responses of great tits (Parus major). Gen Comp Endocrinol 125:197–206
Coulson JC (2002) Colonial breeding in seabirds. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, New York, pp 87–113
Dingemanse NJ, Both C, Drent PJ, Tinbergen JM (2004) Fitness consequences of avian personalities in a fluctuating environment. Proc R Soc Lond Ser B-Biol Sci 271:847–852
Drent PJ, van Oers K, van Noordwijk AJ (2003) Realized heritability of personalities in the great tit (Parus major). Proc R Soc Lond Ser B-Biol Sci 270:45–51
Dugatkin LA, Godin JGJ (1992) Prey approaching predators—a cost-benefit perspective. Ann Zool Fenn 29:233–252
Elgar MA (1989) Predator vigilance and group-size in mammals and birds—a critical-review of the empirical-evidence. Biol Rev 64:13–33
Forslund P (1993) Vigilance in relation to brood size and predator abundance in the barnacle goose, Branta leucopsis. Anim Behav 45:965–973
Friesen VL, Montevecchi WA, Gaston AJ, Barrett RT, Davidson WS (1996) Molecular evidence for kin groups in the absence of large-scale genetic differentiation in a migratory bird. Evolution 50:924–930
García-Borboroglu P, Yorio P (2004) Effects of microhabitat preferences on kelp gull Larus dominicanus breeding performance. J Avian Biol 35:162–169
Gilchrist HG, Gaston AJ, Smith JNM (1998) Wind and prey nest sites as foraging constraints on an avian predator, the Glaucous Gull. Ecology 79:2403–2414
Groothuis TGG, Carere C (2005) Avian personalities: characterization and epigenesis. Neurosci Biobehav Rev 29:137–150
Harris MP (1964) Aspect of the breeding biology of the gulls Larus argentatus, L. fuscus and L. marinus. Ibis 106:432–456
Helfenstein F, Tirard C, Danchin E, Wagner RH (2004) Low frequency of extra-pair paternity and high frequency of adoption in Black-legged Kittiwakes. Condor 106:149–155
Kazama K (2007) Factors affecting egg predation in Black-tailed Gulls. Ecol Res 22:613–618
Kazama K, Fukuda T, Mori T (2009) Adoption of additional chicks from other nests by the Black-tailed Gull, in relation to chick age. Jpn J Ornithol 58:103–107 (In Japanese with English abstract)
Knight RL, Temple SA (1986) Nest defense in the American goldfinch. Anim Behav 34:887–897
Koolhaas JM, Meerlo P, DeBoer SF, Strubbe JH, Bohus B (1997) The temporal dynamics of the stress response. Neurosci Biobehav Rev 21:775–782
Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ (1999) Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev 23:925–935
Kosugi K, Sugimura N, Sato M (2005) Breeding colony status of Black-tailed Gull in Rishiri Island, northern Hokkaido (1) Estimated population from 2002–2004. Rishiri Kenkyu 24:29–35 (In Japanese with English abstract)
Kralj-Fiser S, Scheiber IBR, Blejec A, Moestl E, Kotrschal K (2007) Individualities in a flock of free-roaming greylag geese: behavioral and physiological consistency over time and across situations. Horm Behav 51:239–248
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, New York
Lambrechts MM, Prieur B, Caizergues A, Dehorter O, Galan M-J, Perret P (2000) Risk-taking restraints in a bird with reduced egg-hatching success. Proc R Soc Lond B 267:333–338
Mills JA (1994) Extra-pair copulations in the Red-billed Gull: females with high-quality, attentive males. Behaviour 128:41–64
Montevecchi WA (1979) Predator–prey interactions between Ravens and Kittiwakes. Z Tierpsychol 49:136–141
Montgomerie RD, Weatherhead PJ (1988) Risks and reward of nest defense by parent birds. Q Rev Biol 63:167–187
Pierotti R (1980) Spite and altruism in gulls. Am Nat 115:290–300
R Developmental Core Team (2005) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN3-900051-07-0, URL http://www.R-project.org
Reid ML, Montgomerie RD (1985) Seasonal patterns of nest defence by Baird’s sandpipers. Can J Zool 63:2207–2211
Shealer DA, Spendelow JA, Hatfield JS, Nisbet ICT (2005) The adaptive significance of stealing in a marine bird and its relationship to parental quality. Behav Ecol 16:371–376
Sih A, Bell AM, Johnson JC, Ziemba RE (2004) Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–277
Stenhouse IJ, Gilechrist HG, Montevecchi WA (2005) An experimental study examining the anti-predator behaviour of Sabine’s gulls Xema sabini during breeding. J Ethol 23:103–108
van Oers K, de Jong G, van Noordwijk AJ, Kempenaers B, Drent PJ (2005) Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142:1185–1206
Velando A, Márquez JC (2002) Predation risk and nest-site selection in the Inca tern. Can J Zool 80:1117–1123
Wallin K (1987) Defense as parental care in Tawny Owls Strix aluco. Behaviour 102:213–210
Whittam RM, Leonard ML (1999) Predation and breeding success in roseate terns (Sterna dougallii). Can J Zool 77:851–856
Whittam RM, Leonard ML (2000) Characteristics of predators and offspring in fluence nest defense by Arctic and Common terns. Condor 102:301–306
Wittenberger JF, Hunt GL Jr (1985) The adaptive significance of coloniality in birds. In: Farner DS, King JR, Parkes KC (eds) Avian biology Vol. 8. Academic, New York, pp 1–78
Acknowledgments
We thank Y. Sakurai and A. Takahashi for helpful comments during the course of the study. Thanks are also due to N. Takahashi, H. Sahara, J. Kaji, K. Kosugi, M. Sato, R. Sato, K. Nakaya, and T. Nishijima for their support in the field. The Marine Biomedical Institute of Sapporo Medical University provided space and resources for the fieldwork in 2004. Hokkaido Souya subprefectural office gave us permission (no. 46, 47 in 2004 and no. 7-1, 7-2 in 2005) to work on Black-tailed Gulls and to cut back vegetation in the study areas at Rishiri Island. We are grateful to M. J. Dunn, Y. Suzuki, D. Lyons, editors, and anonymous referees for their very helpful comments and suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Brown
Rights and permissions
About this article
Cite this article
Kazama, K., Watanuki, Y. Individual differences in nest defense in the colonial breeding Black-tailed Gulls. Behav Ecol Sociobiol 64, 1239–1246 (2010). https://doi.org/10.1007/s00265-010-0938-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-0938-8