Abstract
Group living confers both benefits and costs to the individuals involved. Benefits may include enhanced defense, thermoregulation, and increased foraging efficiency while costs often involve competition for resources such as food, shelter, and mates. Communication provides a medium of exchange among individuals engaged in either cooperative or competitive interactions. The functional analysis of signals within groups therefore requires testing both cooperative and competitive functions, although the latter is infrequently done. In this paper, I study the use of two vibrational signals in a gregarious, processionary Australian sawfly larva, Perga affinis: tapping and contractions. Tapping involves striking the substrate with the sclerotized portion of the abdominal tail and a contraction is a fast, whole-body twitch, which is both tactile and vibrational in its transmission. For tapping, I first demonstrate that it is a form of communication, as tapping of one larva elicits tapping in another, and that it is transmitted through substrate vibrations. I then test whether the signal is mostly cooperative or competitive in nature by examining it in light of two hypotheses: (1) the Group Coordination hypothesis, stating that the signal functions to maintain group cohesiveness and (2) the Competitive Signaling hypothesis, stating that tapping serves as a competitive assessment signal between larvae while feeding. For contractions, I test only the group coordination hypothesis that they serve to coordinate and initiate group movement. Results support the group coordination hypothesis for each signal. While feeding, lone larvae (without potential competitors) were significantly more likely to tap than those in groups, and this trend continued in non-feeding situations. Contractions regularly preceded periods of group movement during processions and were given with increasing frequency before departure from preforaging clusters. The vibrational signals in this processionary species likely function cooperatively to maintain group cohesiveness and coordinate movement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Group living involves the act of balancing costs and benefits. Individuals may lessen costs by competing with other group members; alternatively, they may enhance the benefits of association through cooperation. Communication among group members serves as an important tool in either endeavor.
Communication for competitive purposes occurs in many group-living environments, such as those involving colonial insects (Reeve 1991; Tibbetts 2002), breeding congregations (Bourne 1992; Stein and Uy 2006), or foraging aggregations (Radford 2004b). Group-living animals also communicate for more cooperative purposes. Many organisms such as birds, dolphins, schools of fish, or herds of elephants use signals and communication as a way to attract group members and to maintain group structure and cohesiveness while traveling (Moller 1976; Black 1988; Janik and Slater 1998; Langbauer 2000; Radford 2004a). Among insects, many species have gregarious larvae that engage in daily movement, and they use a variety of communication modalities to maintain group structure such as pheromones, silken trails, and/or tactile cues (Fitzgerald 1995; Costa and Louque 2001; Ruf et al. 2001; Costa et al. 2004).
This paper investigates the signals used in communication by a gregarious, processionary Australian sawfly larva, Perga affinis (Pergidae: Hymenoptera). Like other processionary species, tactile cues appear to play an integral role in group structure as larvae remain physically connected (and often overlapping with the tail of one around the head of another) at all times except when feeding; however, there is no evidence of pheromone trails and larvae do not produce silk. Instead of coupling tactile signals with these more commonly described modalities, behavioral observations suggest that P. affinis may use substrate vibrations (Evans 1934; Carne 1962). Vibrational communication is common among insects (Cocroft and Rodriguez 2005); however, the majority of work has focused on adults (i.e., duetting between the sexes and male–male competitive interactions; Cokl and Doberlet 2003; Cocroft and Rodriguez 2005) or on communication between adults and their brood, as seen in wasps (Harding and Gamboa 1998; Savoyard et al. 1998; Cummings et al. 1999). A few studies have documented its presence and function among nymphs or larvae (Russ 1969; Hograefe 1984; Yack et al. 2001; Cocroft 2005; Fletcher et al. 2006). If backed by experimental evidence, larvae of P. affinis would provide an example of a processionary species using vibrational signals to maintain group cohesion and prevent fragmentation while processing.
Although it is easy to assume that any form of communication in a processionary species serves to promote group cohesion and integrity, it is important to consider alternative functions, especially given the context of group living. As a gregarious species, P. affinis faces both the costs and benefits of group association, and therefore, one cannot rule out a potential competitive function of its signals. Many organisms that aggregate or form groups use signals to mediate competition for resources such as mates or food (e.g., Nelson and Fraser 1980; Guerra and Mason 2005). Among insects, acoustic signals are also involved in competitive situations. Several cases have documented larvae using acoustic signals to mediate competitive territorial disputes (Russ 1969; Yack et al. 2001; Fletcher et al. 2006) Alternatively, there are at least two cases involving larval and nymphal insects where acoustic signaling serves a cooperative function by attracting siblings to a good food source (Hograefe 1984; Cocroft 2005). Therefore, I consider both competitive and cooperative hypotheses when investigating the function of a vibratory signal in P. affinis.
P. affinis has two acoustic signals: tapping and contractions. Tapping occurs when a larva strikes the substrate with the sclerotized portion of its abdominal tail, and a contraction is a fast, whole-body twitch. In this paper, I first describe and characterize the signals and also demonstrate that tapping is a form of communication transmitted through the substrate. I then examine their function in light of two hypotheses, a Group Coordination Hypothesis and a Competitive Signaling Hypothesis. For tapping, I test the coordination hypothesis that this signal promotes group cohesiveness by serving as a ‘where are you?’ or ‘I am here’ signal for larvae that become separated from the group. I also test the alternative hypothesis that tapping acts as a competitive signal between larvae for gaining access to a common food source (much like the territorial signal of Drepana; see Yack et al. 2001). This sets up four discriminatory predictions (see Table 1). Predictions 1 and 2 support the coordination hypothesis of a ‘where are you?’ or ‘I am here’ function, as they predict that lone individuals will tap more and have a lower latency to tap than those in groups. This is expected if tapping serves to promote finding and aggregating with other larvae. Prediction 3 is compatible with the competitive hypothesis by predicting that when feeding, a larva on a leaf is more likely to tap when others are present (and hence offer competition) than if it is alone. Prediction 4 is compatible with the coordination hypothesis because a predicted positional effect of tapping while in a group suggests a form of coordination or organization.
For contractions, as the signal is not given in a context that could be considered competitive (i.e., while accessing a shared resource), I only test the coordination hypothesis. Specifically, I examine whether or not contractions function to initiate and orchestrate group movement. Three predictions are tested: (P1) larvae only contract when in a group and not when alone, (P2) contractions precede periods of group movement, and (P3) there is a positional effect in the timing of or initiation of a contraction.
The above predictions for both signals are tested experimentally as well as through quantified observations. Results are discussed for each signal separately.
Materials and methods
Natural history and daily patterns of P. affinis larvae
Adult female sawflies lay clusters of 20–30 eggs in the leaves of several species of Eucalyptus in early April (Carne 1962). After about a month of incubation, larvae hatch together and emerge forming tight cycloalexic formations (a rosette pattern with heads pointed outward and the posterior abdomens pointed inward; see Jolivet et al. 1990) during the day and feeding on the leaf’s perimeter at night. As the larvae grow, they maintain their nocturnal activity patterns but shift their daytime resting site to small twigs and eventually branches, where they form a cluster of overlapping bodies (no longer using cycloalexy). They remain gregarious for their entire larval stage (which lasts up to 6 months).
In their daily movement as nomadic foragers (Fitzgerald and Peterson 1988), the larvae move to different feeding and resting sites by processing as a group. The transition between the tight daytime clusters to the processions involves an intermediate stage that I have referred to as a preforaging cluster (a tight cluster 1–2 h before the procession begins). It is during this stage that one begins to see an increase in activity and signaling behavior in the larvae.
Insects and plants
Colonies of sawfly larvae (P. affinis) were collected from Orange, NSW (2002) and the Australian Capital Territory (2003) and maintained on cuttings from their host plant. Colonies used for observations on preforaging and processionary behavior were kept indoors on a reverse light cycle (L/D 11:13 h) at a constant temperature of 21°C. Larvae used for experiments on the tapping behavior were kept on branch cuttings in an outdoor shade house. All recordings and trials took place on host plant cuttings or branches, thus providing a natural substrate for observations.
Acoustic and video recordings
Preforaging and processionary recordings took place under dark conditions, with a red lamp and infrared light from the video camcorder to monitor behavior, while all tapping experiments were conducted during daylight hours. Vibrational signals and movements of the larvae were picked up by a phonocartridge placed on the substrate. The phonocartridge was attached to a Sound Devices MP-1 preamplifier that fed into a Sony Digital 8 DCR-TRV830 camcorder, thereby allowing simultaneous acoustic and visual information to be recorded. Separate samples of tapping and group constrictions were recorded with a BU Series Knowles accelerometer (model BU-1771) and an LEEM guitar transducer (connected to the preamplifier and the camcorder) for more precise analysis of signal spectral characteristics. Acoustic recordings used for characterization were made using sound files of the tapping signal imported as wave files to a PC and analyzed using Raven 1.2 software from the Cornell Laboratory of Ornithology.
Preforaging and processionary behavior
Ten colonies (ranging in size from 15–45 larvae) were monitored for the description and quantification of preforaging and processionary behavior.
Preforaging clusters are the same as tight daytime clusters but are defined in terms of their temporal proximity to the start of a procession (i.e., they are tight clusters 1–2 h before larvae process to forage). To monitor them, I recorded the timing and relative group involvement (a few individuals, half the group, or the whole colony) of any signaling events before departure. This measured the frequency of signal use over time; it also enabled me to examine prediction 2 (P2) regarding the timing of contractions in relation to periods of group movement (see Table 1).
For the processionary behavior, I examined three procession events for each of six colonies. A procession event begins with waves of contractions that pass through the group followed by walking and ends when the group stops all movement. To test P3 for contractions and P4 for tapping (see Table 1), I also investigated the effect of larval position within the colony on the use of signaling during the procession event. For each colony, I monitored three front, three middle, and three back-positioned larvae. A Mann–Whitney U test was used to compare the proportion of front vs back-positioned larvae per colony that tapped during procession events. A Wilcoxon-signed rank test examined the timing of contractions by comparing the proportion of times contractions moved from front to back individuals (null hypothesis, proportion = 0.5).
Function of tapping
First, I tested to see if tapping serves as a form of communication and examined whether the signal is transmitted through substrate-borne or air-borne vibrations. Then, to examine the function of tapping, I tested a set of predictions based on two alternative hypotheses: the group coordination hypothesis (tapping aids in maintaining group cohesiveness by serving as a ‘where are you?’ or ‘I am here’ signal as well as coordinating movement when processing) and the competitive signaling hypothesis (tapping serves as a competitive signal between larvae gaining access to a common food source; see Table 1). Tapping occurs in a variety of contexts (see Table 2); therefore, I examined the function in three situations: single, lone larva vs a larva in a group (P1, P2), individual and grouped larvae while foraging (P3), and larvae during processions (P4, see the “Preforaging and procession” of Materials and methods). I tallied each prediction according to whether it supported (+) or did not support (−) a given hypothesis and then compared these scores with the observed outcome.
Tapping—a form of communication?
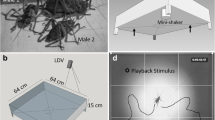
If tapping serves as a signal in communication, then the tapping should elicit some type of behavioral response in the receiver (Bradbury and Vehrencamp 1998). To test this, I conducted a paired experiment with 20 experimental and control trials (both 20-min in duration). In the experimental setup, two larvae were placed on opposite sides of a single eucalyptus branch, each equidistant (10.5 cm) from and facing the center cardboard divider (see Fig. 1). This setup allowed larvae to ‘hear’ each other and receive vibrational cues through the substrate while preventing the transfer of any visual information. The typical propagation ranges for substrate vibrations are 30 cm to 2 m (Michelsen et al. 1982; Cokl and Doberlet 2003; Cocroft and Rodriguez 2005), so the larvae were well within receiving distance for this experiment.
Experimental and control set-up for tapping experiment. For experimental trials, larvae sat on the same branch separated by a cardboard insert. For control trials, larvae sat on separate branches attached to different bottles that were a distance of 50 cm from each other. In both set ups, the larvae were placed 10.5 cm from the center divider
The control trials had a similar setup except the larvae sat on separate branches (placed on different tables 50 cm apart from each other, see Fig. 1). Larvae received neither visual information (due to cardboard blocks) nor vibrational cues through the substrate. The setup, in addition to testing for communication, tested whether the signal was substrate-borne or air-borne. If tapping is perceived through substrate vibrations, the behavior of larvae between the control and experimental trials should differ. If the signal is air-borne, there should be little or no difference.
Data collected from the videotapes involved recording the start and end time (duration) of the following behaviors for each larva: tapping, walking, searching (lifting the head and first pair of thoracic legs off the substrate), and turning around. A Mathematica™ program developed by Dr. Kern Reeve (Cornell University) calculated the total time of tapping overlap (periods where one individual is tapping and the second larva joins in) for each trial. The program, using bootstrap analysis, calculated both the observed and expected overlap (if the overlap occurs randomly), which was then used in a paired t test. As a second comparison, I also looked at the observed tapping overlap in the experimental vs control trials using a paired t test.
Single larva vs a larva in a group
To test the first two predictions (P1, P2) of the tapping hypotheses, I set up a paired experiment involving 31 larvae from 10 different colonies. Each larva was placed in two situations (order of presentation was alternated between larvae), alone on a branch or within a group of larvae (its original colony), for 5 min. To give larvae a period of adjustment after being removed from their original colony, all larvae were individually placed in solitary containers for 5 min before being tested in the two situations. During the trials, I counted the total number of taps given by the larva, the number of taps within each bout of tapping, and the time at which the first tap occurred. Data were analyzed using the paired t test.
Tapping while foraging
To test P3, I video-recorded the foraging behavior of three colonies during the first hour of feeding. I noted whether larvae were alone on a leaf or with at least one other individual and also whether or not any tapping occurred. A two-sample proportion test was used to compare the proportion of individual (alone) larvae (N = 19) vs grouped larvae of two or more (N = 12) that tapped while feeding on a leaf.
Data for examining P4 came from behavioral observations of group processions described earlier in “Materials and methods”.
Function of contractions
To examine a potential function of contractions, I tested a set of predictions based on the group coordination hypothesis (contractions serve to initiate or coordinate group movement; see Table 1). I tallied the observed outcomes of each prediction and compared these scores with those predicted by the coordination hypothesis.
To test the first prediction (P1), that larvae only contract (before walking) when in a group and not when alone, I randomly recorded one walking event for 30 single larvae and scored whether or not it contracted. This was compared with data from larvae in groups. A total of eight to nine larvae were sampled from six colonies and scored for whether or not they contracted before walking; the scores provided a proportion of larvae that contracted per colony. A Mann–Whitney U test compared the proportion of single vs grouped larvae that contracted before walking.
Data for predictions P2 and P3 involved preforaging clusters and processionary behavior (see their respective methods in “Materials and methods”).
Results
Signals and the context in which they occur
The two signals discussed in this paper are tapping and contractions. Tapping is performed by striking the sclerotized anal segment at the tip of the abdomen onto the substrate (Fig. 2a). It normally occurs in a series of successive taps referred to as a bout of tapping. The average number of taps per bout and bout length (for solitary individuals) are 23.4 ± 5.69, N ind = 20 and 8.34 ± 2.1 s, N ind = 20, respectively, with a mean rate of 2.81 ± 0.21 taps per second, N ind = 20 (see Fig. 3b). Being a substrate-borne signal produced through a striking motion, the tap is a broad band signal ranging from 0–12 kHz, with frequencies under 6 kHz carrying the most energy (Fig. 3c).
a Late instar P. affinis tapping on a branch of Eucalyptus sp. Larvae lift their abdomen up to15 mm off the substrate and then swing it down to strike the surface with the sclerotized tip of the abdomen. b Two larvae on a branch demonstrating the typical head-to-tail position of processing larvae. c Back of a procession line showing the overlapping nature of processions. Several larvae are nestled side by side, maintaining anterior, adjacent, and posterior (with the exception of the rear individuals) contact with other colony members. Scale bars: 5 mm
Tapping is used in a variety of contexts as seen in Table 2. Daytime tight clusters of larvae exhibit negligible amounts of signaling aside from the occasional tap or tail flick of individuals. In preforaging clusters, colonies show increased amounts of tapping after the contractions of individuals or the group as a whole. During processions, tapping again occurs after contractions, although mainly the larvae in the back seem to be involved (see Fig. 4 and procession results in “Results”). At the start of foraging, when only one or a few larvae are on a leaf, individuals may tap until several more larvae join the feeding site (this has been noted in Pergagrapta sp. as well; Reid 2004). Tapping also occurs as colonies reassemble in the early morning after having dispersed to forage.
a Oscillogram of a single group contraction recorded with the accelerometer at a distance of 36 cm. b Oscillogram of a substrate-borne tapping signal by P. affinis, recorded with LEEM guitar transducer 12.5 cm from the larva. The y-axis is the amplitude of the signal. c Power spectrum of the above tapping signal; reveals that it is a broad band signal with energy from 0 to over 12 kHz. The y-axis is dB (ticks at 20 dB intervals), but the measurements are relative, as the recording was not calibrated
Contractions are characterized by a whole-body twitch that appears to be transmitted tactilely as well as through the substrate by the thoracic legs. Figure 3a shows an oscillogram of a single group contraction (involving five larvae) recorded by an accelerometer at a distance of 36 cm. The average duration of a group contraction is 0.135 s ± 0.115 (N = 10, sampled from eight groups of five larvae each), although timing varies according to the synchronicity of the larvae and the size of the group. In general, it is an extremely brief signal (on the order of a tenth to a hundredth of a second). It was much harder to get recordings of contractions by individual larvae so no detailed measurements are included; however, visually (via the video recordings) the signal appears to be of a similar duration. As seen in Table 2, contractions occur in preforaging clusters and during procession events and, in both cases, contractions precede tapping (see Fig. 4 for signal timing in procession events). Contractions may be done by single individuals, a subset of the group, or by the colony as a whole (either simultaneously as in preforaging clusters or in a wave of succession as in processions).
Preforaging clusters
Tight daytime clusters are relatively quiet with little movement or signaling occurring. An increase in signaling (tapping and contractions) by individuals as well as tail movement among the larvae occurs before departure. The most conspicuous signal, however, was the group contraction. A group contraction involves the whole colony, where all larvae contract simultaneously. A group will perform one to four of these contractions (mean = 1.72 ± 0.514, N = 51 from four colonies) followed by periods of stillness. The frequency with which group contractions occur increases as the colony nears departure time (Fig. 5). Colonies (ranging in size from 18–25 larvae) were monitored from 2.5 to 4 h before departure and in each case showed a dramatic increase in the occurrence of group contractions. This finding provides support for P2, that contractions occur before periods of group movement and hence may facilitate the eventual transition from clustering to processing.
Processionary behavior
Processions of P. affinis are marked by periods of movement and stillness. A period of movement begins with one to three waves of contractions (average of 2.75 ± 0.97, N = 75) through the group. After the contractions pass through the colony, a subset of the larvae begins tapping, and then the group commences its procession along the branch. Eventually, the group stops all activity for a period of stillness, and then the whole cycle is repeated with the onset of another wave of contractions. Periods of movement during processions last on average for 73.2 ± 32.5 s, N = 74 (from seven colonies) while periods of stillness last for an average of 53.7 ± 38.6 s, N = 67.
Processions range from being single-file (when on thin branches) to 15–25 individuals wide when on a tree trunk. The width generally depends on the diameter of the substrate as well as the size of the group (late in the season when several colonies coalesce the width can be upwards of 20 cm). In either case, the larvae remain in physical contact with others by nestling side-to-side and by placing their abdomens on the heads of the larvae behind them (Fig. 2b,c). The close physical contact appears to aid in the transmission of signals such as contractions, which are both tactile and vibrational in nature and move through the group in a wave-like manner.
Number of group contractions in preforaging clusters recorded at 30-min intervals before a procession. The last point on each line corresponds to the time when the respective colony began to process. A–D represent different colonies (ranging in size from 18–25 larvae), with D1 and D2 depicting sequences for colony D recorded on different days
It appears as though all members of the group contract when the waves of contractions occur. When I sampled three larvae (one in front, one in the middle, and one in the back) in each of 18 procession events, all of them contracted. The contractions generally started in the front and moved to the back (Fig. 4), as seen by the significantly higher proportion of times that the contractions moved from front to back in each of the six colonies (tested against the null hypothesis of proportion = 0.5; Wilcoxon signed-rank test: TS = 21, N = 6, P = 0.036). Position in the group therefore affects the timing of contracting in the sequence but not the actual performance of the signal. This result supports P3 for the function of contractions (see Table 1). Contractions are initiated by those directing the movement of the group, as they are significantly more likely to start from the front than from the middle or back of the colony.
For tapping, instead of a positional effect on signal timing, one sees a positional effect on signal use, namely, position affects the likelihood of an individual tapping after the waves of contraction (Fig. 6; also see Fig. 4). In each of the six colonies, a significantly higher proportion of the back-positioned larvae tapped than those in the front (Mann–Whitney U test: W = 25.5, N = 6, P = 0.0239). This supports P4 for the function of tapping and is consistent with the group coordination hypothesis (see Table 1). The difference in the proportion that tapped in the back vs the middle or the front vs the middle was not significant. Middle-positioned larvae generally tapped if the procession became fragmented and stopped signaling once reconnected with the remaining group members.
Tapping
A form of communication
If tapping is a signal used in communication, it should elicit a predictable response in the receiver. Of all the behaviors recorded, the most striking observation was how the tapping of one larva elicited the tapping of the second larva. To quantify this behavior and to test whether or not a larva responds to tapping by signaling itself, I looked at the total time of tapping overlap (when one larva starts tapping and the second individual joins in) in each experimental trial. The observed overlap for each experimental trial was then compared with the observed overlap in the corresponding control trial as well as the expected overlap if it occurred randomly (calculated by the Mathematica™ program, see “Materials and methods”). The observed tapping overlap in the experimental trials was significantly greater than the overlap in the control trial (paired t test: t 17 = 3.27, P = 0.004, Fig. 7). Similarly, the observed tapping overlap in the experimental trial was significantly greater than the overlap expected if it occurred randomly (paired t test: t 19 = 4.87, P = 0.000). Both results suggest that larvae respond to each other’s tapping by signaling and hence provide evidence of communication. The signal may serve as a ‘where are you?/I am here’ signal for separated larvae. Additionally, the significantly lower tapping overlap in the control vs experimental trials suggests that larvae receive the tapping signal through the substrate as a vibration and not as an air-borne signal.
Tapping overlap (in seconds) between paired larvae in experimental vs control trials. Significantly more tapping overlap occurred between the two larvae when they were on the same branch (experimental) than when they were on separate branches and could not detect each other (control; paired t test: t 17 = 3.27, P = 0.004)
Single larva vs a larva in a group
When alone, larvae tapped a significantly greater number of times during the 5-min trial than when in a group, with a mean of 98.1 and 38.4 taps, respectively (paired t test: t 30 = 5.01, P = 0.000). Single larvae also had a significantly higher number of taps per bout of tapping than when in groups, with a mean of 19.9 and 12.3, respectively (paired t test: t 30 = 3.48, P = 0.001). Both results support P1, which stated that a larva taps more when alone than within a group. Examining the time of the first tap in a trial when larvae were alone vs in a group showed that the lone larvae had a significantly shorter latency to tap than those in a group, with a mean time of 70 vs 136.7 s, respectively (paired t test: t 30 = −3.03, P = 0.003). This supports P2, which stated that larvae would have a shorter latency to tap (i.e., tap sooner) when alone than when in a group. It should be noted that the tapping seen in the group situations mainly occurred because the group started processing after being disturbed to remove and then replace the experimental larva.
Tapping while foraging
Contrary to P3 for tapping, larvae were significantly more likely to tap on a leaf when alone than when in the presence of at least one other individual (see Fig. 8; two-sample proportion test, TS = −3.01, N single larva = 19, N grouped larvae = 12, P = 0.0011). In fact, only 2 of the 12 monitored feeding groups displayed any tapping, and both of these were small groups, two and three larvae each. This result fails to support the competitive signaling hypothesis for tapping and is consistent with the group coordination hypothesis only.
A comparison of signal use (tapping) while foraging on a leaf as a single larva or as a group of larvae (two or more). Single larvae had a significantly higher likelihood of tapping while foraging than grouped larvae (two-sample proportion test: TS = 3.01, N single larvae = 19, N grouped larvae = 12, P = 0.003)
Results for P4 were discussed in “Procession Behavior” of “Results”. Larvae demonstrated a positional effect in the use of tapping during processions, with individuals in the back having a higher probability of tapping than those in front. This supports the group coordination hypothesis.
Function of contractions
Three predictions (P1–P3) were made for the function of contractions and assessed according to their support of the group coordination hypothesis (see Table 1). Prediction 1, that larvae only contract (before walking) when in a group and not when alone, was tested by comparing the proportion of single larvae vs those in groups that contracted before walking. A significantly higher proportion of the larvae within groups contracted before walking than the lone larvae (Mann–Whitney U test: W = 189, N group = 6, N single = 30, P = 0.000). This result supports P1 and is consistent with both the group coordination hypotheses.
Results for P2 and P3 were discussed in “Preforaging Cluster” and “Procession Behavior” in Results, respectively, both supported the group coordination hypothesis.
Discussion
Communication in group-living animals serves many functions ranging from cooperative to competitive purposes. In the case of P. affinis larvae, the two signals, contractions and tapping, have a cooperative function. For both signals, the observed results (Table 1) are consistent with the group coordination hypothesis.
Tapping
Tapping occurs in a variety of contexts (Table 2) and no doubt serves a range of functions. Predictions 1–3 (Table 1) dealt with the contexts of single larvae vs groups of larvae and foraging behavior; the observed results were consistent with the group coordination hypothesis only. In these situations, the signal appears to function in a ‘where are you?’ or ‘I am here’ capacity for separated larvae. A larva taps significantly more and has a shorter latency to tap (i.e., taps sooner) when alone than when in a group. This higher tendency to tap when separated from others would not be expected if it served as a competitive signal; instead, one would expect the stimulus of other larvae (and hence competitors) to induce an increase in signaling behavior.
Similarly, if tapping served as a competitive signal, one would expect the presence of other larvae while foraging on a given leaf (i.e., a limited resource) to invoke the behavior. Although trees have many leaves, they are known to vary in quality (Wheeler and Center 1996; Wheeler 2001), suggesting that a high quality leaf may be a rare find. Additionally, several colonies often inhabit a single tree for up to 6 months and, depending on larval density, they are capable of a defoliating their host (Carne 1962). Despite the reality of a limited food source, observations indicate that the presence of other larvae decreased the likelihood of tapping. The closely related Pergagrapta sp. behaves similarly (Reid 2004), with isolated larvae tapping until four to five individuals join them to forage.
How might the larvae benefit from sharing a leaf with others? In some species, such as the jack pine sawfly (Neodiprion pratti banksianae), grouped larvae have a higher probability of establishing a successful feeding site than solitary individuals (Ghent 1960). When feeding on tough plant cuticles like that of pine needles, playing the numbers game has high payoffs, especially for survival rates of first instars (Ghent 1960). An alternative benefit could arise if aggregative feeding induced a change in host plant suitability, making it more palatable, as seen in pipevine swallowtail larvae (Battus philenor) and their host Aristolochia californica (Fordyce 2003). In this case, the increased suitability is short-lived, making it important to use the resource quickly and hence, further highlighting the benefit of group feeding. If tapping served as a quality indicator for a given food source, as seen in the stridulation behavior of the striped alder sawfly larvae (Hograefe 1984), perhaps larvae would gain genetic benefits. As P. affinis larvae are thought to live in groups of siblings (at least initially, before colonies merge), they may derive selective advantages from advertising and sharing good resources with colony members. Perhaps tapping is a way of alerting and guiding other members to a leaf of high quality, and larvae on lesser quality leaves do not tap to advertise their food source.
Prediction 4 dealt with tapping in the context of group processions, and again, the observed results were consistent with the group coordination hypothesis only. A clear positional effect occurred regarding the use of tapping. Individuals in the back of the procession were significantly more likely to tap than those in the front, suggesting that position in the group elicits the behavior. In other processionary larvae, the nudging and jostling of individuals from behind seem to be a cue to encourage hesitant leaders to begin processing (Fitzgerald and Pescador-Rubio 2002; Fitzgerald 2003; Fitzgerald et al. 2004). Similarly, the tapping of rear individuals could be a mechanism for encouraging those in front to start moving. This differs from the ‘where are you? or ‘I am here’ function of tapping seen in the context of individuals vs groups or during foraging but still fits the coordination hypothesis in that it may function to promote group integrity while processing. Animals are known to have variable walking speeds that can cause fragmentation in group movement (Gueron et al. 1996), so perhaps signaling a ’readiness’ to move increases the chances of all larvae commencing movement at the same time.
Contractions
Contractions occur in the context of a group environment and are not given by lone larvae (i.e., physically separate), suggesting that their use is stimulated by or dependent upon the presence of other larvae. This supports prediction 1, as the signal is only expected to occur in situations where it may coordinate activity. When in use, larvae typically signal in a synchronized manner involving most, if not all, colony members. A similar phenomenon of a group pulsation was observed in the weevil larvae of Phelypera distigma; however, here the signal appears to have a very different function, as it only occurs during or after disturbance by a predator (Costa et al. 2004). Larvae of P. affinis contract in preforaging clusters and while processing, but in neither situation are the contractions elicited by an external stimulus. Instead, contractions occur before periods of coordinated group movement (supporting P2). Larvae in preforaging clusters contract simultaneously and do so with increasing frequency leading up to the time of procession and departure for foraging. Similarly, contractions occur during processions and move through the entire group in a wave-like manner before a bout of walking. In addition to contractions occurring before periods of group movement, we also see an effect of position on the timing of signaling in processions (supporting P3). Larvae in the front of the procession contract before those in the back (Fig. 4), perhaps as a way of initiating group movement.
Another piece of evidence supporting the group coordination hypothesis involves an important characteristic of the processions—larvae undergo periods of movement and stillness at regular intervals. This pattern of movement and stillness is initiated by the individuals in front and could be a mechanism of maintaining group contact while traveling. Intermittent pauses during processions appear to enable stragglers to catch up with the group; cessation of movement does not occur simultaneously and often those in back continue walking a bit longer before stopping. The pauses could serve other purposes such as providing time for the lead individuals to assess the conditions ahead; however, an obvious result of this pattern is its promotion of group cohesiveness.
Why promote group cohesiveness and coordination?
Many organisms live in groups, and each is subject to a range of selective pressures pushing it towards this cooperative effort. All aggregating animals such as tadpoles and herds of ungulates have the advantage of a dilution effect when dealing with predators (Watt et al. 1997; Lingle 2001; Spieler 2005). When foraging, groups often increase their foraging efficiency while also increasing the effectiveness of predator vigilance, as seen in flocks of birds (Boland 2003; Fernandez-Juricic et al. 2004; Dias 2006). And in some instances, the grouping of individuals into a unit creates emergent properties with special functions such as thermoregulation in colonial insects (Simpson 1961; Klingner et al. 2005), aerodynamic lift benefits of formation flying in migrating birds (Lissaman and Shollenberger 1970; Hainsworth 1989), and the creation of spore-bearing structures in slime mold (Parrish and Edelstein-Keshet 1999). Even if individuals vary in the proportion of benefit that they receive (i.e., depending on position in group), they still receive an advantage that is unavailable to the solitary individual.
The larvae of P. affinis share many of the benefits of group living received by other organisms. P. affinis has an effective chemical defense in the form of a regurgitant composed of concentrated eucalyptus oils (Morrow et al. 1976), and its potency no doubt increases as the number of larvae employing the strategy increases. Additionally, like the display functions of other organisms such as the giant Thai honeybees (Apis dorsata) that shimmer in a wave motion across the colony when a predator approaches (Oldroyd and Wongsiri 2006), larvae rear heads in unison and flick their tails, movements which may make them appear as a large coordinated entity thereby deterring attack. Both of these qualities suggest that predation played a role in shaping the gregarious behavior of the larvae. Aggregation is seen as an effective form of defense in insects, especially when combined with chemical deterrence (Vulinec 1990); however, it is worth noting that no major predators of P. affinis are known (Carne 1969). Perhaps, evolutionarily, predators were an important selective pressure, but it is likely that other factors, such as the environment, also played a large role. For example, a potential emergent property of gregariousness in these larvae, like other colonial insects, may be enhanced thermoregulation. This could stem from environmental pressure, as the sawfly larvae are around during the winter months. By congregating in clusters during the day, they may be able to better absorb solar radiation, thus facilitating physiological processes and growth rates.
Traditionally, the evolution of cooperation through group living has been explained in two ways: (1) kinship theory and (2) reciprocation theory (Axelrod and Hamilton 1981). Many eusocial species such as bees or ants with single-queen colonies exhibit a high degree of relatedness due to their haplodiploid genetic structure and to the fact that all the workers are siblings (Holldobler and Wilson 1990; Boomsma and Ratnieks 1996). A eusocial mammal species, the naked mole rat (Heterocephalus glaber), also exhibits high levels of relatedness within colonies by having only one queen and one to three reproductive males at any time (Jarvis et al. 1994). In these systems, individuals gain indirect benefits by cooperating and helping kin. As the group benefits, so does the individual. Larvae of P. affinis may reap similar benefits through kinship, as the initial colonies are likely composed of siblings. Additionally, it is thought that the main mode of reproduction is amphitokous parthenogenesis (Carne 1962; although this has not been confirmed genetically). Carne (1962) demonstrated this by dissecting ovaries from newly emerged females and producing normal larvae after a 30-day incubation on sterile agar. If parthenogenesis is common, it would give early colonies a relatedness value of one, as the siblings would be clones. This level of relatedness, coupled with the overall benefits gained by gregarious living, may help to explain how such a complex signaling system evolved to aid in promoting group integrity. One must note, however, that colonies often merge later in the larval stage, so it is unclear how long the kinship benefits would last.
The second condition under which cooperation may occur is through repeated interactions. A broad range of organisms from complex primate societies to group-living birds utilizes these tactics to ensure a stable cooperative strategy. In female baboons (Papio cynocephalus), there is a highly significant relationship between grooming equality and the strength of social bonds, indicating the effectiveness of reciprocation in building social ties and hence cooperation (Silk et al. 2006). Food sharing in juvenile jackdaws (Corvus monedula) occurs more frequently between individuals that have shared before (and are reciprocating) as well as between those that have exchanged allopreening sessions (De Kort et al. 2006). In both examples, the cooperation directly stems from repeated interactions where reciprocation is possible.
P. affinis larvae also have repeated contact with each other through their daily rhythm of processing and dispersing to feed and then re-aggregating in a tight group during the day. In this context, cooperation through signaling and coordinated movement benefits all individuals, which may explain why larvae continue to cooperate even when the sibling colonies (with kinship benefits) coalesce with other, unrelated colonies later in the season (this generally starts occurring in the early third or fourth instar, personal observation). Alternatively, the environment may be sufficiently adverse for solitary or small groups of larvae that cooperation among unrelated individuals arises from by-product mutualism (Mesterton-Gibbons and Dugatkin 1992). Here, the strategy of cooperation stems from ‘ordinary selfish behavior’ (Eberhard 1975) because defecting (i.e., not signaling or promoting group cohesiveness) could lead to group disintegration, having dire fitness consequences for the individual. In these situations, the benefits of cooperating as a group largely outweigh any costs or conflicts inherent in association.
The adversity of the environment and the corresponding benefits of association are especially pronounced near the end of the larval stage. Larvae descend from their host tree to pupate underground en masse, and it is during this time that they have their highest exposure to parasitoids and may suffer high levels of mortality due to desiccation as they attempt to burrow under the ground (Carne 1966). In fact, the major causes of mortality for P. affinis throughout its entire larval and pre-pupal stages are desiccation, parasitism, and fungal disease (of water-logged cocoons; Carne 1969). Being in a group offers the advantage of the dilution effect (from parasitoids) as well as increased chances that at least one individual will successfully break through the ground to burrow. Their cocoons are lined with regurgitant, offering chemical protection and a sealant to protect pupae from desiccation (Morrow et al. 1976); both functions of the regurgitant are likely to be enhanced by being in a large group. Therefore, individual larvae gain by cooperating with the group and enhancing its coordination through communication.
Overall, this study confirms the use of acoustic signals for cooperative communication in a gregarious species of sawfly larvae, P. affinis. Often, when we study group-living organisms, we are quick to assume benefits and hence cooperation between the members. However, it is important to consider that, as stated by Parrish and Edelstein-Keshet (1999), “what appears to be cooperation resulting in cohesion may in fact be conflict veiled by the necessity to minimize the cost of disintegration.” Although a group may coordinate its efforts, individual members may still be in conflict suggesting that any signals used in communication have the potential to be competitive or cooperative in nature. Testing between these alternative hypotheses allows us to gain a deeper understanding of not only a specific organism but of the forces that govern cooperation and competition within animal societies. The study of communication is but one of many avenues by which one could determine what is important in balancing the cost and benefit scales in social living.
References
Axelrod R, Hamilton WD (1981) The evolution of cooperation. Science 211:1390–1396
Black JM (1988) Preflight signaling in swans—a mechanism for group cohesion and flock formation. Ethology 79:143–157
Boland CRJ (2003) An experimental test of predator detection rates using groups of free-living emus. Ethology 109:209–222
Boomsma JJ, Ratnieks FLW (1996) Paternity in eusocial Hymenoptera. Philos Trans R Soc Lond B Biol Sci 351:947–975
Bourne GR (1992) Lekking behavior in the neotropical frog Ololygon rubra. Behav Ecol Sociobiol 31:173–180
Bradbury JW, Vehrencamp SL (1998) Principles of animal communication. Sinuaer Associates, Sunderland
Carne P (1962) The characteristics and behaviour of the sawfly Perga affinis affinis (Hymenoptera). Aust J Zool 10:1–34
Carne P (1966) Primitive forms of social behaviour and their significance in the ecology of gregarious insects. Proc Ecol Soc Aust 1:75–78
Carne P (1969) On the population dynamics of the eucalypt-defoliating sawfly Perga affinis affinis Kirby (Hymenoptera). Aust J Zool 17:113–141
Cocroft RB (2005) Vibrational communication facilitates cooperative foraging in a phloem-feeding insect. Philos Trans R Soc Lond B Biol Sci 272:1023–1029
Cocroft RB, Rodriguez RL (2005) The behavioral ecology of insect vibrational communication. Bioscience 55:323–334
Cokl A, Doberlet MV (2003) Communication with substrate-borne signals in small plant-dwelling insects. Annu Rev Entomol 48:29–50
Costa J, Louque R (2001) Group foraging and trail following behavior of the red-headed pine sawfly Neodiprion lecontei (Fitch; Hymenoptera: Symphyta: Diprionidae). Ann Entomol Soc Am 94:480–489
Costa JT, Fitzgerald TD, Pescador-Rubio A, Mays J, Janzen DH (2004) Social behavior of larvae of the Neotropical processionary weevil Phelypera distigma (Boheman; Coleoptera: Curculionidae: Hyperinae). Ethology 110:515–530
Cummings DLD, Gamboa GJ, Harding BJ (1999) Lateral vibrations by social wasps signal larvae to withhold salivary secretions (Polistes fuscatus, Hymenoptera: Vespidae). J Insect Behav 12:465–473
De Kort SR, Emery NJ, Clayton NS (2006) Food sharing in jackdaws, Corvus monedula: what, why and with whom? Anim Behav 72:297–304
Dias RI (2006) Effects of position and flock size on vigilance and foraging behaviour of the scaled dove Columbina squammata. Behav Processes 73:248–252
Eberhard MJW (1975) Evolution of social behavior by kin selection. Q Rev Biol 50:1–33
Evans J (1934) Notes on the behavior of the larval communities of Perga dorsalis Leach (Hymenoptera, Tenthredinidae). Trans R Entomol Soc Lond 82:455–460
Fernandez-Juricic E, Siller S, Kacelnik A (2004) Flock density, social foraging, and scanning: an experiment with starlings. Behav Ecol 15:371–379
Fitzgerald T, Peterson S (1988) Cooperative foraging and communication in caterpillars. BioScience 38:20–25
Fitzgerald TD (1995) The tent caterpillars. Comstock, Ithaca
Fitzgerald TD (2003) Role of trail pheromone in foraging and processionary behavior of pine processionary caterpillars Thaumetopoea pityocampa. J Chem Ecol 29:513–532
Fitzgerald TD, Pescador-Rubio A (2002) The role of tactile and chemical stimuli in the formation and maintenance of the processions of the social caterpillar Hylesia lineata (Lepidoptera: Saturniidae). J Insect Behav 15:659–674
Fitzgerald TD, Pescador-Rubio A, Turna MT, Costa JT (2004) Trail marking and processionary behavior of the larvae of the weevil Phelypera distigma (Coleoptera: Curculionidae). J Insect Behav 17:627–646
Fletcher LE, Yack JE, Fitzgerald TD, Hoy RR (2006) Vibrational communication in the cherry leaf roller caterpillar Caloptilia serotinella (Gracillarioidea: Gracillariidae). J Insect Behav 19:1–18
Fordyce JA (2003) Aggregative feeding of pipevine swallowtail larvae enhances hostplant suitability. Oecologia 135:250–257
Ghent AW (1960) A study of the group-feeing behavior of larvae of the jack pine sawfly, Neodiprion pratti banksianae Roh. Behaviour 16:110–147
Gueron S, Levin SA, Rubenstein DI (1996) The dynamics of herds: from individuals to aggregations. J Theor Biol 182:85–98
Guerra PA, Mason AC (2005) Male competition and aggregative behaviour are mediated by acoustic cues within a temporally unstructured aggregation. Behaviour 142:71–90
Hainsworth FR (1989) Wing movements and positioning for aerodynamic benefit by Canada geese flying in formation. Can J Zool 67:585–589
Harding BJ, Gamboa GJ (1998) The sequential relationship of body oscillations in the paper wasp, Polistes fuscatus (Hymenoptera: Vespidae). Great Lakes Entomol 31:191–194
Hograefe T (1984) Subtrat-stridulation bei den koloniebildended Blattwespenlarven von Hemichroa crocea (Geoff.) (Hymenoptera: Tenthredinidae). Zool Anz 213:234–241
Holldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Janik VM, Slater PJ (1998) Context-specific use suggests that bottlenose dolphin signature whistles are cohesion calls. Anim Behav 56:829–838
Jarvis JUM, Oriain MJ, Bennett NC, Sherman PW (1994) Mammalian eusociality—a family affair. Trends Ecol Evol 9:47–51
Jolivet P, Vasconcellos-Neto J, Weinstein P (1990) Cycloalexy: a new concept in the larval defence of insects. Insecta Mundi 4:133–142
Klingner R, Richter K, Schmolz E, Keller B (2005) The role of moisture in the nest thermoregulation of social wasps. Naturwissenschaften 92:427–430
Langbauer WR (2000) Elephant communication. Zoo Biol 19:425–445
Lingle S (2001) Anti-predator strategies and grouping patterns in white-tailed deer and mule deer. Ethology 107:295–314
Lissaman PB, Shollenberger C (1970) Formation flight of birds. Science 168:1003–1005
Mesterton-Gibbons M, Dugatkin LA (1992) Cooperation among unrelated individuals-evolutionary factors. Q Rev Biol 67:267–281
Michelsen A, Flemming F, Gogala M, Traue D (1982) Plants as transmission channels for insect vibrational songs. Behav Ecol Sociobiol 11:269–281
Moller P (1976) Electric signals and schooling behavior in a weakly electric fish, Marcusenius cyprinoides L (Mormyriformes). Science 193:697–699
Morrow P, Bellas T, Eisner T (1976) Eucalyptus oils in the defensive oral discharge of Australian sawfly larvae (Hymenoptera: Pergidae). Oecologia 24:193–206
Nelson MC, Fraser J (1980) Sound production in the cockroach, Gromphadorhina portentosa—evidence for communication by hissing. Behav Ecol Sociobiol 6:305–314
Oldroyd BP, Wongsiri S (2006) Asian honey bees: biology, conservation and human interactions. Harvard University Press, Cambridge
Parrish JK, Edelstein-Keshet L (1999) Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284:99–101
Radford AN (2004a) Vocal coordination of group movement by green woodhoopoes (Phoeniculus purpureus). Ethology 110:11–20
Radford AN (2004b) Vocal mediation of foraging competition in the cooperatively breeding green woodhoopoe (Phoeniculus purpureus). Behav Ecol Sociobiol 56:279–285
Reeve HK (1991) Polistes. In: Ross KG, Matthews RW (eds) The social biology of wasps. Comstock, Ithaca, pp 99–148
Reid SF (2004) Group foraging, navigation and morphology of the sawfly larvae Pergagrapta (Hymenoptera: Symphyta: Pergidae). In: School of botany and zoology. University of Australia, Canberra, pp 56
Ruf C, Costa JT, Fiedler K (2001) Trail-based communication in social caterpillars of Eriogaster lanestris (Lepidoptera: Lasiocampidae). J Insect Behav 14:231–245
Russ K (1969) Beiträge zum Territorialverhalten der Raupen des Springwurmwicklers, Sparganothis pilleriana Schiff (Lepidoptera: Tortricidae). Pflanzenschutz Ber Wein 40:1–9
Savoyard JL, Gamboa GJ, Cummings DLD, Foster RL (1998) The communicative meaning of body oscillations in the social wasp, Polistes fuscatus (Hymenoptera, Vespidae). Insectes Soc 45:215–230
Silk JB, Alberts SC, Altmann J (2006) Social relationships among adult female baboons (Papio cynocephalus) II. Variation in the quality and stability of social bonds. Behav Ecol Sociobiol 61:197–204
Simpson J (1961) Nest climate regulation in honey bee colonies-honey bees control their domestic environment by methods based on their habit of clustering together. Science 133:1327–1333
Spieler M (2005) Can aggregation behaviour of Phrynomantis microps tadpoles reduce predation risk? Herpetol J 15:153–157
Stein AC, Uy JAC (2006) Plumage brightness predicts male mating success in the lekking golden-collared manakin, Manacus vitellinus. Behav Ecol 17:41–47
Tibbetts EA (2002) Visual signals of individual identity in the wasp Polistes fuscatus. Proc R Soc Lond B Biol Sci 269:1423–1428
Vulinec K (1990) Collective security: aggregation by insects in defense. In: Evans L, Schmidt J (eds) Insect defenses. State University of New York Press, Albany, pp 251–288
Watt PJ, Nottingham SF, Young S (1997) Toad tadpole aggregation behaviour: evidence for a predator avoidance function. Anim Behav 54:865–872
Wheeler GS (2001) Host plant quality factors that influence the growth and development of Oxyops vitiosa, a biological control agent of Melaleuca quinquenervia. Biol Control 22:256–264
Wheeler GS, Center TD (1996) The influence of Hydrilla leaf quality on larval growth and development of the biological control agent Hydrellia pakistanae (Diptera: Ephydridae). Biol Control 7:1–9
Yack J, Smith M, Weatherhead P (2001) Caterpillar talk: acoustically mediated territoriality in larval Lepidoptera. Proc Natl Acad Sci USA 98:11371–11375
Acknowledgments
I would like to thank the Gurr Lab at University of Sydney at Orange and the Foley lab at the Australian National University for hosting me during my fieldwork, Rex Cocroft for the phonocartridge and instruction on recording vibrations, Bob Grotke for his help with the recording equipment, and Tom Eisner, Kern Reeve, Jayne Yack, Cole Gilbert, Meredith Cosgrove, Michael Braby and two anonymous reviewers for comments on the manuscript. I also thank Anita Tseng for help with video data analysis. Funding for this work was provided by an NSF Predoctoral Fellowship and a Sigma Xi grant. The experiments comply with the current laws in Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Moore
Rights and permissions
About this article
Cite this article
Fletcher, L.E. Vibrational signals in a gregarious sawfly larva (Perga affinis): group coordination or competitive signaling?. Behav Ecol Sociobiol 61, 1809–1821 (2007). https://doi.org/10.1007/s00265-007-0414-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-007-0414-2