Abstract
Some non-human primates produce acoustically distinct alarm calls to different predators, such as eagles or leopards. Recipients respond to these calls as if they have seen the actual predator, which has led to the notion of functionally referential alarm calls. However, in a previous study with free-ranging putty-nosed monkeys (Cercopithecus nictitans martini), we demonstrated that callers produced two acoustically distinct alarm calls to eagle shrieks and leopard growls, but both alarm calls were given to both predators. We can think of two basic explanations for this surprising result, a methodological and theoretical one. Firstly, acoustic predator models may not always be suitable to test alarm call behaviour in primates, sometimes causing uncharacteristic behaviour. Secondly, referential alarm calling may not be a universal feature of primate alarm call systems. Considering the methodological and theoretical importance of these possibilities, we conducted a follow-up study using life-sized leopard, eagle, and human models on the same population and compared the resulting vocal responses to those given to acoustic predator models. We compared the alarm call series given to each of these predator model types and found a considerable degree of consistency suggesting that the mode of presentation did not affect anti-predator calling strategies. However, evidence for audience effects on calling behaviour was inconclusive. While it appears that predator class is reliably encoded by different call series types irrespective of the mode of presentation, observations of these same call series given in non-predatory contexts indicate that predator class is unlikely to be the relevant organising principle underlying the alarm-calling behaviour in this species. We conclude by offering an alternative, non-referential, account of the alarm-calling system exhibited by this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Like many other African forest primates, male putty-nosed monkeys produce loud and conspicuous vocalisations that carry over considerable distances (Gautier-Hion et al. 1999). These vocalisations, usually referred to as ‘loud calls’, are normally given by adult males in response to disturbances or as part of a circadian pattern (Gautier and Gautier 1977). Work on Diana monkeys (Cercopithecus diana) and Campbell’s monkeys (Cercopithecus campbelli) has shown that male loud calls can also function as predator alarm calls. In Diana monkeys, male loud calls differ in a number of subtle acoustic features that co-vary with the type of predator spotted by the caller (Riede and Zuberbühler 2003a, b; Riede et al. 2005). These vocalisations have consequently been termed ‘leopard’ or ‘eagle alarm calls’, implying that they function as referential labels for the different predator types, the perceptual bases of a semantic alarm call system. Functionally referential alarm calls were first described in East African vervet monkeys, Cercopithecus aethiops. In this species, individuals produce acoustically distinctive alarm calls to different types of predators and recipients respond to these calls as they would to direct evidence of the corresponding predator (Seyfarth et al. 1980). Because of these and similar observations, it has been argued that primate alarm calls can function as referential, or semantic, signals in the sense that recipients are able to link a particular vocalisation with an external object or event (Zuberbühler et al. 1999a; Seyfarth and Cheney 2003).
Although such findings suggest that non-human primates may share some of the core cognitive capacities required for language, current empirical evidence does not warrant solid conclusions. Firstly, while functionally referential alarm-calling systems have now been reported for a few New World monkey species (Fichtel et al. 2005; Digweed et al. 2005; Kirchhof and Hammerschmidt 2006) and prosimians (Pereira and Macedonia 1991; Fichtel and Kappeler 2002; Fichtel and van Schaik 2006), the strongest evidence for highly predator-specific alarm calls stems from the guenon family (Seyfarth et al. 1980; Zuberbühler et al. 1999a; Zuberbühler 2000a). However, a recent study has found that free-ranging male putty-nosed monkeys, a close relative of Diana, Campbell’s and vervet monkeys, produced long call series consisting of combinations of two types of loud alarm calls, hacks and pyows, in response to two common predators, eagles and leopards, and that callers regularly used both call types to both predators (Arnold and Zuberbühler 2006a). Thus, it is not clear whether the functionally referential alarm-calling systems is representative of primates in general and whether reference is a universal feature of primate communication and cognition (Zuberbühler 2003).
Secondly, as predation events are rarely witnessed and systematic observations are difficult to obtain, especially in forest habitats, one popular alternative method of study has been to present predator models to study anti-predator behaviour in primates. Many field experiments have employed acoustic stimuli in which pre-recorded predator vocalisations were played back to unwary animals from a hidden speaker (Macedonia and Yount 1991; Zuberbühler 2000a, 2001; Fichtel and Kappeler 2002; Eckhardt and Zuberbühler 2004; Rainey et al. 2004; Fichtel and van Schaik 2006; Arnold and Zuberbühler 2006a). However, there are a number of methodological concerns about using acoustic predator models to study primate alarm-call behaviour. It should not be assumed that callers recognise the playback stimulus as a representation of a particular class of predators. Acoustic models are short-lived and might not provide subjects with sufficient evidence of the presence of the predator. Moreover, the predator’s presence and location can never be confirmed visually or otherwise, and this may have important effects on calling behaviour. It is also relevant that ambush predators are unlikely to vocalise while hunting, which might reduce the effectiveness of attempts to simulate their presence using this method. Finally, broadcasting predator vocalisations in essence renders subsequent warning signals redundant: When hearing the vocalisations of a potential predator, both the caller and his potential audience will learn about the predator’s whereabouts simultaneously, perhaps leading to callers’ adopting a different behavioural strategy in these instances.
A useful alternative strategy to address the concerns outlined above is to use realistic, visual models of predators, which have the advantage of providing sustained visual stimulation and thus avoid some of the problems outlined (Cheney and Seyfarth 1985; Macedonia and Polak 1989; Pereira and Macedonia 1991; Brown et al. 1992; Ramakrishnan and Coss 2000; Wich and Sterck 2003; Fichtel and van Schaik 2006). In the present paper, we attempt to achieve this goal by studying the alarm-calling behaviour of putty-nosed monkeys, Cercopithecus nictitans martini, of Gashaka Gumti National Park, Nigeria, in response to life-size models of natural predators, leopards (Panthera pardus), crowned eagles (Stephanoaetus coronatus), and human poachers. We then compare various aspects, including the structure, of the alarm-call series given to acoustic and visual predator models to determine whether differences in the mode of presentation result in differences in alarm-calling behaviour. While we predict that the monkey’s vocal responses to visual predator models will be broadly similar, in terms of the structure of the call sequences given to each predator category, to those given in response to acoustic predator models, there should also be some subtle differences. Specifically, we predict that if alarm calls are given to warn group members, then subjects should respond more strongly by giving more calls when presented with visual models compared with acoustic models as many group members will be ignorant of the presence of a visual predator model, while acoustic models inform all group members simultaneously.
Materials and methods
Study site and species
Field experiments were conducted in Gashaka Gumti National Park, Nigeria, between January and May 2004, by YP. The research was conducted as part of a larger project aimed to investigate the cognitive processes underlying alarm call behaviour of Nigerian forest primates. The study area consisted of approximately 10 km2 of primary rain forest in the Kwano region of the park, near the Gashaka Primate Research Station (7°19′N, 11°35′E). Putty-nosed monkeys live in one-male groups of up to about 20 individuals, with 6–9 adult females and their offspring (K Arnold unpublished data). Group density in the area has been estimated at 3–4 groups per km2 (Dunn 1993).
Predators
Putty-nosed monkeys at Gashaka are hunted by leopards, crowned eagles, and sometimes also humans. These predators vary in their hunting techniques and predation pressure. Forest leopards rely on surprise for a successful hunt, usually close to the ground, and monkeys can easily escape through canopy into safety (Zuberbühler et al. 1999b; Jenny and Zuberbühler 2005). This is not the case for other predators, including large eagles and humans, which can essentially reach monkeys at all heights. Although hunting pressure by humans is probably relatively low at Gashaka Gumti compared to other parts of West Africa, illegal poaching does occur. Crowned eagles can attack monkeys in most parts of the forest canopy, including the forest floor, provided the monkey is sufficiently exposed to an aerial attack (Shultz and Thomsett 2007). Eagles are likely to represent the greatest threat to this population of putty-nosed monkeys because the density of leopards is quite low in the study area and also because eagles can attack at all heights, whereas leopards rely on ambush from the ground.
Alarm calls of male putty-nosed monkeys
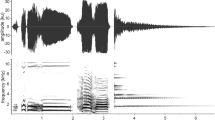
Male putty nosed monkeys regularly produce two different kinds of loud calls, ‘hacks’ and ‘pyows’ (Struhsaker 1970; Gautier-Hion et al. 1999; Eckhardt and Zuberbühler 2004; Arnold and Zuberbühler 2006a; Fig. 1). Both vocalisations are loud and conspicuous, discrete call types that carry over considerable distances in a rainforest habitat and that can easily be distinguished by ear (audioclips S1, S2). Statistical analyses differentiating the acoustic structure of these call types are presented elsewhere (Arnold and Zuberbühler 2006a).
Spectrographic illustration of representative exemplars of the two types of male putty-nosed loud alarm calls. Pyows are acoustically more variable than the hacks, but both call types are perceptually discrete (Arnold and Zuberbühler 2006a)
In an earlier study, we have shown that male putty-nosed monkeys produce apparently complex calling patterns in response to predators. However, these call series are made up of a number of distinct alarm call series types that are either given alone or are combined (Arnold and Zuberbühler 2006a). For each call sequence, we distinguished between three forms: pure hack series, comprised only of hacks; pure pyow series, comprised of only pyows; and transitional series, beginning with a series of hacks followed by a series of pyows. These call series were relatively long and rather variable in terms of the number of calls produced (hack series, mean±SD = 19.8 ± 17.1, n = 17; pyow series, mean±SD = 11.8 ± 12.0, n = 41; transitional series, mean±SD = 11.4 ± 5.3, n = 11). A fourth structure, the pyow–hack sequence, a discrete unit usually comprised of between one and four pyows followed by between one and four hacks, was also given in response to predator models. These comparatively short sequences of calls (mean±SD = 3.1 ± 1.0, n = 39) either preceded, or were inserted within, alarm-call sequences or were given alone. When inserted within an alarm-call series, the beginning and end of the sequence is marked by conspicuous pauses, the durations of which were minimally the mean + 2SD of all preceding inter-call intervals but were typically much longer (Arnold and Zuberbühler 2006a,b). Such pauses were clearly perceptible and enabled pyow–hack sequences to be easily distinguished when situated within a series of similar calls. The same criterion was applied for identifying pauses within series made up of one call type and also within transitional series where a pause usually occurred at the transition point between the hacks and the subsequent pyows. In cases where the pyow–hack sequence was followed by pyows, pauses were sometimes omitted, and the switch from hacks to pyows served to mark the end of the sequence.
Female and juvenile male putty-nosed monkeys tend to respond to experimental stimuli with only one alarm call type, a chirp, irrespective of the predator category (K Arnold, unpublished data). Although it is possible that we may have missed more subtle vocal responses, it is doubtful that these calls have the potential to act as referential signals, and so data concerning their responses are not presented in this paper.
Predator models
A custom-made model of a crowned eagle served as the visual eagle stimulus. The head and torso were constructed using wire mesh and then filled with straw. Two 0.8-m long wings, also made of wire mesh, matched the 1.5–1.7-m wingspan of an adult bird. The body was then covered with a paste of paper and glue to obtain a smooth surface. The fine structure of the head, wings, tail feather base, and claws were moulded from the paste. The bird’s posture resembled that of an individual waiting to ambush prey from dense vegetation. Its wings were folded over its back, crossing each another at the tips. The body and head were covered with chicken feathers, while real tail feathers of an adult crowned eagle, collected from the forest floor, were used for the tail. The chicken feathers, the head, and the claws were painted with commercial paint to match the real patterns of an adult crowned eagle. Finally, a stick was attached to the base to facilitate handling and mounting.
For the visual leopard model, we used a commercially produced replica of about 1.0 m torso length and 0.5 m height. Size, shape, posture, and colouration of both models matched those of real animals well (Fig. 2).
Experimental protocol
The experimenter (YP) systematically searched the study area for monkey groups with the help of a field assistant. Groups were located using acoustic cues, either their vocalisations or noises originating from individuals moving through the canopy. Once a group was located, the experimenter determined their geographical location using a GARMIN 12XL GPS receiver. Most groups were located when animals were moving away from or towards their sleeping trees in the morning or evening, or while moving between feeding sites during the day. After locating a group, the experimenters estimated the most likely direction of the group progression. They then circumnavigated the group at a distance far enough to avoid detection and positioned the predator model along their anticipated path. The models were positioned in relatively open locations, either on the ground (<1 m) or in a tree (range 1.0–13.0 m, average 4.0 m). The experiment simulated a natural situation as both predators are encountered on the ground and in trees. An experimental trial was usually terminated 45 min after detection of a stationary model, 20 min after detection of moving models, or when the group moved away.
Experiment 1. Responses to stationary predators
After positioning the predator model, the experimenter and field assistant moved away and, while hiding under a camouflage cover, started recording the approaching monkeys’ vocal behaviour for at least 5 min, but often longer, before the first monkey detected the model. Vocalisations were tape-recorded with a SONY WMD6C cassette recorder and a SENNHEISER ME62 directional microphone, using standard 90 min Chrome cassette tapes. Calls were digitised on a personal computer, using a CMI8738/C3DX PCI Audio Device sound card and the software COOLEDIT Pro 2.1 (Syntrillium Software Corporation, Phoenix, AZ) with a sampling rate of 44.1 kHz/16-bit accuracy.
Experiment 2. Responses to moving ground predators
To investigate whether putty-nosed monkeys responded differentially to different types of ground predators, we conducted the following experiment. A field assistant approached a group of putty-nosed monkeys wearing either (a) olive-brown clothing as typically used by local hunters, or (b) a large piece of leopard-print fabric, which completely covered the body. A small slit in the fabric allowed the assistant to see, while moving with a hunched posture.
Sample sizes
As none of the study groups were habituated to human presence, and the boundaries of home ranges were unknown, the exact number of groups tested could not be specified. However, experimental groups were drawn from a large pool of about 40 different groups that occupied home ranges throughout the study area. It is therefore unlikely that a particular group experienced any of the four predator models more than once. In experiment 1, the eagle model was presented in an area of 4.3 km2, which potentially incorporated the home ranges of about 15 different groups. The leopard model was presented in an area of 7.3 km2, covering the home ranges of about 25 different groups. The moving leopard model trials were conducted in an area of 9.8 km2, equalling about 35 different groups’ home ranges, while the human poacher trials were conducted in an area of 4.4 km2, equalling about 15 different groups’ home ranges. Although we could not exclude the possibility that a particular group was tested more than once, the location of many of the core areas occupied by each group was known, and care was taken only to test groups within each area once so that the likelihood of retesting a given group with the same stimulus was low.
Data analyses
A number of trials were excluded due to equipment failure (N = 9), interference by real eagles (N = 2), or responses from non-target males (N = 2). Trials were also excluded if the male did not call (eagle, N = 2; stationary leopard, N = 4; moving human, N = 16; moving leopard, N = 0) or called from a distance and never approached the site (eagle, N = 4; stationary leopard, N = 17; moving human, N = 1; moving leopard, N = 1). We excluded these trials because it was impossible to ensure that the male ever directly saw the model, as opposed to, for example, responding to other individuals’ alarm calls. The fact that moving humans caused such a large proportion of silent responses is probably meaningful finding, as it suggests that cryptic behaviour is the more adaptive response to this predator. However, in this study, we were only interested in the males’ selection of alarm call types, not their overall calling strategies.
The distributions of call sequence types given in response to each of the model types were analysed at two levels. Responses fell into one of three main categories: (1) pure hack sequence; (2) transitional sequence; (3) pure pyow sequence. We have shown previously that a fourth sequence, the pyow–hack sequence, is a distinct unit that functions to elicit group movement in both predatory and non-predatory contexts (Arnold and Zuberbühler 2006b). Given that pyow–hack sequences can play a specific role in anti-predator strategies, we were interested in the proportion of responses in which these sequences occurred. These units were often inserted within the other three sequence categories (given above). Consequently, we also compared the distribution of all responses in which pyow–hack sequences occurred, whether given alone or inserted within the three major sequence types as a fourth category, and further distinguished between those responses in which pyow–hack sequences were given alone and those where they were inserted within the three major sequence types.
For comparisons of the number of calls, calling duration, call rate and the proportion of hacks given in response to the models, we used exact Mann–Whitney U tests. We compared the distribution of response types using Fisher’s exact probability tests. All tests were two-tailed with α set at 0.05 except where comparisons were made of responses to different predator categories within both stationary visual and acoustic model types and between visual and acoustic model types within each predator type (Tables 1 and 2). In these cases, a Bonferroni correction was applied resulting in α = 0.025 (0.05/2).
Results
Responses to stationary predator models
Male putty-nosed monkeys reliably produced loud calls in response to the visual models of the crowned eagle and leopard. After excluding a number of trials (see methods), the final data set included 17 leopard and 8 eagle trials. The responses of adult males to the visual models of the predators were similar in many respects to those given to acoustic models (Arnold and Zuberbühler 2006a) and are compared in Table 1.
Calls were given at similar rates irrespective of model type. Male responses to the visual and acoustic models of crowned eagles did not differ in terms of the number of calls given and call series duration, whereas responses to the acoustic leopard model were significantly shorter than those given to the visual leopard model in these respects. These relatively short responses given to the acoustic leopard model also accounts for the significant difference in the number of calls given and call series duration between acoustic predator stimuli which was absent between visual eagle and leopard models. The proportion of hacks given to each of the model types was dependent upon the predator category, with more hacks being given to eagle models, but was unaffected by whether the model was acoustic or visual (Fig. 3).
Raw data of calling patterns of the first 11 calls given in response to visual and acoustic models of a crowned eagle and a leopard. As alternation in call types was found only during the first 11 calls, we present a maximum of 11 calls. Any additional calls are identical to the 11th call type. Trials are depicted in chronological order. N = total number of alarm calls given. Different call series types are indicated by coloured boxes: dark grey pyow series; white hack series; light grey pyow–hack sequence; transitional series consist of a series of hacks followed by a series of pyows. Significantly long pauses (mean + 3SD of pauses between all preceding calls) between sequences are indicated by marking the first call of the sequence in bold
Figure 3 shows the distribution of call-series types produced in response to visual and acoustic models simulating the presence of either eagle or leopard predators.
Presentation of either visual or acoustic models did not affect male alarm-calling behaviour in terms of the relative frequency with which the different call sequences were given. The distribution of call series types produced in response to visual and acoustic models of crowned eagles and leopards are given in Table 2 together with statistical comparisons of the frequencies with which call-series types were produced both within and between model types. Pure hack series were given significantly more often to acoustic eagle models than to acoustic leopard models, whereas transitional series, which begin with a series of hacks, were significantly more often associated with visual eagle models than with visual leopard models. Pure pyow series were given significantly more often to leopard models than to eagle models irrespective of whether visual or acoustic models were used.
Pyow–hack sequences were given significantly more often in response to acoustic leopard models than to acoustic eagle models. This distinction was less clear for visual leopard and eagle models because males more often inserted the pyow–hack sequence within a longer alarm call series when presented with the visual leopard model but more often gave the pyow–hack sequence but no further alarm calls to the acoustic leopard model (Fisher’s exact test, P = 0.066).
Response to moving ground predator models
We were also interested in whether the alarm-call response was affected by the type of ground predator encountered, i.e. a leopard or human poacher. After excluding a number of trials (see methods) the final data set included 11 leopard and 6 human trials. Again, males produced hacks and pyows to both predators. We found no difference in the number of calls given (median leopard vs median human = 5 vs 8.5; U = 25.5, n = 17, P = 0.469), in the duration of alarm calling (median leopard vs median human = 25.6 s vs 90.9 s; U = 26, n = 17, P = 0.525), in the call rate (median leopard vs median human = 0.17 calls/s vs 0.15 calls/s; U = 30, n = 17, P = 0.808), or in the proportion of hacks given to each predator type (median leopard vs median human = 0 vs 0.19; U = 16.5, n = 17, P = 0.085). All statistical tests were two-tailed Mann–Whitney U tests.
In this experiment, the two model types elicited very similar calling patterns in males (Fig. 4). Pure pyow series were the most common in the case of both ground predator models (proportion of responses consisting of a pure pyow sequence given to leopard vs human = 0.91 vs 0.83, Fisher’s exact P = 1.000). Pyow–hack sequences often preceded or were inserted within pure pyow series given to the human model but were less frequently given in response to the leopard model. In one case, a pyow–hack sequence but no other calls was given to the human model. Thus, pyow–hack sequences were given significantly more often to the human model than to the leopard model (proportion of responses in which pyow–hack sequences were given to leopard vs human = 0.13 vs 0.83, Fisher’s exact P = 0.050).
Raw data of calling patterns of the first 11 calls given in response to approaching leopard and human predator models. As alternation in call types was found only during the first 11 calls, we present a maximum of 11 calls. Any additional calls are identical to the 11th call type. Trials are depicted in chronological order. N = total number of alarm calls given. Different call-series types are indicated by coloured boxes: dark grey pyow series; white hack series; light grey pyow–hack sequence; transitional series consist of a series of hacks followed by a series of pyows. Significantly long pauses (mean + 3SD of pauses between all preceding calls) between sequences are indicated by marking the first call of the sequence in bold
Discussion
Several field studies have relied on using acoustic predator models to study anti-predator behaviour in non-human primates, and we have mentioned reasons to remain cautious about results obtained by using this technique. Most importantly, adult male monkeys often approach the source of predator vocalisations, presumably in an attempt to locate and then drive the predator away (Zuberbühler et al. 1997). By their nature, acoustic stimuli prevent callers from confirming the location of a predator, which may impact on their calling behaviour. It is also questionable whether potential attackers vocalise during a hunt, raising more general concerns about the use of vocalisations to simulate predator presence in field experiments. However, despite these and other concerns, we found that the alarm-calling patterns of male putty-nosed monkey to visual and acoustic models were remarkably similar, suggesting that previous studies using acoustic predator models produced reliable results. Contrary to our predictions, callers responded equally strongly, in terms of the number of calls given and call duration, to eagle models regardless of the mode of detection and the caller’s subsequent ability to confirm the presence or location of the predator visually. The fact that vocal responses did not differ between acoustic and visual predator models suggests that they do not take into account their audience’s knowledge. If callers produced calls with the intent to warn ignorant group members, visual models should elicit stronger responses than auditory models as acoustic predator information is simultaneously available to all group members (see Cheney and Seyfarth 1990). In contrast, an audience effect may have played a role in responses given to leopard models as the visual model did elicit stronger responses than the acoustic model, although it would make little sense to suggest that audience effects operate for one predator but not the other. This conundrum can be resolved, however, if we consider the audience to be not only other group members but also the predator itself. It has previously been suggested that alarm calls may be as much a message for the predator indicating that it has been detected, as for conspecifics (Zuberbühler et al. 1997). This would explain why a visually detected leopard model would elicit more calls than an acoustic one which cannot be located. It has also been suggested that alarm calls to terrestrial predators may also function to elicit mobbing behaviour by the rest of the group (Fichtel et al. 2005; Digweed et al. 2005; see also Palleroni et al. 2005). Taken together, these compatible explanations can adequately explain the difference in the strength of responses to visual vs acoustic leopard models.
Production of call series types
As in a previous study (Arnold and Zuberbühler 2006a), males produced both hacks and pyows in response to all predator model types with a bias in favour of producing hacks to eagles, regardless of model type. In the majority of trials, alarm-call series to eagles were made up entirely of hacks or were transitional series, which began with a series of hacks followed by a series of pyows. Acoustic and visual leopard models generally elicited alarm-call series made up of pyows. As predicted, callers produced these distinct call-series types in response to these two predators, regardless of whether they were detected in the acoustic or visual mode. In addition to these three main alarm-call series types, males regularly produced a fourth structure, the ‘pyow–hack’ sequence, although much more often in response to the leopard than to the eagle models. We suspect that this was due to the difference in the hunting strategies employed by the two predators and the degree of threat they posed for the monkeys after being detected. As mentioned previously, forest leopards are ambush predators that are unable to chase monkeys in the canopy and usually abandon hunting attempts once detected (Zuberbühler et al. 1999b). Movement through the canopy and away from the threat is a viable strategy for the monkeys after detecting a leopard. However, when crowned eagles are present, travel is likely to be dangerous, especially for smaller animals, as it may expose them to attack. Unlike leopards, eagles can attack at all heights, often by sitting in dense canopy and waiting to locate vulnerable individuals. Pyow–hack sequences given in response to acoustic and visual eagle models were equally rare, probably as a consequence of prior experience of eagle hunting techniques as hidden eagles are likely to be just as dangerous as located ones. In response to leopard models, pyow–hack sequences were either given alone or accompanied by pyows.
In line with the suggestion that alarm calls given to terrestrial predators can function as mobbing calls (Fichtel et al. 2005; Digweed et al. 2005), it should be noted that pyows were given preferentially to leopard models, more often accompanied pyow–hack sequences given to visual as opposed to acoustic leopard models and made up the latter part of transitional series which were given more often in response to visual eagle models that to acoustic ones.
Responses to the moving models, humans and leopards, were very similar. Both models elicited series of pyows (except in one case where the moving leopard elicited a series consisting only of hacks). However, the moving leopard model, unlike the human model, regularly elicited responses consisting entirely of pyows, with no pyow–hack sequences. Pyow–hack sequences were recorded in both contexts, although significantly more often to the human model, suggesting that callers found it more appropriate to move away from a human than from a moving leopard.
The question of referential signalling
At the proximate level, a common conceptual strategy is to contrast ‘referential’ with ‘emotional’ or ‘affect-based’ calling systems. This dichotomy is probably unhelpful until the psychological processes that determine both call production and comprehension are understood. In human language, the assumption is that signallers and receivers operate within the same referential space, a phenomenon that is made possible because of our ability to understand each other’s intention to communicate (Grice 1969). Comparable evidence is not available for non-human primates, and it is entirely possible that all of non-human primate communication is composed of ‘affect-based’ signallers, who only have limited abilities to take the audience into account, and ‘referential’ recipients, who have learned to interpret particular calling patterns to predict relevant objects and events in the environment (Seyfarth and Cheney 2003).
In putty-nosed monkeys, predator class had some effect on the monkeys’ choices of alarm calls, but these effects were only probabilistic: hacks were regularly produced in response to real eagles and eagle stimuli, while pyows were associated with leopard stimuli. At the level of the individual calls, therefore, it seems safe to conclude that male putty-nosed monkey alarm calls do not function as referential signals, in striking contrast to what has been reported from closely related Diana monkeys and vervet monkeys: calls do not provide reliable information about the nature of the eliciting stimuli, even in the broadest sense. At the level of the call sequence, the relationship between calling behaviour and external events is more complex. Hack series and transitional series clearly play a role in advertising the presence of eagles, while pyow series are employed to indicate the presence of leopards. However, can we conclude from these data that putty-nosed monkey alarm call series function as referential signals?
It is important to point out that, over the course of our fieldwork, we observed a number of cases in which males produced hack and transitional series in response to non-predatory events, such as falling branches or raucous baboon fights. We also observed males giving pyow series during intergroup encounters or apparently spontaneously, suggesting that this call series is also triggered by non-predatory events (K Arnold unpublished data; see also Arnold and Zuberbühler 2006a). It is interesting that white-faced capuchin monkeys (Cebus capuchinus), redfronted lemurs (Eulemeur fulvus rufus) and Verreaux’s sifakas (Propithecus verreauxi verreauxi) also produce one alarm-call type to a range of terrestrial disturbances, including inter-group encounters (Fichtel and Kappeler 2002; Fichtel et al. 2005). These observations raise important questions about the semantic content conveyed by these call series, making it exceedingly difficult to delineate a ‘referential space’ to explain all these cases.
It seems safe to assume that, for a putty-nosed monkey, encountering a leopard is a conceptually different matter than meeting a neighbouring group and that the underlying psychological processes are likely to be different. Equally, where eagles require specific anti-predator strategies, falling trees do not. One way to avoid maladaptive responses in recipients is for callers to produce vocalisations in concerted interaction with the ongoing context. For example, if males call in immediate response to the loud noise of a falling tree, then the meaning of these calls can be determined primarily by the loud sound of the event, and confusion with eagle presence should not occur. As receivers, monkeys are very good at taking context into account when responding to each others’ alarm calls (Zuberbühler et al. 1999b; Zuberbühler 2000b,c). In one study with Diana monkeys, the receivers’ default response to guinea fowl terrestrial alarm calls was to react as if a leopard were present, the most likely cause of the birds’ alarm calls. However, if the birds’ alarm calls were elicited by a human poacher, the monkeys’ response to the same alarm calls was very different, demonstrating that receivers responded to the underlying cause of the alarm calls, not the alarm calls themselves (Zuberbühler 2000b). Analogously, in putty-nosed monkeys, receivers may interpret series of pyows or hacks differently depending upon the available context (Smith 1965); for instance, whether or not a neighbouring group is present or whether the sound of a falling tree precedes a hack series. Field experiments will have to address this issue, for example, by investigating the monkeys’ response to a particular call series, presented with or without additional contextual information.
References
Arnold K, Zuberbühler K (2006a) The alarm calling system of adult male putty-nosed monkeys, Cercopithecus nictitans martini. Anim Behav 72:643–653
Arnold K, Zuberbühler K (2006b) Semantic combinations in primate calls. Nature 441:303
Brown MM, Kreiter NA, Maple JT, Sinnott JM (1992) Silhouettes elicit alarm calls from captive vervet monkeys (Cercopithecus aethiops). J Comp Psychol 106:350–359
Cheney DL, Seyfarth R (1985) Vervet monkey alarm calls: manipulation through shared information? Behaviour 93:150–166
Cheney DL, Seyfarth R (1990) How monkeys see the world. University of Chicago Press, Chicago
Digweed SM, Fedigan LM, Rendall D (2005) Variable specificity in the antipredator vocalizations and behavior of the white-faced capuchin, Cebus capuchinus. Behaviour 142:997–1021
Dunn A (1993) The large mammals of Gashaka Gumpti National Park, Nigeria. Unpublished report to NCF, WWW-UK
Eckhardt W, Zuberbühler K (2004) Cooperation and competition in two forest monkeys. Behav Ecol 15:400–411
Fichtel C, Kappeler PM (2002) Anti-predator behavior of group-living Malagasy primates: mixed evidence for a referential alarm call system. Behav Ecol Sociobiol 51:262–275
Fichtel C, van Schaik CP (2006) Semantic differences in sifaka (Propithecus verreauxi) alarm calls: a reflection of genetic or cultural variants? Ethology 112:839–849
Fichtel C, Perry S, Gros-Louis J (2005) Alarm calls of white faced capuchin monkeys: an acoustic analysis. Anim Behav 70:165–176
Gautier JP, Gautier A (1977) Communication in old world monkeys. In: Sebeok TA (ed) How animals communicate. Indiana University Press, Bloomington, Indiana, pp890–964
Gautier-Hion A, Colyn M, Gautier JP (1999) Histoire naturelle des primates d’Afrique centrale. Ecofac, Libreville, Gabon
Grice HP (1969) Utterers’ meaning and intentions. Philos Rev 78:147–177
Jenny D, Zuberbühler K (2005) Hunting behaviour in West African forest leopards. Afr J Ecol 43:197–200
Kirchhof J, Hammerschmidt K (2006) Functionally referential alarm calls in tamarins (Sanguinus fuscicollis and Sanguinus mystax)—evidence from playback experiments. Ethology 112:346–354
Macedonia JM, Polak JF (1989) Visual assessment of avian threat in semi-captive ringtailed lemurs (Lemur catta). Behaviour 111:291–304
Macedonia JM, Yount PL (1991) Auditory assessment of avian predator threat in semi-captive ringtailed lemurs (Lemur catta). Primates 32:169–182
Palleroni A, Hauser M, Marler P (2005) Do responses of galliform birds vary adaptively with predator size? Anim Cogn 8:200–210
Pereira ME, Macedonia JM (1991) Ringtailed lemur anti-predator calls denote predator class, not response urgency. Anim Behav 41:543–544
Rainey HJ, Zuberbühler K, Slater PJB (2004) Hornbills can distinguish between primate alarm calls. Proc R Soc Lond B 271:755–759
Ramakrishnan U, Coss RG (2000) Age differences in the response to adult and juvenile alarm calls by bonnet macaques (Macaca radiata). Ethology 106:131–144
Riede T, Zuberbühler K (2003a) Pulse register phonation in Diana monkey alarm calls. J Acoust Soc Am 113:2919–2926
Riede T, Zuberbühler K (2003b) The relationship between acoustic structure and semantic information in Diana monkey alarm calls. J Acoust Soc Am 114:1132–1142
Riede T, Bronson E, Hatzikirou H, Zuberbühler K (2005) Vocal production mechanisms in a non-human primate: morphological data and a model. J Human Evol 48:85–96
Seyfarth RM, Cheney DL (2003) Signalers and receivers in animal communication. Annu Rev Psychol 54:145–173
Seyfarth RM, Cheney DL, Marler P (1980) Monkey responses to three different alarm calls: Evidence of predator classification and semantic communication. Science 210:801–803
Shultz S, Thomsett S (2007) Interactions between African crowned eagles and their primate prey community. In: McGraw WS, Zuberbühler K, Noë R (eds) Monkeys of the Taï forest: an African monkey community. Cambridge Univ. Press, Cambridge, pp 171–193
Smith WJ (1965) Message, meaning, and context in ethology. Am Nat 99:405–409
Struhsaker TT (1967) Ecology of vervet monkeys (Cercopithecus aethiops) in the Masai-Amboseli Game Reserve, Kenya. Ecology 48:891–904
Struhsaker TT (1970) Phylogenetic implications of some vocalisations of Cercopithecus monkeys. In Napier JR, Napier PH (eds) Old world monkeys: evolution, systematics and behaviour. Academic Press, London, pp 365–407
Wich SA, Sterck EHM (2003) Possible audience effect in Thomas langurs (Primates; Presbytis thomasi): an experimental study on male loud calls in response to a tiger model. Am J Primatol 60:155–159
Zuberbühler K (2000a) Referential labelling in Diana monkeys. Anim Behav 59:917–927
Zuberbühler K (2000b) Causal cognition in a non-human primate: field playback experiments with Diana monkeys. Cognition 76:195–207
Zuberbühler K (2000c) Causal knowledge of predators’ behaviour in wild Diana monkeys. Anim Behav 59:209–220
Zuberbühler K (2001) Predator specific alarm calls in Campbell’s guenons. Behav Ecol Sociobiol 50:414–422
Zuberbühler K (2002) A syntactic rule in forest monkey communication. Anim Behav 63:293–299
Zuberbühler K (2003) Referential signalling in non-human primates: cognitive precursors and limitations for the evolution of language. Adv Study Behav 33:265–307
Zuberbühler K, Noë R, Seyfarth RM (1997) Diana monkey long-distance calls: messages for conspecifics and predators. Anim Behav 53:589–604
Zuberbühler K, Cheney DL, Seyfarth RM (1999a) Conceptual semantics in a nonhuman primate. J Comp Psychol 113:33–42
Zuberbühler K, Jenny D, Bshary R (1999b) The predator deterrence function of primate alarm calls. Ethology 105:477–490
Acknowledgements
We are grateful to Volker Sommer for his permission and support to conduct research on monkey vocalisations as part of the Gashaka Primate Project. We thank the Nigerian National Parks Service for providing the research permits and the Nigerian Conservation Foundation (NCF) for help with logistics. We thank Dan Azumi and Ali Tappare for their invaluable help in the field. We also thank Robert Seyfarth and three anonymous reviewers for helpful comments and criticisms. This research has been funded by research grants of the Wenner Gren Foundation, the European Science Foundation (‘Origins of Man, Language, and Languages’), the British Academy, the Leverhulme Trust, and the EC Pathfinder FP6 (‘Origins of referential communication’).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Setchell
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arnold, K., Pohlner, Y. & Zuberbühler, K. A forest monkey’s alarm call series to predator models. Behav Ecol Sociobiol 62, 549–559 (2008). https://doi.org/10.1007/s00265-007-0479-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-007-0479-y