Abstract
We used a brood-size manipulation to test the effect of rearing environment on structural coloration of feathers grown by eastern bluebird (Sialia sialis) nestlings. Ultraviolet (UV)-blue structural coloration has been shown to be sexually selected in this species. Our experimental design took advantage of the growth of UV-blue wing feathers in nestlings that are retained as part of the first nuptial plumage. We cross-fostered nestlings to create enlarged and reduced broods with the purpose of manipulating parental feeding rates and measured the effect on nestling growth and plumage coloration. Brood size influenced feeding rates to offspring, but the effect varied with season. In general, male nestlings reared in reduced broods were fed more often, weighed more, and displayed brighter structural plumage compared to nestlings reared in enlarged broods. Female nestlings appeared to experience less adverse affects of brood enlargement, and we did not detect an effect of brood-size manipulation on the plumage coloration of female nestlings. Measures of plumage coloration in both males and females, however, were correlated to hatching date and nestling mass during feather development. These data provide empirical evidence that environmental quality can influence the development of the blue structural coloration of feathers and that males may be more sensitive to environmental fluctuations than females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Models of sexual selection propose that ornamentation can be used to evaluate mate quality because ornaments are costly and covary positively with condition (Zahavi 1975; Hamilton and Zuk 1982; Kodric-Brown and Brown 1984). Such a covariation between condition and ornament expression arises when the environment experienced by an individual during trait production has the potential to impact expression of the ornamental traits. Understanding the specific environmental variables that affect aspects of ornament display is crucial to understanding what facets of individual condition are being signaled by ornamentation.
Plumage coloration is one of the most widespread ornamental traits in birds and is among the best-studied types of ornamentation. The ornamental coloration of feathers can result either from pigments deposited in feathers or from the microstructures of feathers interacting with ambient light. A large and growing number of studies have shown that pigment-based plumages of birds commonly function as honest indicators of quality (Hill 2006). It is still unclear, however, whether noniridescent structural coloration can serve as a condition-dependent signal of individual quality. A few studies have shown that environmental perturbations can reduce structural color display (McGraw et al. 2002; Johnsen et al. 2003; Hill et al. 2005, Jacot and Kempenaers 2007; Peters et al. 2007) and that the precision of the arrangement of tissues at a nanostructural scale affects coloration (Shawkey et al. 2003, 2005), but Prum (2006) argued that the mechanisms by which such structural coloration is produced makes condition dependency unlikely.
Sexual selection is probably responsible for driving the elaboration of structural plumage coloration in male and female eastern bluebirds (Sialia sialis). Male bluebirds have brilliant blue plumage on their heads, backs, rumps, wings, and tails and deep rusty coloration on their breasts. Females have the same color pattern as males but with drabber blue and rust coloration. Males and females that express more colorful UV-blue plumage pair earlier, feed chicks more often, and gain higher reproductive success (Siefferman and Hill 2003, 2005a). Moreover, bluebirds are obligate cavity-nesting passerines and the most-ornamented males, regardless of age, are better able to compete successfully for limited nest sites (Siefferman and Hill 2005b). Experimental manipulations of food availability and paternal investment suggest that structurally based plumage coloration is a condition-dependent trait in both female (Siefferman and Hill 2005a) and male (Siefferman and Hill 2005c) adult bluebirds.
The blue coloration displayed by both male and female bluebirds during their first breeding season is produced as feathers are grown in the nest and after fledging while they are still being fed by parents. These colorful rectrices and remiges begin to emerge within 11 days after hatching, and by 14 days after hatching, the color of remiges can be quantified (Siefferman and Hill, personal observation). Significantly, young bluebirds retain these juvenile wing and tail feathers, as part of their first nuptial plumage-only contour feathers are replaced in the first prebasic molt (Gowaty and Plissner 1998).
The plumage coloration of rectrices and remiges measured on the same individuals as nestlings and again as second-year birds is highly positively correlated (Siefferman and Hill, unpublished data). Older male bluebirds are in better body condition and express more-ornamented (greater UV chroma and brighter) structural coloration (Siefferman et al. 2005). In females, however, a similar relationship between age, body condition, and coloration was not detected (Siefferman and Hill 2005a). In this population, approximately 50% of breeding males and females are second-year birds. Older females and more-colorful females and males experience higher reproductive success (Siefferman and Hill 2003, 2005a).
To test the hypothesis that nutrition during feather production affects the structural coloration of feathers, we manipulated brood sizes of eastern bluebirds and hence experimentally altered the per capita food delivery to young birds growing blue feathers. We then compared the effects of the brood-size manipulation on the production of structurally based UV-blue plumage coloration and on growth parameters in male and female nestlings. Because it is relatively uncommon in birds for nuptial coloration to develop while individuals are under the care of parents, this is one of the first direct tests of rearing environment on nuptial plumage coloration (but also see Jacot and Kempenaers 2007).
Materials and methods
Field manipulation
We conducted an experimental manipulation of brood size in 2000 and 2001 in a population of eastern bluebirds breeding in nest boxes in Alabama, USA. A brood size of four nestlings is typical in our population (mean±SD = 3.75 ± 1.1, range = 1–6). Brood sizes were enlarged and reduced by pairing nests that had four nestlings and a common hatch date. We randomly assigned one nest in each pair to an enlarged-brood treatment and the other nest to a reduced-brood treatment. We reduced and enlarged 60 pairs of nests, but some nests were lost to predators such that we analyzed data from 47 reduced and 40 enlarged broods. The majority of these unsuccessful broods were depredated either by gray rat snakes (Elaphe obsolete, n = 29) or eastern flying squirrels (Glaucomys volans, n = 2), whereas two reduced broods died because of female depredation or desertion. Nestlings that were placed in enlarged broods did not differ by sex (Fisher’s Exact Test P = 0.35) or body mass (t = 0.44, n = 178,81, P = 0.66) from their siblings that remained in reduced broods. Nestlings were cross-fostered on the second day after the first egg hatched (day first egg hatched = day 1). Nests in the enlarged-brood treatment were increased in size by two nestlings, and nests in the reduced-brood treatment were decreased in size by two nestlings, and only enlarged broods had foster nestlings. Thus, after chick swapping, there were six nestlings in enlarged broods and two nestlings in reduced broods. In some nests (enlarged n = 21, reduced n = 19), parental activity was recorded at the nest for 4 continuous hours between 06:00 and 11:00 when chicks were 7 days of age. Parental provisioning rates were quantified as the number of provisioning trips per hour per nestling. As bluebirds deliver individual prey items to offspring, feeding frequency should be a reliable estimator of quantity of prey items delivered. Whenever possible, we identified the relative size of prey items (small, medium, and large) and calculated the mean size of prey items per brood.
Mass of nestlings was measured to the nearest 0.1 g on days 2, 8, and 14 after hatching. At age 14, the right tarsus and wing were measured to the nearest 0.1 mm. From the time they hatch until they are about 11 days old, nestlings increase rapidly in mass, but by age 13 days, the mass of nestlings begins to asymptote (Pinkowski 1975). Hence, the mass of a nestling 14 days after hatching is an accurate estimate of fledging mass. We used the residuals of a linear regression of mass on tarsus length as a proxy of body condition at age 14. Nestlings generally fledge from the nest between ages 15 and 18 days. Nestlings at age 8 days have feather sheaths, at age 11 days, feathers begin to emerge from the feather sheaths, and at age 14 days, 2 cm of the wing feathers have emerged from the sheaths. The fifth primary is the longest feather at this age, and remiges are less than 1 cm long. At age 14 days, feather samples were collected from nestlings for spectrophotometric plumage analysis. At day 8, a blood sample was collected, and we determined the sex of all young genetically using primers designed to amplify the sex-specific locus (Fridolfsson and Ellegren 1999).
Plumage measurements
Plumage reflectance was measured from a point 2 cm below the tip of the right fifth primary. The feathers were stored in envelopes in a climate-controlled environment until spectrophometric analyses were conducted. One researcher (Siefferman) recorded spectral data with an Ocean Optics S2000 spectrometer (range 250–880 nm; Dunedin, FL) using a micron fiber-optic probe at a 90° angle to the feather surface. Reflectance data were summarized by calculating three standard descriptors of reflectance spectra: mean brightness, UV chroma, and hue. Mean brightness was calculated as the mean of the summed reflectance from 300 to 700 nm, UV chroma as the proportion of the total reflectance that is in the UV range (∫300–400/∫300–700), and hue as the wavelength (nm) corresponding to maximal reflectance.
Statistical analyses
Normality of variables was tested using Shapiro–Wilk tests, and data conformed to a normal distribution. To analyze the effect of the brood-size manipulation on parental feeding rates, we used analysis of covariance (ANCOVA) with hatch date as a covariate. To analyze the effects of the experimental treatment on juvenile body mass, structural size, and plumage coloration, we performed mixed-effect models with crossed random effects. All models a priori include the sex of the nestlings and the brood-size manipulation as fixed factors, hatch date as a covariate, and nests of origin and rearing as random factors. For model simplification, we used a stepwise backward procedure and tested two-way interactions between the covariate and the fixed factors. When covariate interaction terms were not significant, interactions were removed from models. In models with significant covariate interactions terms, we performed separate analyses for each group (Engqvist 2005). SPSS v.13.0 software was used to analyze the data, and all tests were two tailed.
Results
Effects of treatment and hatch date on rates of parental provisioning
We used ANCOVA to test the effects of brood size and hatch date on parental feeding rates to offspring. We found a significant interaction between experiment and day of year on provisioning rate (F 3, 36 = 10.7, P < 0.001), suggesting that effect of the brood-size manipulation on parental feeding rates varied with season. Parents rearing reduced broods showed a decrease in feeding rates as the season progressed (F 1, 17 = 9.9, P = 0.006), while parents of enlarged broods did not (F 1, 19 = 0.01, P = 0.96), suggesting that parents rearing enlarged broods were probably feeding offspring at a maximum rate throughout the season. In general, the brood-size manipulation significantly changed the per capita feeding rates; offspring in reduced broods were fed more often than offspring from enlarged broods (4.2 ± 1.44 vs 2.5 ± 0.78 feeds/h per nestling; ANOVA F 1, 38 = 21.9, P < 0.001). Thus, the brood manipulation was successful in creating ‘good’ and ‘poor’ environmental conditions during the period when colorful primary feathers were grown. For a subset of nests, we were able to distinguish the approximate size of prey items that parents fed to offspring. Within nests, we did not detect a significant negative association between the parental provisioning rates and prey size (r = −0.14, n = 40, P = 0.39). We did not detect a significant interaction between experiment and season on mean size of prey items (ANCOVA; F 3, 27 = 0.49, P = 0.5). The size of prey items increased with season (F 1, 29 = 14.7, P = 0.001), but the size of the prey did not vary with brood size (F 1, 29 = 0.03, P = 0.89). Offspring were fed smaller prey (Lepidoptera larvae, small insects, and spiders) earlier in the season and larger prey (usually adult Orthoptera) later in the season.
Effects of treatment and sex on nestling mass, size, and body condition
Before the experimental manipulation, there was no difference in the mass of nestlings that would make up enlarged and reduced broods (F 1, 58 = 0.65, P = 0.46). By 8 days after hatching, however, we found a significant interaction between brood-size manipulation and sex (F 1, 216 = 7.28, P = 0.008, Table 1) suggesting that male and female nestlings responded differently to the brood-size manipulation. Males reared in enlarged broods weighed less than males from reduced broods (F 1, 58 = 11.71, P = 0.001). We found no concurrent effect of brood-size manipulation on the mass of females at age 8 days (F 1, 52 = 0.56, P = 0.46). At day 8, males in enlarged broods were not significantly heavier than females (F 1, 186 = 0.76, P = 0.38); however, in reduce broods, males were heavier than females (F 1, 45 = 12.88, P = 0.001). Finally, there was no effect of hatch date on mass at age 8 days (Table 1).
At day 14, bluebirds reared in enlarged broods exhibited lower mass and were in poorer body condition compared to nestlings reared in reduced broods (Table 1). We detected no significant sexual dimorphism on day 14 mass or body condition (Table 1). We detected no effect of the brood-size manipulation on tarsus length; however, males exhibited longer tarsi than females (Table 1). Hatch date did not influence mass at day 14, tarsus length, or body condition (Table 1).
We found a significant interaction between sex and brood-size manipulation on wing length (F 1, 213 = 6.22, P = 0.01) suggesting that male and female nestlings responded differently to the brood-size manipulation. Males reared in enlarged broods grew shorter wings than male nestlings from reduced broods (F 1, 52 = 7.37, P = 0.009). In females, we found no concurrent effect of brood-size manipulation on wing length (F 1, 58 = 0.2, P = 0.89). In enlarged broods, we detected no sexual dimorphism in wing length (F 1, 170 = 0.67, P = 0.41); however, in reduce broods, males had longer wings than females (F 1, 43 = 16.0, P < 0.001). Finally, offspring that hatched later in the season grew longer wings (Table 1).
Effects of treatment and sex on plumage coloration of nestlings
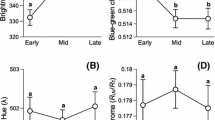
Although males expressed more UV chromatic coloration and hues with peak reflectance at shorter wavelengths compared to females (Fig. 1, Table 1), we detected no effect of brood size on UV chroma or hue (Table 1). Later in the season, however, nestlings displayed, although not significantly, coloration with greater UV chroma and shorter wavelength hues (Table 1).
We found a significant interaction between brood-size manipulation and sex on brightness of plumage coloration (F 1, 254 = 3.7, P = 0.05) indicating that male and female nestlings responded differently to the brood-size manipulation. Comparisons of the plumage color of male offspring from enlarged and reduced broods showed that nestlings from reduced broods were brighter than nestlings from enlarged broods (Fig. 2, F 1, 53 = 8.6, P = 0.005). Thus, the poor-rearing environment experienced by male chicks in enlarged broods caused a reduction in ornamentation of wing feathers. We found no concurrent effect of the brood-size manipulation on brightness of female plumage (F 1, 56 = 0.15, P = 0.69). In both enlarged and reduced broods, males were significantly brighter than females (enlarged: F 1, 202 = 23.8, P < 0.001; reduced: F 1, 56 = 28.0, P < 0.001, Figs. 1, 2). Finally, nestlings were brighter later in the breeding season (Table 1).
Box plots of brightness of plumage coloration of nestling male (diagonal lines) and female (white) eastern bluebirds from reduced and enlarged broods. The line within each box represents the median color score; the upper and lower borders of each box represent the 25 and 75% percentiles; the lower and upper bars are the 10 and 90% percentiles
Nest monitoring indicated that parents of all broods cleaned fecal material from nests, so the cleanliness of the nests probably did not influence plumage color. We also investigated whether nestlings that were cross-fostered into enlarged broods suffered greater environment stress compared to foster siblings that remained in the natal nest with the same number of nest mates. Moving from the natal nest had no significant influence on morphology or coloration of either males or females (all t < 0.8, P > 0.2).
Relationships between nestling mass on day 8 and plumage coloration
The relationships between nestling body mass at day 8 (when the feathers were developing) and brightness and UV chroma did not differ with sex (brightness: F 1, 261 = 0.1, P = 0.75; UV chroma: F 1, 259 = 0.001, P = 0.98). Male and female nestlings that were heavier exhibited brighter plumage (Fig. 3; F 1, 183 = 32.6, P < 0.001) and had, although not significantly, more UV chromatic color (F 1, 198 = 2.8, P = 0.09). The relationship between nestling body mass at day 8 and hue did differ with sex (F 1, 262 = 7.0, P = 0.01). Females that were heavier at day 8 exhibited shorter wavelength hues (F 1, 124 = 6.6, P = 0.01), while there was no significant relationship between mass at day 8 and male hue (F 1, 72 = 0.25, P = 0.88).
Discussion
When we manipulated the sizes of broods of eastern bluebirds, we found a significant effect on the rate at which offspring were fed; hence, our brood-size manipulation was a manipulation of food available to young birds as they grew feathers with blue coloration. We measured the mass of nestlings at day 8, when the growth trajectory is at its steepest (Pinkowski 1975), and again at age 14, days just before fledging. We found that males and, to a lesser extent, females showed negative effects of being reared in enlarged broods. When we measured the coloration of the feathers that young birds grew under different brood sizes, we found that male chicks from smaller broods with access to more food grew flight feathers with brighter structural coloration. We did not detect a concurrent effect of the brood-size manipulation on plumage coloration of female nestlings.
We also found that offspring hatched later in the breeding season, when food resources were likely more abundant, grew plumage that was brighter and exhibited, although not significantly more UV chromatic coloration with hues shifted toward shorter wavelengths compared to nestlings hatched earlier in the breeding season. This seasonal effect overlaid our experimental effects: In reduced broods, feeding rates decreased in later-hatching nests, but in enlarged broods, parents continued to feed offspring at similar rates throughout the season. The size of the prey items did not vary with brood size or with parental feeding rates but changed with season—Later in the season, parents brought offspring larger food items and a greater ratio of adult Orthoptera to Lepidoptera larvae.
Our data are consistent with results from an experiment that manipulated parental effort in male adult bluebirds and found a greater affect of stress on feather brightness compared to UV chroma and hue (Siefferman and Hill 2005c). The current study demonstrates that brightness of structural coloration in nestling eastern bluebirds is influenced by food availability during molt. Age day 8 represents the point at which nestlings are growing the fastest and the point in development when feather shafts are emerging from the dermis. When we directly tested the relationship between all three measures of plumage coloration and nestling mass at day 8, we found that larger male and female nestlings exhibited more-exaggerated coloration. Thus, although UV chroma and hue may be less sensitive than brightness to food availability, all three color parameters varied significantly with the rearing environment. Because the size and type of prey items varied with season, spectral shape may be less influenced by quantity of food but more influenced by the quality of food (possibly protein content of food) that likely change with season. In male nestling blue tits (Parus caeruleus), there is a negative relationship between UV chroma of blue feathers and protein levels in the blood, suggesting that individuals that withdraw more protein from blood are better able to grow more-ornamented feathers (Peters et al. 2007).
The differential effects of the brood-size manipulation on brightness, UV chroma, and hue may be due the feather anatomy responsible for these different aspects of color. The UV-blue feathers of eastern bluebirds are composed of a spongy medullary layer of feather barbs lying beneath a keratin cortex and above a layer of melanin granules surrounding large central vacuoles (Shawkey et al. 2003). Brightness is related to the thickness of the keratin cortex that surrounds the medullary layer, such that birds with brighter plumage have feather barbs with thinner cortex (Shawkey et al. 2005). Measures of spectral shape (UV chroma and hue), however, appear to be determined more by the precision of structural elements (keratin and air spaces) within the spongy layer (Shawkey et al. 2003, 2005).
It is interesting that male nestlings were more adversely affected by the brood-size manipulation than females. Male nestlings, as well as adult bluebirds, display more exaggerated coloration (brighter, more chromatic, and reflect maximal light in the shorter wavelengths) compared to females. Male nestlings are also larger than females at age 8 and have longer wings and tarsi at fledgling. Thus, although the plumage coloration of males and females is differently affected by the brood size, it may be that the added demands on males to grow faster and develop more exaggerated plumage color cause males to suffer more from being reared in enlarged broods. These results are consistent with predictions from the sexual selection theory that more-exaggerated ornaments will be more sensitive to environmental conditions (Cotton et al. 2004). An alternative explanation is that in the enlarged broods, females are better able to compete for the limited food resources than males. The larger size of male nestlings, however, should give them the competitive advantage. Unfortunately, we were unable to tally the amount of food received by individual nestlings.
The greater environmental sensitivity of male eastern bluebird nestlings is similar to the response of great tit (Parus major) nestlings; male great tits are more susceptible to parasites and less able to mount cell-mediated immunity compared to female great tits (Tschirren et al. 2003). Moreover, as in our study of bluebirds, the UV-blue structurally colored tail feathers of male but not female blue tit nestlings are adversely affected by brood enlargement (Jacot and Kempenaers 2007). According to the sexual allocation theory, sexual dimorphism of juveniles influences the relative costs of rearing male and female offspring (Fisher 1930; Trivers and Willard 1973). It would be interesting, therefore, to investigate whether natural variation in environmental conditions—including the females’ perception of the quality of her mate—influence brood sex ratios or parental effort in eastern bluebirds.
One limitation of the brood manipulation was that not all chicks were moved between nests; the enlarged broods were partially cross-fostered (of mixed origin), while the nestlings in the reduced brood were not cross-fostered. A better design would have used a reciprocal cross-fostering design (Jacot and Kempenaers 2007), but the relatively small brood sizes of the eastern bluebird made that technique less feasible. It is also possible, therefore, that cross-fostered nestlings reared in enlarged broods suffered disproportionately because they were moved from the natal environment. In the enlarged broods, however, we did not find evidence that cross-fostered nestlings suffered disproportionately compared to the nestlings that had remained in the natal nest.
Our study indicates that structural plumage coloration of nestling bluebirds is a condition-dependent trait that can be influenced by environmental variables including parental effort, brood size, nestling condition, and seasonality. Because juvenile bluebirds retain these ornamental wing and tail feathers as part of their first nuptial plumage, maternal and paternal investment strategies have the potential to influence the elaboration of sexually selected plumage coloration in this species. This study also suggests natural variation in parental investment could influence offspring ornamentation in eastern bluebirds; by manipulating parental effort, brood size, and timing of reproduction, parents could influence offspring ornamentation. Indeed, maternal and paternal affects have been found to influence sexually selected traits in birds, mammals, and insects (reviewed in Qvarnström and Price 2001). Brighter plumage should allow yearling eastern bluebirds advantages in acquiring nest sites (Siefferman and Hill 2005b) and translate into higher reproductive success (Siefferman and Hill 2003, 2005a). Because male and female bluebirds honestly advertise parental quality via structural plumage coloration (Liu et al. 2007; Siefferman and Hill 2003, 2005a) and because offspring color is influenced by parent quality, selection may drive the elaboration of this trait (Wolf et al. 1999; Qvarnström and Price 2001).
References
Cotton S, Fowler K, Pomiankowsi A (2004) Do sexual ornaments show heightened condition-dependence expression as predicted by the handicap hypothesis? Proc R Soc Lond B 771:78
Engqvist L (2005) The mistreatment of covariate interaction terms in linear model analyses of behavioral and evolutionary ecology studies. Anim Behav 967–971
Fisher RA (1930) The genetical theory of natural selection. Oxford Univ Press, Oxford
Fridolfsson A-K, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Gowaty PA, Plissner JH (1998) Eastern bluebird, Sialia sialis. In: Poole A, Gill GP (eds) Birds of North America no. 381. The Birds of North America, Philadelphia, PA, pp 1–32
Hamilton W, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Hill GE (2006) Environmental regulation of ornamental coloration. In: Hill GE, McGraw KJ (eds) Bird coloration vol. I: mechanisms and measurements. Harvard Univ Press, Cambridge, MA, pp 507–560
Hill GE, Doucet SM, Buchholz R (2005) The effect of coccidial infection on iridescent plumage coloration in wild turkeys. Anim Behav 69:387–394
Jacot A, Kempenaers B (2007) Effects of nestling condition on UV plumage traits in blue tits: an experimental approach. Behav Ecol 18:34–40
Johnsen A, Delhey K, Andersson S, Kempenaers B (2003) Plumage colour in nestling blue tits: sexual dichromatism, condition dependence and genetic effect. Proc R Soc Lond B 270:1263–1270
Kodric-Brown A, Brown J (1984) Truth in advertising: the kinds of traits favored by sexual selection. Am Nat 124:309–323
Liu M, Siefferman l, Hill GE (2007) An experimental test of female choice relative to male structural coloration in eastern bluebirds. Behav Ecol Sociobiol 61:623–630
McGraw KJ, Mackillop EA, Dale J, Hauber ME (2002) Different colors reveal different information: how nutritional stress affects the expression of melanin and structurally based ornamental plumage. J Exp Biol 205:3747–3755
Peters A, Delhey K, Johnsen A, Kempenaers B (2007) The condition-dependent development of carotenoid-based and structural plumage in nestling blue tits: males and females differ. Am Nat 169:S122–S136
Pinkowski B (1975) Growth and development of eastern bluebirds. Bird-band 46:273–289
Prum RO (2006) Anatomy, physics and evolution of avian structural colours. In: Hill GE, McGraw KJ (eds) Bird coloration vol. I: mechanisms and measurements. Harvard Univ Press, Cambridge, MA, pp 295–353
Qvarnström A, Price TD (2001) Maternal effects, paternal effects and sexual selection. Trends Ecol Evol 16:95–100
Shawkey MD, Estes AM, Siefferman LM, Hill GE (2003) Nanostructure predicts intraspecific variation in structural plumage colour. Proc R Soc Lond B 270:1455–1460
Shawkey MD, Estes AM, Siefferman LM, Hill GE (2005) The anatomical basis for sexual dichromatism in non-iridescent ultraviolet-blue colouration of feathers. Biol J Linn Soc 84:259–271
Siefferman L, Hill GE (2003) Structural and melanin plumage coloration indicate parental effort and reproductive success in male eastern bluebirds. Behav Ecol 14:855–861
Siefferman L, Hill GE (2005a) Evidence for sexual selection on structural plumage coloration in female eastern bluebirds. Evolution 59:1819–1828
Siefferman L, Hill GE (2005b) UV-blue structural coloration and competition for nest boxes in male eastern bluebirds. Anim Behav 69:67–72
Siefferman L, Hill GE (2005c) Male eastern bluebirds trade future ornamentation for current reproductive investment. Biol Lett 1:208–211
Siefferman L, Hill GE, Dobson S (2005) Ornamental plumage coloration and condition are dependent on age in eastern bluebirds Sialia sialis. J Avian Biol 36(5):428–435 DOI 10.1111/j.0908-8857.2005.03401.x
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary sex-ratio of offspring. Science 179:90–92
Tschirren B, Fitze PS, Richner H (2003) Sexual dimorphism in susceptibility to parasites and cell-mediated immunity in great tit nestlings. J Anim Ecol 72:839–845
Wolf JB, Brodie III ED, Moore AJ (1999) The role of maternal and paternal effects in the evolution of parental quality by sexual selection. J Evol Biol 12:1157–1167
Zahavi A (1975) Mate selection—a selection for a handicap. J Theor Biol 53:205–214
Acknowledgments
We thank E. Gering, T. Robinson, and B. Staton for field assistance and C. Ariail, A. King, and H. Mays Jr. for laboratory assistance. We are grateful to R. Montgomerie for the use of his spectral processing program, to I. Cuthill and J. McGlothlin for statistical advice, and to M. Liu for comments on the manuscript. This research was conducted according to an animal use permit from Auburn University, banding permits to GEH, and complies with the laws of the USA. Funding came from NSF grants IBN 9722171, IBN 0235778, DEB 0077804 to GEH, a NSF-NIH grant R01-AI49724 to GEH and American Ornithological Union, Animal Behaviour Society, and Birmingham Audubon Society grants to LS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. Cuthill
Rights and permissions
About this article
Cite this article
Siefferman, L., Hill, G.E. The effect of rearing environment on blue structural coloration of eastern bluebirds (Sialia sialis). Behav Ecol Sociobiol 61, 1839–1846 (2007). https://doi.org/10.1007/s00265-007-0416-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-007-0416-0