Abstract
Combining chemical analysis and odour preference tests, we asked whether two closely related sympatric species of sac-winged bats use odour for species recognition. Males of the two sister species Saccopteryx bilineata and Saccopteryx leptura have pouches containing an odoriferous liquid in their antebrachial wing membrane, which is used in S. bilineata during courtship displays. Although both species occasionally share the same daytime roosts and are morphologically similar, there is no evidence for interbreeding. We compared the production and composition of the wing sac odorant in male S. leptura and S. bilineata and performed odour preference tests with female S. bilineata. Similar to male S. bilineata, male S. leptura cleansed and refilled their wing sacs with secretions, but they spent more time each day in doing so than male S. bilineata. Chemical analysis by gas chromatography and mass spectrometry revealed that male Saccopteryx carried species-specific scents in their wing sacs. Binary choice tests confirmed that female S. bilineata preferred the wing sac scents of male S. bilineata to those of the sister species, suggesting that the species specificity of male wing sac scents maintain the pre-mating isolation barrier between these closely related species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A basic but nonetheless crucial prerequisite to increasing one’s own fitness is to identify a mate of the same species. Thus, species recognition is a fundamental but often not trivial aspect of mate choice (Andersson 1994). Mating traits used in interspecific mate choice (species recognition) are especially important when closely related species occur in sympatry to prevent these species from hybridisation. Mate choice, however, includes the ability to find not only a genetically compatible mate but also a mate of high quality, so that interspecific and intraspecific (sexual selection) mate choices are not independent of each other (Ryan and Rand 1993; Boake et al. 1997; Pfennig 1998; Higgie and Blows 2007, 2008; Kozak et al. 2008). The investigation of mating signals that are involved in both interspecific and intraspecific mate recognition might consequently help in understanding how mating signals have evolved.
The importance of chemical signals in social communication and mate choice in mammals is well documented (e.g. Albone 1984; Burger 2005). Many mammalian species have species-specific odours, enabling individuals to recognise conspecifics based on olfactory cues (Nevo et al. 1976; Welsh et al. 1988; Heth et al. 1996; Heth and Todrank 2000; Bininda-Emonds et al. 2001; Zhang et al. 2002, 2003; Ganem et al. 2008). Studies on mammalian olfactory communication, however, are often either based on odour preference tests or on chemical analysis of the signal, whereas studies combining both are still scarce. In addition, most of our knowledge of mammalian communication is based on studies conducted with captive rodents. For rodents, it has been argued that the nocturnal and cryptic lifestyle promoted the evolution of olfaction as a major modality of communication (Burger 2005). These lifestyle characteristics are especially applicable for bats, but studies on olfactory communication in bats are rare. The few existing studies demonstrated the potential use of volatiles for kin recognition (Gustin and McCracken 1987), individual recognition (Caspers et al. 2008) or detection of colony members (DeFanis and Jones 1995; Bouchard 2001; Bloss et al. 2002; Safi and Kerth 2003). In this study, we used a multidisciplinary approach to test whether olfactory signals of Saccopteryx bilineata, which are involved in courtship displays (Voigt and von Helversen 1999; Voigt 2005; Voigt and Schwarzenberger 2008), are also used for interspecific communication. We investigated whether male wing sac odorants differ between two sympatric sister species, whether females use male odours to distinguish between individuals of their own and a closely related species and, consequently, which aspects of the signal might be important for species recognition.
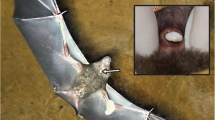
In S. bilineata, odours play an important role during courtship displays and probably also during mate choice (Voigt and von Helversen 1999; Voigt 2005; Caspers et al. 2008). We investigated whether the wing sac odour is used for species recognition in two species of sac-winged bats (S. bilineata and Saccopteryx leptura) that occur sympatrically throughout Latin America. S. bilineata has originated from the same ancestor as S. leptura and can thus be considered a sister species (Lim et al. 2007). These species are similar in morphology and visual appearance, and occasionally, they share the same roosts (Bradbury and Emmons 1974; Voigt 2003). In both species, only males possess antebrachial wing sacs, which contain odoriferous secretions (Bradbury and Emmons 1974; Voigt 2005). Histological studies of the wing sacs, however, revealed that antebrachial wing sacs are free of glandular tissues (Starck 1958; Scully et al. 2000). Male S. bilineata fill their sacs with various odoriferous liquids and secretions, and this particular behaviour has been named perfume blending (Voigt and von Helversen 1999). Perfume-blending behaviour is a stereotypical time-consuming process, which may last up to an hour and includes two separate phases. During the first phase, males take up urine orally and afterwards lick their wing sacs intensively. During the second phase, males deposit some secretions onto the chin by pressing their chin onto their genitalia. The secretion droplet attached to the chin is then smeared into one of the wing sacs. Each phase is characterised by several iterations of bending movements, licking or secretion transfer movements, and both phases are interrupted by several minutes of resting (Voigt and von Helversen 1999; Voigt 2002). Currently, it is unknown whether males of other species within the genus Saccopteryx show a similar behaviour.
Male S. bilineata display their wing sac odour several times a day and exhibit a large repertoire of behaviours during which the wing sacs are opened (Bradbury and Emmons 1974; Bradbury and Vehrencamp 1976; Voigt and von Helversen 1999; Voigt 2005). For example, every morning, males hover in front of females that returned to their harem (Bradbury and Emmons 1974; Voigt and von Helversen 1999). This behaviour is repeated several times during the day and again in the late afternoon prior to emergence from the daytime roost (Voigt 2002). Hovering flights as well as the chemical signals may act as a kind of courtship display, since males hover more frequently prior to and during the mating season than afterwards (Voigt et al. 2007), and hovering flights precede copulations. Furthermore, scent profiles of wing sacs show differences among individuals (Caspers et al. 2008), suggesting that wing sacs bear the potential to be involved in mate recognition.
We compared the production and composition of wing sac odorants in the two species. We observed male S. leptura in their daytime roost during the afternoon hours to find out whether they show a perfume-blending behaviour similar to the one in S. bilineata. Then, we analysed the compounds of the wing sac odorant using gas chromatography and mass spectrometry (GC-MS) and compared the scent profiles of male S. bilineata and S. leptura using a non-parametric variance analysis. We predicted that production and composition of male wing sac odorant differs between the two sister species. Finally, to find out whether the wing sac odorant is involved in species recognition, we performed binary odour preference tests with free-living female S. bilineata. In these odour preference tests, we tested the female preference for the odour of an unfamiliar male of its own species (S. bilineata) and for the odour of an unfamiliar male of the sister species (S. leptura). We predicted that female S. bilineata prefer the odour of a male conspecific (S. bilineata) to that of a male S. leptura.
Materials and methods
We performed our study in the vicinity of “La Selva” Biological Station, which is located in the Caribbean lowlands of northern Costa Rica (Heredia province, Costa Rica; 10°25′ N, 84°00′ W) and surrounded by tropical and premontane wet forest. Individuals used in our study were from different colonies (S. leptura: three colonies; S. bilineata: five colonies) to ensure that colony-specific differences would not bias our results.
Behavioural observations
We observed the perfume-blending behaviour in five male S. leptura roosting in three different colonies. Activities of colony members were documented between December 2003 and January 2004, in December 2005 and in November 2007 by videotaping the activities of bats between 1300 hours and 1600 hours using a Sony Mini DV camcorder (DCR-TRV 14E, Sony, Tokyo, Japan). For each focus male, we analysed four different parameters as defined by Voigt (2002) to compare the perfume-blending behaviour between male S. bilineata and male S. leptura: duration of phase 1 (min), number of urine uptake movements (n), duration of phase 2 (min), and number of secretion uptake movements (n).
Sample collection
We collected samples of the wing sac odorant for chemical analysis from January through March 2005. S. leptura were captured either during the late afternoon (1600–1700 hours) or at dawn (0600–0630 hours) when bats foraged along trails close to their daytime roost. Male S. leptura were captured in the vicinity of three different colonies. We captured S. bilineata when they emerged from (1700–1800 hours) or returned to (0500–0530 hours) their daytime roost. We used nylon mist nets (Avinet, Dryden, NY, USA; net length 2.6 m, height 2.6 m, mesh width 38 mm) to catch the bats in flight. We collected scent samples from adult male S. bilineata and S. leptura by wiping out each wing sac with a piece of cotton (DIN 61640-CO, 100% cotton; Hartmann, Heidenheim, Germany), resulting in two scent samples per individual. Prior to scent collection, the cotton was cleansed with dichloromethane (99.9%) and dried at ambient temperature. Samples were transferred into Teflon-capped glass vials (2 ml, Rotilabo®, Roth, Karlsruhe, Germany). We added 100 μl dichloromethane (99.9%) as a preservative to all samples that were assigned to later chemical analysis. We stored all samples at −20°C until use in experiments or chemical analysis. For chemical analysis, we collected samples of the wing sac odorant from eight adult male S. bilineata and from four adult male S. leptura. We marked each individual with a small plastic band to allow recognition of recaptured bats (A.C. Hughes Ltd., Middlesex, UK; size XCL). We estimated the age of the bats based on the colour of the interior of the wing sacs, which are black in juvenile S. bilineata and whitish in adult S. bilineata (Voigt and von Helversen 1999). All bats were released at the site of capture immediately after scent collection or preference tests.
Chemical analyses
The wing sac odour is an airborne distributed signal, and thus, we analysed the volatile substances since we think that these are of biological interest. Scent samples of S. bilineata and S. leptura were analysed with a GC-MS (Hewlett-Packard 5890 gas chromatograph, Hewlett-Packard Corporation, Palo Alto, CA, USA) equipped with a 30-m DB5-coated capillary column (J and W Scientific, Folsom, CA, USA) attached to a Hewlett-Packard mass selective detector (70 eV EI). Prior to analysis, we extracted the samples from the cotton with a syringe (50-μl syringe, blunt needle point, Hamilton, Bonaduz, Switzerland). We squeezed out the cotton ball by pressing the blunt point syringe on it. Using that method, we extracted the dichloromethane-extracted compounds from the cotton ball and transferred them into another glass vial (2 ml, Rotilabo®, equipped with a 100-μl inlet; Rotilabo®). We condensed the extract to a maximum volume of 5 μl by evaporation. We injected 1.5 μl of the sample into the GC and collected data under the following conditions: splitless injection, helium as carrier gas, 60°C inlet temperature, 3-min initial time, 10°C min−1 rate, 280°C final temperature, 20-min final time. Peaks were matched by retention time and mass spectra (Wiley Library, John Wiley & Sons Ltd., UK) or by synthesis of compound candidates and comparative GC-MS analysis (Schroeder et al., unpublished). For further statistical analyses, the relative proportion (%) of each peak to the total peak area was calculated. We defined substances as species-specific if they occurred in the wing sac odour of all individuals of the species and in none of the samples collected from the other species.
Odour preference tests
We performed odour preference tests from January through March 2005 and in November 2007. Odour sample collection followed the same protocol as described above with the exception that no solvent was added to the samples. For odour preference tests, we collected odour samples from ten adult male S. bilineata and nine adult male S. leptura. We tested ten adult female S. bilineata in a two-choice preference test to find out whether female S. bilineata are able to distinguish between the odour of male S. bilineata and S. leptura. Female S. bilineata were caught in their daytime roost (0600–1600 hours) using a hand-held mist net and immediately transferred to a choice arena (Fig. 1a). We performed all preference tests in the vicinity of the daytime roost. Thus, bats were exposed to ambient temperature and humidity. The choice arena was set up in the shade to ensure that bats were not exposed to direct sunlight, which may affect the side preference of the bats. The choice arena consisted of a conventional plastic bucket with the base removed (35-cm diameter; 40-cm height) and divided into two compartments (Fig. 1a). The wall of the upper compartment was lined with metal mosquito gauze, enabling the animals to cling to the wall. The lower compartment was attached to the ground, whereas the upper compartment with the roosting bat could be turned relative to the lower compartment and thus relative to the two odour sources. For habituation of animals to the experimental conditions in the choice arena, individuals were released into the upper compartment at least 5 min before odours were presented. At the onset of the experiment, we attached two odour-impregnated cotton balls to opposite sides of the inner wall of the lower compartment, 1 cm below the mosquito gauze bottom of the upper compartment. The upper compartment was rotated in relation to the lower compartment so that roosting bats were positioned equidistant to both odour sources. One cotton ball contained the odour of a male S. bilineata and the other the odour of a male S. leptura. The side on which each sample was placed was chosen at random. We used a small fan for the first minute to blow the stimulus odours upwards into the upper compartment, where the bat hung. Each experiment lasted 10 min. We recorded all movements of the bat during this time using an infrared video camera (Sony DCR-TRV 14E, Sony, Tokyo, Japan). The camera was mounted 1 m above the arena on a tripod. We cleaned the choice arena after each trial using dichloromethane-soaked cotton.
Odour preference test arena (a) consisting of two compartments: the upper compartment could be turned in relation to the lower compartment. The test animal roosted in compartment I, which was completely lined with metal mosquito gauze. The stimulus odours were set into the sample holders in compartment II, and the fan propelled the odour into the upper compartment. b Top-down view of the odour preference test arena. A 10-cm radius was drawn around each stimulus odour to outline the preference zone (grey area). Prior to the test, compartment I was turned in relation to the lower compartment so that the bat was equidistant from both stimulus odours and outside of the preference zones (white area)
To quantify a female’s preference, we defined two preference zones as a 10-cm radius around each stimulus odour (Fig. 1b). This actually reflects the average distance between two individuals in a harem group. In addition, we used a 10-cm radius to warrant that there was a non-preference zone of about the size of the hanging bat between the two preferences zones, enabling us to set the bat in a non-preference zone at the beginning of the test by turning the upper compartment. We started to measure the time when a female moved with her whole body into a preference zone. We analysed the amount of time a female spent in each preference zone in relation to the total time the female spent in both preference zones, and we based statistical tests on the binary information (more or less than 50% of time present in the S. bilineata preference zone).
We used only odours of unfamiliar males, i.e. we offered odours only from males originating from colonies located at least 0.8 km away from the females’ colonies. Each male was used only once as a donor of the stimulus odour, except for one male S. leptura which was used twice as a donor for the stimulus odour. Each odour sample was used only once.
Statistical analyses
We used the data about perfume blending in S. bilineata from Voigt (2002) to assess the species specificity of perfume-blending behaviour. Perfume-blending parameters were compared between the two species using a Mann–Whitney U test (MWU). To calculate the number of substances in the species’ scent profiles, we considered only substances whose peak areas contributed more than 0.1% to the total peak area. We compared the number of peaks between male S. bilineata and male S. leptura using a MWU test. Using all selected peaks, we compared odour sample composition by computing a similarity matrix based on the Bray–Curtis similarity index (Clarke and Warwick 1994). Potential differences between the species were then evaluated by applying non-parametric variance analysis (ANOSIM and SIMPER—see Clarke and Warwick 1994) to these similarities with species as the factor. Additionally, we plotted the data in two dimensions using multi-dimensional scaling (MDS). A binomial test was used to compare the proportion of successes (>50% of time with male of own species) in the odour preference test with an expected probability of P 0 = 0.5. We used a directed test because we expected females to choose the odour of the conspecific male (see Rice and Gaines 1994). We used a directed test instead of a one-tailed test to warrant that we do preclude a rejection if the results come out in the unexpected direction. Results are given as mean ± 1 SE. Statistical analyses were performed using PRIMER 5.0 (Primer-E 2000, Plymouth, UK) and StatXact 7.0 (Cytel, Cambridge, MA, USA). The significance level was set to α = 0.05.
Results
Comparison of perfume blending
Similar to male S. bilineata, male S. leptura cleansed and refilled their wing sacs during a two-stage process. During phase 1, males bent their head towards their genital region, took up urine orally and licked their wing sacs afterwards. During phase 2, males also showed bending movements of the head towards the genital region, but in contrast to phase 1, males transferred secretions into the wing sac by a sideward movement of the head along the wing sacs, during which their chin briefly touched the wing sac. Similar to male S. bilineata, male S. leptura did not lick the wing sacs during phase 2. We found no significant difference in the duration of phase 1, between male S. leptura and male S. bilineata, but the number of urine uptake movements was significantly higher in male S. leptura than in male S. bilineata (see Table 1). Additionally, the duration of phase 2 was significantly longer and the number of secretion uptake movements was significantly higher in male S. leptura than in male S. bilineata (see Table 1).
Comparison of male wing sac odours between species
Chemical analysis using gas chromatography and mass spectrometry revealed that male S. bilineata and male S. leptura had a similar number of substances in the wing sac odorant (S. bilineata, 51.3 ± 5.6 compounds; S. leptura, 41.5 ± 10.9 compounds; MWU test: U = 10.5; N 1 = 8; N 2 = 4; p = 0.37). Both species shared the majority of substances (63%). The scent profiles of male S. bilineata and male S. leptura shared six of the nine male-specific substances found in S. bilineata, namely, indole, 2-aminoacetophenone, anthranilic acid, indole-3-carboxaldehyde, tryptanthrin and pyrocoll, whereas three of the male-specific substances, namely, indole-3-carboxylic acid, 2,6,10-trimethyl-3-oxo-6,10-dodecadienolide and C15H24O2, were absent in S. leptura. Scent samples of male S. leptura contained two unidentified species-specific substances, which were not present in the odour samples of male S. bilineata (Fig. 2). Analyses of the wing sac contents of four adult male S. leptura and eight adult male S. bilineata showed that the scent profiles of the wing sac liquids differed between the two species (ANOSIM: p = 0.006). The similarity of odour samples was on average higher among individuals of the same species (SIMPER, average within group similarity S. bilineata, 65.72%; average within group similarity S. leptura, 57.12%) than among individuals of different species. Differences between the two species were also due to different relative amounts of substances present in the wing sac odour of each species, such as tryptanthrin (S. bilineata, 6.9 ± 3.8%; S. leptura, 17.5 ± 6.9%) or pyrocoll (S. bilineata, 1.0 ± 0.9%; S. leptura, 10.8 ± 7.2%). A comparison of the MDS-transformed chromatogram data illustrates (Fig. 3) that the species-specific odour profiles are grouped in the two-dimensional plot, thus indicating a difference in the composition of the wing sac odorant between the species.
Two-dimensional MDS plot of the scent samples of male S. bilineata (black triangle) and S. leptura (white triangle). The plot is based on Bray–Curtis similarities of the relative abundances of all substances. The wing sac odour samples of S. bilineata (black triangle) and S. leptura (white triangle) form two distinct groups according to an ANOSIM (global R = 0.6, p = 0.006)
Odour preference tests
We tested ten adult female S. bilineata for their preference for either of the wing sac odorants from the two species. Two females did not move in the choice arena during the 10-min experimental period, and we therefore excluded them from our analyses. Females spent on average 201.9 ± 128.9 s in both preference zones and spent on average three times more time in the vicinity of the odour of male S. bilineata (154.8 ± 54.0 s) than they did in the vicinity of the odour of male S. leptura (47.1 ± 12.8 s; binomial test, N = 8, p < 0.05; directed test; Fig. 4).
Relative proportion of time female S. bilineata spent in the preference zone of the odour of a male conspecific (S. bilineata) or a male of the sister species (S. leptura). The left part of the graph shows the preference area for S. leptura and the right part shows the preference area for S. bilineata. Female S. bilineata spent more time in the vicinity of the odour of a male S. bilineata significantly than they did close to the odour of a male S. leptura (binomial test; N = 8, p < 0.05, directed test)
Discussion
Our results support that olfactory cues are involved in social communication and species recognition in bats. Male S. leptura blended the perfume of their wing sacs in a similar way like male S. bilineata, but spent even more time in doing so. The wing sac odour composition of males differed between species, and female S. bilineata preferred the odour of male S. bilineata to that of S. leptura.
Perfume blending
Male S. leptura show a similar perfume-blending behaviour to that of male S. bilineata, i.e. they cleanse and refill their wing sacs with odoriferous secretions from various body regions during two stereotypic behavioural sequences. Secretions from different body regions may differ in their information content, and therefore, the overall odorant may include multiple information (Johnston et al. 1993). Previous chemical analysis revealed that wing sac odorant of male S. bilineata is a multi-component and composite signal enabling males to transfer information in different situations by using the same signal (Caspers et al. 2008). It is likely that the wing sac odorant of S. leptura is also a multi-component signal given its compound origin.
Thus far, we have neither observed S. leptura using wing sac odorant, nor are any observations reported in the literature to our knowledge. However, the observation of perfume blending in colonies of S. leptura suggests that olfaction is of major importance for social communication in this species as well. Perfume blending lasted even longer in S. leptura than in S. bilineata, even though S. leptura spent less time in the daytime roost than S. bilineata. Thus, S. leptura put more effort into perfume blending than S. bilineata not only on an absolute time scale but also in relation to the overall time spent in the daytime roost.
Species-specific odours
Chemical analysis revealed differences in the composition of the wing sac odour between S. bilineata and S. leptura. Differences in the composition of the wing sac odour could be attributed to several factors. (1) Differences in odour composition might emerge from different glands contributing to the wing sac odorant of the two species. In male S. bilineata, at least two types of glands are associated with perfume blending: an intermandibular gland and a gular gland (Caspers et al. 2009). Since the gular gland is missing in S. leptura (Scully et al. 2000), some of the compounds specific to S. bilineata may originate from this gland. (2) Differences in odour composition might also be the result of dietary differences. S. bilineata may hunt slightly larger insects than S. leptura, since species size correlates positively with the size of its insect prey (Bradbury and Vehrencamp 1976). These dietary differences might convert into subtle, but still significant, difference in the species-specific odour profiles. (3) Bacterial metabolism as a cause of differences in the species-specific odour profiles seems unlikely. Microbial analyses of the wing sacs in S. bilineata showed a high individual variation in the microbial floral of the wing sacs (Voigt et al. 2005), with, on average, only two different microbial species per individual, the converse of what we would expect if species-specific odours are largely influenced by microbial metabolism.
The differences in the composition of the wing sac odour of the two Saccopteryx species were due to species-specific substances that were absent in the wing sac compounds of the other species and to the relative amounts of some shared substances. Species-specific odours are known for a variety of other mammals (Microtus: Welsh et al. 1988; Mastomis: Apps et al. 1990; mongoose: Decker et al. 1992, Mustela: Zhang et al. 2003). Microtus montanus and Microtus pennsylvanicus, for example, differ in the composition of preputial gland secretions (Welsh et al. 1988), and chemical analysis revealed differences in the anal gland volatiles of Mustela eversmanni and Mustela sibirica (Zhang et al. 2003). Overall, species-specific odours may be omnipresent in mammals and may play a major role during mate choice even in taxa like Chiroptera that are better known for their acoustic capabilities.
Preference tests
Using odour preference tests, we showed that female S. bilineata were able to distinguish between the male odours of the two species, i.e. female S. bilineata preferred the odour of male conspecifics to the odour of male S. leptura. These findings support that the wing sac odorant might be used in mate recognition and might function as a pre-mating isolation barrier. In general, odours are predisposed to be honest signals in mate recognition or mate choice, including species recognition (Albone 1984; Penn and Potts 1998; Johansson and Jones 2007). Furthermore, it has been shown that there is a link between genetic relatedness and similarities in odour composition within as well as between species (Heth and Todrank 2000; Heth et al. 2003; Todrank and Heth 2003). Wing sac odour in S. bilineata is used during hovering displays, which precede copulations, and males keep their wing sacs open during copulation. It may therefore be one of the last opportunities for females to recognise a non-conspecific male.
Speciation and sexual selection have long been considered as agonistic factors, leading to the assumption that a single trait cannot be shaped by both factors because sexually selected traits ought to vary with mate quality whilst remaining sufficiently constant to encode species-specific information. Our finding that the wing sac odour, which is most likely affected by sexual selection (Voigt and von Helversen 1999; Voigt et al. 2005, 2008), is also used in species recognition is in line with other studies which showed that traits used in mate choice and species recognition are often multi-component (Ryan and Rand 1993) or multimodal (Kozak et al. 2008). Using a multi-component or multimodal trait, the signaller can transfer different information at the same time. The signal, or aspects of the signal, can be sufficiently constant within a population to be used for species recognition, whereas other aspects can vary with male quality. In S. bilineata, the existence of the species-specific substances might be used to recognise a mate of one’s own species, whilst the quantities of substances within the scent are specific for individuals and might therefore be used for individual recognition (Caspers et al. 2008). The multi-component characteristic of mammalian odours in general and the wing sac odour in S. bilineata in particular seem to be an adaptation to the combined needs for species recognition and for finding an appropriate mate among individuals of its own species.
References
Albone ES (1984) Mammalian semiochemistry. Wiley, New York
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, New Jersey
Apps PJ, Gordon DH, Viljoen HW, Pretorius V (1990) Chromatographic analysis of species specific odor profiles in Mastomys natalensis and M. coucha (Rodentia: muridae). J Chem Ecol 16:2667–2676
Bininda-Emonds ORP, Decker-Flum DM, Gittleman JL (2001) The utility of chemical signals as phylogenetic characters: an example from the Felidae. Biol J Linnean Soc 72:1–15. doi: 10.1006/bijl.2000.0492
Bloss J, Acree TE, Bloss JM, Hood WR, Kunz TH (2002) Potential use of chemical cues for colony-mate recognition in the big brown bat, Eptesicus fuscus. J Chem Ecol 28:819–833
Boake CRB, DeAngelis MP, Andreadis DK (1997) Is sexual selection and species recognition a continuum? Mating behavior of the stalk-eyed fly Drosophila heteroneura. PNAS 94:12442–12445
Bouchard S (2001) Sex discrimination and roostmate recognition by olfactory cues in the african bats Mops condylurus and Chaerephon pumilus. J Zool 254:109–117
Bradbury JW, Emmons L (1974) Social organisation of some Trinidad bats. I. Emballonuridae. Z Tierpsychol 36:137–183
Bradbury JW, Vehrencamp SL (1976) Social organization and foraging in emballonurid bats I. Field studies. Behav Ecol Sociobiol 1:337–381
Burger BV (2005) Mammalian semiochemicals. Top Curr Chem 240:231–278
Caspers B, Franke S, Voigt CC (2008) The wing sac odour of male greater sac-winged bats (Saccopteryx bilineata) as a composite trait: seasonal and individual differences. In: Hurst JL, Beynon RJ, Roberts SC, Wyatt TD (eds) Chemical signals in vertebrates XI. Springer, New York, pp 151–160
Caspers B, Wibbelt G, Voigt CC (2009) Histological examinations of facial glands in the greater sac-winged bat Saccopteryx bilineata (Chiroptera, Emballonuridae) and the potential use in territorial marking. Zoomorphology. doi:10.1007/s00435-008-0072-6
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory
Decker DM, Ringelberg D, White DC (1992) Lipid components in anal scent sacs of 3 mongoose species (Helogale-Parvula, Crossarchus-Obscurus, Suricata-suricatta). J Chem Ecol 18:1511–1524
De Fanis E, Jones G (1995) The role of odour in discrimination of conspecifics by pipstrelle bats. Anim Behav 49:835–839
Ganem G, Litel C, Menormand T (2008) Variation in mate preference across a house mouse hybrid zone. Heredity 100:594–601
Gustin MK, McCracken GF (1987) Scent recognition between females and pups in the bat Tadarida brasiliensis mexicana. Anim Behav 35:13–19
Heth G, Todrank J (2000) Individual odour similarities across species parallel phylogenetic relationships in the S. ehrenbergi superspecies of mole-rats. Anim Behav 60:789–795. doi:10.1006/anbe.2000.1538
Heth G, Nevo E, Todrank J (1996) Seasonal changes in urinary odors and in responses to them by blind subterranean mole rats. Physiol Behav 60:963–968
Heth G, Todrank J, Busquet N, Baudoin C (2003) Genetic relatedness assessment through individual odour similarities in mice. Biol J Linn Soc 78:95–603
Higgie M, Blows MW (2007) Are traits that experience reinforcement also under sexual selection? Am Nat 170:409–420. doi:10.1086/519401
Higgie M, Blows MW (2008) The evolution of reproductive character displacement conflicts with how sexual selection operates within a species. Evolution 62:1192–1203. doi:10.1111/j.1558-5646.2008.00357.x
Johansson BG, Jones TM (2007) The role of chemical communication in mate choice. Biol Rev 82:265–289. doi:10.1111/j1469-185X.200700009.x
Johnston RE, Derzie A, Chiang G, Jernigan P, Lee H-C (1993) Individual scent signatures in golden hamsters: evidence for specialization of function. Anim Behav 45:1061–1070
Kozak HL, Cirino LA, Ptacek MB (2008) Female mating preferences for male morphological traits used in species and mate recognition in the Mexican sailfin mollies, Poecilia velifera and Poecilia petenensis. Behav Ecol 19:463–474. doi:10.1093/beheco/arm139
Lim BK, Engstrom MD, Bickham JW, Patton JC (2007) Molecular phylogeny of New World sheath tailed bats (Emballonuridae:Diclidurini) based on loci from the four genetic transmission systems in mammals. Biol J Linnean Soc 93:189–209
Nevo E, Bodmer M, Heth G (1976) Olfactory discrimination as an isolating mechanism in speciating mole rats. Experientia 32:1511–1512
Penn D, Potts WK (1998) Chemical signals and parasite-mediated sexual selection. TREE 13:291–296
Pfennig KS (1998) The evolution of mate choice and the potential for conflict between species and mate-quality recognition. Proc R Soc Lond B 265:1743–1748
Rice WR, Gaines SD (1994) ‘Heads I win, tails you’: testing directional alternative hypotheses in ecological and evolutionary research. TREE 9:235–237
Ryan MJ, Rand AS (1993) Species recognition and sexual selection as a unitary problem in animal communication. Evolution 47:647–657
Safi K, Kerth G (2003) Secretions of the interaural gland contain information about individuality and colony membership in the Bechstein’s bat. Anim Behav 65:363–369. doi:10.1006/anbe.2003.2067
Scully WMR, Fenton MB, Saleuddin ASM (2000) A histological examination of the holding sacs and glandular scent organs of some bat species (Emballonuridae, Hipposideridae, Phyllostomidae, Vespertilionidae and Molossidae). Can J Zool 78:613–623
Starck D (1958) Beitrag zur Kenntnis der Armtaschen und anderer Hautdrüsenorgane von Saccopteryx bilineata Temminck 1838 (Chiroptera, Emballonuridae). Gegenbaurs Morphol Jahrb 99:3–25
Todrank J, Heth G (2003) Odor–genes covariance and genetic relatedness assessments: rethinking odor based “recognition” mechanisms in rodents. Adv Stud Behav 32:77–130
Voigt CC (2002) Individual variation of perfume blending in male sac-winged bats. Anim Behav 63:31–36
Voigt CC (2003) Sac-winged bats, sheath-tailed bats, and ghosts bats (Emballonuridae). Grizmek’s life encyclopedia: mammals II, vol 13 (edited by Kleiman DG, Geist V, Hutchins M, McDade MC). Gale Group, Farmington Hills, Michigan, pp 355–365
Voigt CC (2005) The evolution of perfume blending and wing sacs in emballonurid bats. In: Mason RT, LeMaster MP, Müller-Schwarze D (eds) Chemical signals in vertebrates X. Springer, New York, pp 93–100
Voigt CC, Schwarzenberger F (2008) Female reproductive endocrinology of a small tropical bat (Saccopteryx bilineata; Emballonuridae) monitored by fecal hormone metabolites. J Mammal 89:50–57
Voigt CC, von Helversen O (1999) Storage and display of odor in male Saccopteryx bilineata Emballonuridea. Behav Ecol Sociobiol 47:29–40
Voigt CC, Caspers B, Speck S (2005) Bats, bacteria, and bat smell: sex-specific diversity of microbes in a sexually selected scent organ. J Mammal 86:745–749
Voigt CC, Streich JW, Dehnhard M (2007) Assessment of fecal testosterone metabolite analysis in free ranging Saccopteryx bilineata (Chiroptera; Emballonuridae). Acta Chiropt 9:463–476
Voigt CC, Behr O, Caspers B, Helversen von O, Knörnschild M, Mayer F, Nagy M (2008) Songs, scents, and senses: sexual selection in the greater sac-winged bat, Saccopteryx bilineata. J Mammal 89:1401–1410
Welsh CJ, Moore RE, Bartelt RJ, Jackson LL (1988) Novel, species-typical esters from preputial glands of sympatric voles, Microtus montanus and M. pennsylvanicus. J Chem Ecol 14:143–158
Zhang J-X, Sun L, Zhang Z-B, Wang Z-W, Chen Y, Wang R (2002) Volatile compounds in anal gland of Sibirian weasels (Mustela sibirica) and steppe polecats (M. eversmanni). J Chem Ecol 28:1287–1297
Zhang J-X, Ni J, Ren X-J, Sun L, Zhang Z-B, Wang Z-W (2003) Possible coding for recognition of sexes, individuals and species in anal gland volatiles of Mustela eversmanni and M. sibirica. Chem Senses 28:381–388
Acknowledgements
We thank the Costa Rican Authorities (MINAE) and especially Javier Guevara for research and collecting permits. We are also thankful to Maike Dieckmann who helped in the field. Dina Dechmann, Nicole Burgener, Detlev Kelm, Tomer Czaczkesmi, and two anonymous referees gave helpful comments on earlier versions of the manuscript. The study was financed by a grant from the Deutsche Forschungsgemeinschaft (DFG) to Christian C. Voigt (VO890/3 and VO890/7) and a grant from the Berliner Chancengleichheitsprogramm to Barbara Caspers. We declare that the experiments complied with the current laws of Costa Rica.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kappeler
Rights and permissions
About this article
Cite this article
Caspers, B.A., Schroeder, F.C., Franke, S. et al. Odour-based species recognition in two sympatric species of sac-winged bats (Saccopteryx bilineata, S. leptura): combining chemical analyses, behavioural observations and odour preference tests. Behav Ecol Sociobiol 63, 741–749 (2009). https://doi.org/10.1007/s00265-009-0708-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0708-7