Abstract
The local resource enhancement (LRE) model predicts that in cooperatively breeding species, sex ratios will be biased in favor of the more helpful sex. In this study, we assess the assumptions underlying the LRE model in a population of cooperatively breeding wild dogs (Lycaon pictus) in Northern Botswana monitored over a 15-year period. In this population, litter size and pup survival to 1 year are strongly affected by pack size and the breeding female’s age, but adult males have a stronger and more linear effect on females’ reproductive performance than do adult females. This asymmetry in the benefits derived from male and female helpers is reflected in male-biased sex ratios in litters at the time pups emerge from the den. Sex ratio biases are most pronounced in the litters of the youngest mothers who live in significantly smaller packs than older females. The presence of potential rivals for the dominant female’s position depresses pup production at the time of emergence, suggesting that competition among females for breeding positions may also contribute to the selective forces affecting birth sex ratios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In obligate cooperatively breeding species, such as meerkats (Suricata suricatta), red cockaded woodpeckers (Picoides borealis), and wild dogs (Lycaon pictus), group members help rear the offspring of a single breeding pair (Clutton-Brock 2002). In some cooperatively breeding species, members of one sex remain in their natal groups longer or provide more help than members of the other sex (e.g., Allainé et al. 2000; Gowaty and Lennartz 1985; Komdeur 1996; Malcolm and Marten 1982). When parents gain more benefits from helpers of one sex than the other, they are expected to adjust their sex ratios in favor of the more helpful sex (Emlen et al. 1986). The magnitude of sex ratio biases will depend on the relative benefits derived from male and female helpers (Emlen et al. 1986) and the opportunities available to dispersing offspring of each sex. The magnitude of sex ratio biases will also be influenced by the magnitude of selection within groups, which favors the less common sex, and selection between groups which can favor sex ratio biases (Wilson and Colwell 1981). Local resource enhancement is expected to favor uniform biases in population sex ratios if the conditions that favor skewed sex ratios do not vary across parents, but sex ratios may be biased in any direction if conditions favoring sex ratio biases vary and parents adjust the sex ratio of their progeny facultatively in relation to their current need for help (Pen and Weissing 2000).
To test predictions derived from the local resource enhancement (LRE) model, Griffin et al. (2005) compiled data on 11 cooperatively breeding bird and mammal species. For each species, they estimated the magnitude of relationships between (1) the number of helpers and offspring production or survival and (2) the number of helpers and offspring sex ratio. They showed that species in which the number of helpers had the greatest effect on offspring production or survival were also the species in which the number of helpers had the largest correlation with offspring sex ratios. They concluded that the benefits derived from helpers were linked to the magnitude of sex ratio adjustments within groups, as predicted from the LRE model.
African wild dogs are one of two mammalian species included in the meta-analysis of Griffin et al. Wild dogs form packs with a single dominant breeding pair, subdominant adults (who are generally related to the same sex member of the breeding pair), and immature offspring. Females produce large litters in relation to their body size (Geffen et al. 1996), sometimes numbering as many as 22 pups. Subordinate females occasionally breed, but rarely raise pups successfully (Girman et al. 1997; Woodroffe et al. 2004). Larger packs are reported to hunt more efficiently and to be more successful in producing and rearing pups than smaller packs (Creel and Creel 2002; Creel et al. 2004; Fuller et al. 1992; Maddock and Mills 1994). Helpers provision the breeding female while she is nursing pups in the den, bring back meat for pups after they emerge from the den, guard pups at the den, and help defend pups against potential predators (Creel and Creel 2002). Males usually remain in their natal packs longer than females do and are therefore able to provide help to their parents over a longer period than females (Malcolm and Marten 1982; McNutt 1996). Adult sex ratios within packs are typically skewed toward males (Creel and Creel 2002). This may be due to sex differences in survivorship (Creel & Creel 2002; Maddock and Mills 1994; McNutt 1996) or skews in birth sex ratios (Fuller et al. 1992; Malcolm and Marten 1982).
If local resource enhancement shapes sex ratios in wild dogs, then we would expect male helpers to have a more pronounced positive effect on pup production or pup survival than females do. It is not known whether such asymmetries exist in wild dogs. The only available evidence suggests, in fact, that adult females may have a greater impact on pup survivorship than adult males (Creel and Creel 2002; Maddock and Mills 1994). In this paper, we examine the effects of male and female helpers on pup production and pup survivorship in a population of wild dogs in northern Botswana observed over a 15-year period. We also assess the factors that influence birth sex ratios in this population.
Materials and methods
The study area is located in the northeastern part of the Okavango Delta in northern Botswana and encompasses an area of approximately 3,000 km2. Wild dogs range throughout this region, but are concentrated in the wildlife areas associated with the Okavango Delta and the Kwando/Linyantii Rivers. For more details about the habitat, see McNutt (1996). During this study period, the wild dog population in northern Botswana was estimated to range between 700 and 986 adults in 78–88 packs (McNutt 2001).
Packs are defined as groups that contain a potential breeding pair, meaning at least one adult male and one unrelated adult female. All individuals are known by unique color markings on their pelage. Pack composition was assessed at the beginning of the denning season, which typically occurs in June. Pups are born underground and first emerge from the den at about 4 weeks of age. The size and composition of litters included in this analysis were recorded within 8 weeks of emergence.
McNutt and colleagues compiled information on 115 litters produced by 55 females in 45 packs over the course of a 15-year period (females reproduce in only one pack; the identity of the breeding male and female in a pack may change). The analyses presented here focus on 84 litters born to 40 females in 33 packs for which complete information about pack composition (number of adult males, adult females, yearlings, age of the breeding female, litter size, and litter sex ratio) was recorded. Pup survivorship to 1 year was known for 71 of these litters. Sample sizes reported below reflect the number of litters for which the relevant information was available.
The parity of breeding females was known for all but one of the litters in this sample. The age and parity of breeding females were very closely linked (β = 0.9929, p < 0.001, R 2 = 0.78; N = 83 litters). We use female age in our analyses because it is known for every litter. Readers should note that we cannot distinguish between the effects of parity and age.
We used parametric pairwise correlations to assess the relationship between female age, pack size, and pack composition. In most analyses of count variables, such as litter size, we used Poisson regression to assess the sources of variation. Poisson regression is a form of the generalized linear model (GLM) which is appropriate for data that conform to the Poisson distribution in which the mean is approximately equal to the variance and there are relatively few zeros. The Poisson regression is also appropriate for rate variables, such as litter sex ratios, in association with an exposure variable that provides a measure of the total number of events corresponding to a particular unit of investigation. For analyses of sex ratio and survivorship, the dependent variables are the number of male pups and the number of surviving pups, respectively, and the exposure variable for both analyses is litter size. In each case, we used goodness of fit tests to confirm that the Poisson model was appropriate.
Approximately half of the females produced multiple litters (mean ± SD = 2.10 ± 1.49, range 1–7, n = 40), and litters produced by the same female are not independent. Mixed models with random effects (GLMM) are recommended in such cases (Krackow and Tkadlec 2001). We examined our dataset to determine whether such models were appropriate. We found little variation in litter size or sex ratio among females (likelihood ratio test of alpha = 0, p > 0.5), but sizable variation among females in pup survival (p < 0.002). Therefore, we used a GLMM Poisson model with random effects to assess the sources of variation in pup survival and used the GLM Poisson model to examine the sources of variation in litter size and litter sex ratios. We tested for nonlinear effects by adding quadratic terms to the regression models; when quadratic terms were nonsignificant, they were dropped from the model and are not presented below.
Coefficients derived from Poisson regression models are based on the differences in the logs of expected counts (or rates). For a one unit change in the predictor variable, the difference in the logs of expected counts is expected to change by the respective regression coefficient when other predictor variables in the model are held constant.
We defined pack size as the number of adults (≥1 years of age) present. Adult females were further divided into two categories. “Potential competitors” are non-dominant breeding adult females, usually sisters of the breeding female, who are unrelated to the breeding male. “Noncompetitors” are non-dominant adult females, usually daughters of the current breeding pair. Yearlings are not included in our measure of pack size because preliminary analyses revealed that the number of yearlings had no significant effect on pup production, pup survival, or litter sex ratios.
We defined the adult sex ratio as the number of adult males divided by pack size. Similarly, we defined litter sex ratio as the number of male pups divided by the total number of male and female pups. The analysis of litter sex ratio is limited to complete litters in which all pups were sexed before any recorded litter reduction.
All statistical analyses were performed with STATA 9.0. Means and standard deviations are reported below.
We found no significant variation in litter size, pup survivorship, or litter sex ratio across years of the study, so we did not consider this factor further in the analyses reported below.
Results
Pack size and pack composition
Packs varied in size from 2 to 30 adults, with an average pack size of 10.4 ± 5.4. On average, there were slightly more adult males in the population than adult females (annual means = 0.51 ± 0.02, range 0.33–0.61, n = 17). Within packs, the average number of adult males typically exceeded the average number of adult females (males, 5.5 ± 3.3; females, 4.7 ± 2.9).
On average, breeding females produced their first litter when they were 2.7 ± 1.0 years old (range = 1 to 5, N = 27 females). The size and composition of breeding females’ packs changed as they got older (Fig. 1). As females aged, their packs got bigger (r = 0.4535, p < 0.001, n = 84). Increases in pack size were due to increases in the numbers of adults of both sexes (males: r = 0.3191, p < 0.003, females: r = 0.4505, p < 0.001; n = 84). As females got older, their own daughters matured, and some of their own sisters died. As a consequence, the number of potential competitors declined as females aged (r = −0.2760, p = 0.011) and the number of non-competing females increased (n = 0.5842, p < 0.001).
Pack composition as females age. The average number of adult males (black), potential competitors for the breeding females’ position (white), non-competitive adult females (striped), and yearlings (gray) is plotted against female age. As females age, their packs get larger and the number of potential competitors declines
Offspring production
Litter size at emergence (litter size, hereafter) varied from 3 to 16 pups, with a mean of 9.5 ± 2.9 pups per litter (N = 84). Both maternal age and pack size affected litter size. Older females produced larger litters than younger females, and females living in large packs produced significantly more pups than females living in small packs (GLM Poisson: age, β = 0.1656, z = 3.31, p = 0.001; age2, β = −0.0163, z = −2.86, p = 0.004; pack size, β = 0.05088, z = 3.31, p = 0.001, pack size2, β = −0.0009, z = −2.174, p = 0.030). The significance of the quadratic terms indicates that the effects of pack size and female age on litter size tapered off as packs got large (Fig. 2a) and females reached older ages (Fig. 2b).
Sources of variation in litter size. a Females who live in large packs have larger litters than females who breed in smaller packs, although the benefits of additional pack members taper off at larger pack sizes. The line represents a quadratic regression fitted to the data. Each point represents one litter, and the size of the symbol is proportional to the number of litters represented. b Older females have larger litters than younger females (other conventions as in a). c The number of adult males has a linear effect on litter size. d The number of adult females increases pup survivorship, but the incremental effects of additional females decline as the number of adult females increases
Pack composition also contributed to variation in litter size. When the age of the breeding female was held constant, the number of adult males in packs had a strong and linear impact on litter size (β = 0.0492, z = 5.47, p < 0.001, Fig. 2c). Packs with more adult females also produced more pups than packs with fewer females, but the benefits derived from additional females declined significantly as the number of females increased (β = 0.0598, z = 2.25, p = 0.024; age2: β = −0.0045, z = −2.36, p = 0.018; Fig. 2d).
The status of non-breeding adult females within the pack affected pup production. When the number of adult males in the pack was held constant, the presence of potential competitors had a negative effect on pup production, and this effect became more pronounced as the number of potential competitors increased (β = −0.0747, z = −2.11, p = 0.035; competitors2: β = 0.0164, z = 3.04, p = 0.002). In contrast, the number of non-competitive adult females had a positive effect on pup production, although this effect declined as their numbers increased (β = 0.0763, z = 2.30, p = 0.021; non-competitors2: β = −0.0067, z = −2.48, p = 0.013).
Litter sex ratio
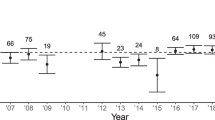
Overall, the sex ratio of pups at emergence was significantly biased toward males (433♂:367♀ = 0.54; binomial p = 0.0215). Sex ratios within litters ranged from 0.11 to 1.00, with a mean of 0.54 ± 0.18 (N = 84 litters; Fig. 3). Younger mothers had significantly more male-biased litters than older females (β = −0.1601, z = −2.31, p = 0.021; age2 = 0.0158, z = 2.48, p = 0.013), but other factors were unrelated to litter sex ratios (pack size: β = 0.0084, z = −1.61, p = 0.107; litter size: β = 0.01987, z = 1.60, p = 0.109; proportion of adult males: β = 0.1987, z = 1.05, p = 0.294). The youngest mothers (1–2 years old) had substantially more male-biased litters than older females (young, 0.65 ± 0.16, N = 12 litters; older, 0.52 ± 0.16, N = 72 litters).
Pup survival
The number of surviving pups per litter varied from 0 to 13, with a mean of 4.5 ± 0.37 (N = 71 litters). On average, less than half of the pups in each litter survived to 1 year of age (0.43 ± 0.03, range = 0 to 1.00). For these litters, we examined the effects of pack size, litter size, pack composition, and the age of the breeding female on pup survival.
The number of surviving pups from each litter was positively influenced by both the size of the litter and the size of the pack. More pups were reared from large litters than from small ones, but this effect diminished as litter size increased (GLMM Poisson: litter size, β = 0.4498, z = 3.31, p = 0.001; litter size2 β = −0.0144, z = −2.25, p = 0.025). Larger packs raised significantly more surviving pups than smaller packs (β = 0.0301, z = 2.50, p = 0.012). The number of surviving pups was not affected by the age of the breeding female (β = −0.0612, z = −171, p < 0.087) or the proportion of adult males in the pack (β = 0.1137, z = 0.29, p = 0.774).
The proportion of pups in each litter that survived to 1 year was directly related to the size of the pack (β = 0.0278, z = 2.33, p = 0.020) and the size of the litter (β = 0.0464, z = 1.95, p = 0.052). Again, the age of the breeding female and the proportion of adult males in the pack did not have a significant impact on pup survival rates (age: β = −0.0571, z = −1.59, p = 0.111; proportion of adult males: β = 0.1279, z = 0.32, p = 0.747).
Discussion
The local resource enhancement model predicts that birth sex ratios will reflect asymmetries in the benefits derived from male and female helpers. For wild dogs in northern Botswana, this prediction seems to hold. Adult males generally outnumber adult females in packs, and adult males contribute more to pack reproductive success than adult females. As in other wild dog populations, females who breed in large packs produce larger litters and have higher survivorship among their pups than females who breed in smaller packs. Young breeding females, who live in small packs with relatively more rivals for the breeding position, produce smaller and more male-biased litters than older females with fewer competitors. Similarly, primiparous females live in smaller packs and have more male-biased litters than multiparous females in the Selous (Creel et al. 1998) and in Hluhluwe-iMfolozi, South Africa (Gusset 2006). This fits the prediction that groups with fewer helpers will produce relatively more of the more helpful sex than groups with larger numbers of helpers (Griffin et al. 2005). However, females did not adjust the sex ratio of their litters in relation to the current size and composition of their packs, and it is not clear whether wild dogs can facultatively adjust litter sex ratios as do the cooperatively breeding Seychelles warblers, Acrocephalus seychellensis, (Komdeur 1996).
Litter size is a major component of variation in female reproductive performance because the number of pups born is significantly related to the number of pups that are alive at 1 year of age. Our data indicate that the number of adult males in packs has a stronger and more linear effect on litter size than does the number of adult females. Sex differences in the effectiveness of male and female helpers could be due to several different factors. First, adult males might be more effective hunters than adult females, so breeding females who live in packs with more adult males are better fed and able to produce larger litters. However, there is no apparent effect of sex on hunting skill, and no significant effects of pack composition on hunting success or efficiency in the Selous (Creel and Creel 2002) or in the Botswana population (McNutt, unpublished data). Second, females living in packs with more adult males might anticipate having more assistance in rearing pups. If so, we would expect the composition of packs to influence pup survivorship as well as litter size, but this is not the case. Pack size, but not the proportion of adult males in packs, is significantly linked to pup survivorship, indicating that females may be just as helpful as males when it comes to provisioning and protecting pups.
Lastly female–female competition may have a negative impact on the number of pups produced, thereby reducing the cumulative benefits derived from female helpers. Reproductive suppression of potential competitors requires active behavioral domination before and during the mating period (Creel et al. 1997), an energetic expense born only by the dominant breeding female. The results presented here suggest that these costs may rise as the number of potential competitors increases, and this may explain the negative relationship between litter size and the number of potential competitors within packs. However, after pups emerge from the den, they are fed and protected by all pack members. Our data suggest the costs associated with the presence of potential competitors are largely eliminated after pups emerge from the den. Pup survival to 1 year of age depends on the size of the pack, not its composition. Although it might be beneficial for subdominant females to compete with their sisters over breeding opportunities, it would not be advantageous for females to neglect closely related pups after they have been born. Thus, female–female competition may be most intense when reproductive suppression of subordinates is most intense: before pups are born or while they are in the den.
If this reasoning is correct, then the effects of LRE may be amplified by the effects of local resource competition in wild dogs. The local resource competition model predicts that sex biases in competition for local resources will favor sex ratio biases in favor of the dispersing sex (Clark 1978; Silk 1984). In this population, males disperse further, later, and in larger groups than females (McNutt 1996). Intense competition for resources provided by helpers may favor the production of male-biased litters, particularly when females are young and most of the other adult females in their packs are rivals for the breeding position.
Changes in the magnitude of sex ratios as females age may help to explain observed variation in birth sex ratios across populations. Males outnumber females at emergence from the den in four of the five wild populations and in captive populations (Table 1), but the extent of the bias varies considerably. Some of the variation may reflect differences in the age structure of populations or differences in the ages of females sampled, as populations with more younger females (or samples with more younger females) would be expected to have more male-biased birth sex ratios than populations (or samples) with more older reproductive females. It is also possible, however, that population-level values could reflect variation in the conditions that individual females encounter and the benefits that they derive from male and female helpers (Pen and Weissing 2000). However, our findings suggest that females do not adjust the sex ratio of their litters in relation to the current size or composition of their packs.
Taken together, the results presented here suggest that birth sex ratios in wild dogs are biased in favor of males and that this bias reflects asymmetries in the costs and benefits derived from male and female pack members. Both local resource enhancement and local resource competition may favor selection for male-biased sex ratios in this species. Further research is needed to determine whether the patterns observed here characterize other populations of wild dogs and to understand the causal factors that shape birth sex ratio biases in wild dogs and other cooperatively breeding mammals.
References
Allainé D (2004) Sex ratio variation in the cooperatively breeding alpine marmot, Marmota marmota. Behav Ecol 15:997–1002
Allainé D, Brondex F, Graziani L, Coulon J, Till-Bottraud I (2000) Male-biases sex ratio in litters of Alpine marmots supports the helper repayment hypothesis. Behav Ecol 11:507–514
Clark AB (1978) Sex ratio and local resource competition in a prosimian primate. Science 201:63–165
Clutton-Brock TH (2002) Behavioral ecology-breeding together: kin selection and mutualism in cooperative vertebrates. Science 296:69–72
Creel S, Creel NM (2002) The African wild dog: behavior, ecology, and conservation. Princeton University Press, Princeton
Creel S, Creel NM, Mills MGL, Monfort SL (1997) Rank and reproduction in cooperatively breeding African wild dogs: behavioral and endocrine correlates. Behav Ecol 8:298–306
Creel S, Creel NM, Monfort SL (1998) Birth order, estrogens and sex ratio adaptation in African wild dogs (Lycaon pictus). Anim Reprod Sci 53:315–320
Creel S, Mills MG, McNutt JW (2004) Demography and population dynamics of African wild dogs in three critical populations. In: Macdonald DW, Sillero-Zubiri C (eds) Biology and conservation of wild canids. Oxford University Press, Oxford, pp 337–350
Emlen ST, Emlen JM, Levin SA (1986) Sex-ratio selection in species with helpers-at-the-nest. Am Nat 127:1–8
Frame LH, Malcolm JR, Frame GW, van Lawick H (1979) Social organization of African wild dogs (Lycaon pictus) on the Serengeti Plains, Tanzania, 1967–1978. Z Tierpsychol 50: 225–249
Fuller TK, Kat PW, Bulger JB, Maddock AH, Ginsberg JR, Burrows R, McNutt JW, Mills MGL (1992) Population dynamics of African wild dogs. In: McCullough DR, Barrett RH (eds) Wildlife 2001: populations. Elsevier Applied Science, London, pp 1125–1138
Geffen E, Gompper ME, Gittleman JL, Luh H-K, MacDonald DW, Wayne RK (1996) Size, life-history traits, and social organization in the Canidae: a reeveluation. Am Nat 147:140–160
Girman DJ, Mills MGL, Geffen E, Wayne RK (1997) A molecular genetic analysis of social sructure, dispersal, and interpack relationships of the African wild dog (Lycaon pictus). Behav Ecol Sociobiol 40:187–198
Gowaty PA, Lennartz MR (1985) Sex ratios of nestling and fledgling red-cockaded woodpeckers (Picoides borealis) favor males. Am Nat 126:347–353
Griffin AS, Sheldon BC, West SA (2005) Cooperative breeders adjust offspring sex ratios to produce helpful helpers. Am Nat 166:628–632
Gusset M (2006) The re-introduction of endangered African wild dogs (Lycaon pictus): a multi-disciplinary evaluation. Ph.D. thesis, University of KwaZulu-Natal
Koenig WD, Walters JR (1999) Sex-ratio selection in species with helpers at the nest: the repayment model revisited. Am Nat 153:124–130
Komdeur J (1996) Facultative sex-ratio biases in the offspring of the Seychelles warblers. Proc R Soc Lond B 263:661–666
Krackow S, Tkadlec E (2001) Analysis of brood sex ratios: implications of offspring clustering. Behav Ecol Sociobiol 50:293–301
Maddock AH, Mills MGL (1994) Population characteristics of African wild dogs Lycaon pictus in the eastern Transvaal lowveld, South Africa, as revealed through photographic records. Biol Conserv 67:67–62
Malcolm JR, Marten K (1982) Natural selection and the communal rearing of pups in African wild dogs (Lycaon pictus). Behav Ecol Sociobiol 10:1–13
McNutt JW (1996) Sex-biased dispersal in African wild dogs, Lycaon pictus. Anim Behav 52:1067–1077
McNutt JW (2001) African wild dogs in northern Botswana. In: Proceedings of the National Technical Predator Management and Conservation Workshop in Botswana. Department of Wildlife and National Parks, October 9–12, pp 18–24
Pen I, Weissing J (2000) Sex-ratio optimization with helpers at the nest. Proc R Soc Lond B 267:539–543
Silk JB (1984) Local resource competition and the evolution of male-biased sex ratios. J Theor Biol 108:203–213
Wilson DS, Colwell RK (1981) Evolution of sex ratio in structured demes. Evolution 35:882–897
Woodroffe R, McNutt JW, Mills MGL (2004) African wild dog Lycaon pictus. In: Sillero-Zubiri C, Hoffmann M, Macdonald DW (eds) Canids: foxes, wolves, jackals and dogs: status survey and conservation action plan. IUCN, Gland, pp 174–183
Acknowledgments
This work was supported by Frankfurt Zoological Society—Help for Threatened Wildlife, grants from Conservation International, National Geographic Society, Woodland Park Zoo, Zoological Society of Philadelphia, and the Moore Family Foundation. We are grateful to Botswana’s Ministry of Environment, Wildlife and Tourism and Department of National Parks for permission to conduct wildlife research in Botswana. We are also grateful for helpful comments on earlier drafts of this manuscript from Markus Gusset and Stuart West, and thank Michael Somers for providing us with access to unpublished work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schulte-Hostedde
Rights and permissions
About this article
Cite this article
McNutt, J.W., Silk, J.B. Pup production, sex ratios, and survivorship in African wild dogs, Lycaon pictus . Behav Ecol Sociobiol 62, 1061–1067 (2008). https://doi.org/10.1007/s00265-007-0533-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-007-0533-9