Abstract
Flowers exhibit great intra-specific variation in the rewards they offer. At any one time, a significant proportion of flowers often contain little or no reward. Hence, foraging profitably for floral rewards is problematic and any ability to discriminate between flowers and avoid those that are less rewarding will confer great advantages. In this study, we examine discrimination by foraging bees among flowers of nasturtium, Tropaeolum majus. Bee visitors included carpenter bees, Xylocopa violacea, which were primary nectar robbers; honeybees, Apis mellifera, which either acted as secondary nectar robbers or gathered pollen legitimately and bumblebees, Bombus hortorum, which were the only bees able to gather nectar legitimately. Many flowers were damaged by phytophagous insects. Nectar volume was markedly lower in flowers with damaged petals (which were also likely to be older) and in flowers that had nectar-robbing holes. We test whether bees exhibit selectivity with regards to the individual flowers, which they approach and enter, and whether this selectivity enhances foraging efficiency. The flowers approached (within 2 cm) by A. mellifera and B. hortorum were non-random when compared to the floral population; both species selectively approached un-blemished flowers. They both approached more yellow flowers than would be expected by chance, presumably a reflection of innate colour preferences, for nectar standing crop did not vary according to flower colour. Bees were also more likely to accept (land on) un-blemished flowers. A. mellifera gathering nectar exhibited selectivity with regards to the presence of robbing holes, being more likely to land on robbed flowers (they are not able to feed on un-robbed flowers). That they frequently approached un-robbed flowers suggests that they are not able to detect robbing holes at long-range, so that foraging efficiency may be limited by visual acuity. Nevertheless, by using a combination of long-range and short-range selectivity, nectar-gathering A. mellifera and B. hortorum greatly increased the average reward from the flowers on which they landed (by 68% and 48%, respectively) compared to the average standing crop in the flower population. Overall, our results demonstrate that bees use obvious floral cues (colour and petal blemishes) at long-range, but can switch to using more subtle cues (robbing holes) at close range. They also make many mistakes and some cues used do not correlate with floral rewards.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Efficiently gathering floral rewards is problematic as the reward per flower varies greatly between plants of a single species and between flowers on a single plant. Variation may be due to micro-environmental influences, genetic variation, age of the plant or age of the flower and also a result of the pattern of depletion of rewards by previous visitors (reviewed in Goulson 1999). At any one time, many flowers may be empty (Wetherwax 1986; Real and Rathcke 1988; Cresswell 1990; Waser and Mitchell 1990).
If foragers can distinguish between more and less rewarding flowers of their preferred species, they can enhance their foraging success (reviewed in Goulson 1999, 2003). The time it takes for a bumblebee forager to handle a flower varies greatly according to floral morphology, from as little as 1 s for simple flowers to up to 10 s for complex flowers (e.g. Heinrich 1979b; Pyke 1979; Hodges 1981; Best and Bierzychudek 1982; Osborne 1994; Cresswell 1999). If the flower contains little or no reward, this time is wasted and because foraging in larger bee species such as bumblebees requires considerable expenditure of energy, visiting flowers with little or no reward is costly (Ellington et al. 1990). Hence, there is strong selection pressure on bees to evolve efficient means of choosing the more rewarding flowers.
Both bumblebees and honeybees are often seen to hover in front of a flower, sometimes briefly touching the corolla and then depart without probing into the flower structure. These rejected flowers contain, on average, less nectar than flowers, which are probed (Heinrich 1979a; Corbet et al. 1984; Wetherwax 1986; Kato 1988; Duffield et al. 1993). Several mechanisms may be in operation. Where the flower structure is open and the anthers are clearly visible, bumblebees are able to directly assess the pollen content of open flowers visually (Zimmerman 1982; Cresswell and Robertson 1994). It has been suggested that they may be able to determine the nectar content of some flower species in the same way (Thorp et al. 1975, 1976; Kevan 1976). It has also been proposed that they may be able to assess nectar volumes from the scent of the nectar itself or the scent of fermentation products from yeasts in the nectar (Crane 1975; Williams et al. 1981; Heinrich 1979a). They could plausibly detect nectar volumes from humidity gradients surrounding the flower (Corbet et al. 1979). However, apart from visual detection of pollen availability, none of these mechanisms of direct detection of floral rewards have been demonstrated.
It has long been known that bumblebees, honeybees, solitary bees, hover flies and butterflies are able to discriminate between age classes of flowers using visual cues, which correlate with reward (Jones and Buchmann 1974; Kevan 1978; Thomson et al. 1982; Weiss 1995a). Discrimination among flowers according to their age may be facilitated by clear visual cues given by the plant itself, particularly by colour changes, which variously occur in part or all of the flower (Kevan 1983; Gori 1983, 1989; Delph and Lively 1992; Weiss 1995a, b; Weiss and Lamont 1997; Nuttman et al. 2006). For example, flowers of Pulmonaria sp. change from red to blue, enabling bumblebees and flower bees (Anthophora pilipes) to select the more rewarding red flowers (Oberrath et al. 1995). These age-dependent preferences can be flexible; honeybees select 3-day-old capitula of Carduus acanthoides in the early morning, and switch to 2-day-old capitula later in the day. This accurately targets the time of maximum nectar production in capitula, which is from mid-way through their second day until early on their third (Giurfa and Núñez 1992).
Rates of nectar production may vary with flower age, but there is no general pattern to the changes in nectar production with age. Commonly, nectar production declines after the flower opens (Voss et al. 1980) or reaches an early peak and then declines (Bond and Brown 1979; Frost and Frost 1981; Bertin 1982; Pleasants and Chaplin 1983; Southwick and Southwick 1983; Cruzan et al. 1988; Nuttman et al. 2006), but in some species nectar production increases with flower age (Pyke 1978; Brink and De Wet 1980; Corbet and Willmer 1980; Best and Bierzychudek 1982; Robertson and Wyatt 1990).
To date, studies have either examined the relationship between floral cues and insect visitation rates or have studied the close range behaviour of bees with regards to their response to scent marks left on flowers by previous foragers. Few attempts have been made to assess the scale over which discrimination between flowers operates. In this study, we examine the foraging choices of both legitimately foraging bumblebees and nectar-robbing honeybees to floral characteristics, which correlate with reward and test whether foraging decisions result in improved foraging efficiency.
Materials and methods
Experiments were conducted at Quinta de Sao Pedro Field Study Centre in Lisbon, Portugal, during June 2006. The site consists of 4 ha of grassland and open woodland.
Tropaeolum majus L. Tropaeolaceae was one of the most abundant herbaceous plant species within the wooded areas. It is an annual plant, native to South America, but widely naturalised in Europe. It flowers in May and June, producing polymorphic red/orange or yellow flowers. Nectar is located at the end of a deep (∼10–12 mm) and narrow tube and so is not readily accessed by most insect species. Within the study site, three bee species commonly visit T. majus: honeybees, Apis mellifera L.; carpenter bees, Xylocopa violacea L. and bumblebees, Bombus hortorum L. X. violacea is by far the scarcest of the three species and is a primary nectar robber, biting a slit in the nectar tube to gain access to the nectaries. A. mellifera are both secondary nectar robbers and also gather pollen by accessing the flower legitimately. B. hortorum has a very long tongue (∼15 mm) and is the only bee species able to collect nectar legitimately. At the study site, phytophagous insects, notably larvae of Pieris spp. (Lepidoptera), damaged many flowers.
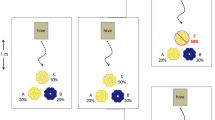
To quantify the frequency of nectar-robbed flowers within the population and of the two colour morphs, 200 nasturtium flowers in the study site were randomly selected using random number tables to generate coordinates. Damage to flowers was also recorded on a three point scale: 1 = complete and un-blemished; 2 = slight damage to petals; 3 = heavy damage and/or some petals shrivelled. The volume of nectar in each flower was measured using a micro-capillary tube.
Bee foraging was recorded as follows. For both A. mellifera and B. hortorum, the flowers that each bee approached were recorded. An approach was defined as flying within 2 cm of a flower. Flowers were scored as rejected if the bee did not land or feed or accepted if the bee landed and attempted to gather pollen or nectar. Each flower that had been approached was then scored as above for colour, damage and nectar robbery. Bees were then caught and marked with a queen marking disc (E.H. Thorne, Wragby, Lincs, UK) to avoid observing the same bee on subsequent occasions. Data were collected for B. hortorum, all of which were collecting nectar, and A. mellifera, which were either collecting nectar as secondary robbers or collecting only pollen (no bees switched between tasks). Too few X. violacea were present to obtain useful data. The probability of bees accepting or rejecting flowers according to floral characteristics was analysed in the generalised linear interactive modelling (GLIM) software using binomial errors, using flower colour, damage category and whether the flower had previously been robbed as potential explanatory factors. Analysis of variance (ANOVA) was used to compare the effects of damage and robbery on nectar levels per flower.
Results
Of the 200 randomly selected flowers, 71% were orange and 29% were yellow. Robbed flowers comprised 42.7% of the population (57.3% un-robbed). There was no association between colour and whether a flower had been robbed (\( \chi ^{2}_{1} = 1.42,\,p{\text{ $>$ }}0.05 \)). Approximately equal numbers of flowers fell into each damage category (32.1%, 33.2% and 34.7% for damage categories 1, 2 and 3, respectively). There was no association between colour and damage category of a flower (\( \chi ^{2}_{2} = 1.14,\,p{\text{ $>$ }}0.5 \)). However, there was a strong association between damage category and whether a flower had been robbed; 40.1% of flowers with undamaged petals had been robbed compared to 65.6% and 65.2% for damaged flowers in categories 2 and 3, respectively (\( \chi ^{2}_{2} = 10.8,\,p < 0.01 \)).
Nectar levels did not differ between orange and yellow flowers (F 1,195 = 0.014, p = 0.652). Nectar levels were significantly lower in robbed flowers compared to un-robbed (F 1,193 = 26.7, p < 0.001) and were lower in damaged flowers compared to undamaged (F 2,195 = 35.9, p < 0.001; Fig. 1). There was no significant interaction between effects of robbing and petal damage on nectar levels (F 2,195 = 1.72, p = 0.141).
Bee foraging behaviour can be examined at two levels: whether the flowers that they approached were a random sample of the population and whether the decision to land on (accept) a flower after an approach was influenced by floral characteristics. Both A. mellifera and B. hortorum approached significantly more yellow flowers than would be expected from the frequency (29.0%) of yellow flowers in the population. For A. mellifera, 40.8% of 358 approaches were to yellow flowers (\( \chi ^{2}_{1} = 23.9,\,p < 0.001 \)). For B. hortorum, 37.7% of 204 approaches were to yellow flowers (\( \chi ^{2}_{1} = 7.53,\,p < 0.01 \)).
Approaches to flowers were also not random with regards to petal damage. For A. mellifera, 56.4% of approaches were to flowers with un-blemished petals, which comprised just 32.1% of the floral population (\( \chi ^{2}_{1} = 90.7,\,p < 0.001 \); Fig. 2). There was no difference in the behaviour of bees collecting pollen vs those collecting nectar in this respect (\( \chi ^{2}_{2} = 3.55,\,p > 0.5 \)). For B. hortorum, 52.5% of visits were to un-blemished flowers, more than would be expected by chance (\( \chi ^{2}_{1} = 40.1,\,p < 0.001 \); Fig. 2).
Numbers of approaches made by foraging A. mellifera (n = 358) and B. hortorum (n = 204) to flowers according to damage category: 1 = complete and un-blemished; 2 = slight damage to petals; 3 = heavy damage and/or some petals shrivelled. It should be noted that approaches include flowers that were rejected by the bee without landing. Also shown is the frequency of flowers in each category within the population
A. mellifera collecting nectar and those collecting pollen did not differ significantly in their likelihood of approaching robbed vs un-robbed flowers (\( \chi ^{2}_{1} = 2.96,\,p > 0.5 \)) and, overall, the proportion of robbed and un-robbed flowers that were approached did not differ from the proportion of robbed and un-robbed flowers in the random sample of the flower population (\( \chi ^{2}_{1} = 1.31,\,p > 0.5. \); Fig. 3). B. hortorum (which were all foraging for nectar) approached slightly more un-robbed flowers than would be expected by random foraging (\( \chi ^{2}_{1} = 4.22,\,p < 0.05 \); Fig. 3).
After an approach, A. mellifera were more likely to land on un-blemished flowers (\( \chi ^{2}_{2} = 54.4,\,p < 0.001 \)), regardless of whether they were foraging for nectar or pollen (proportion of flowers accepted 0.717, 0.517 and 0.239 for flowers in categories 1, 2 and 3, respectively). However, there was a significant interaction between whether bees were collecting nectar or pollen and their response to whether the flower had already been robbed (\( \chi ^{2}_{1} = 11.4,\,p < 0.01 \)) (Fig. 4). Nectar foragers were more likely to land on robbed flowers (\( \chi ^{2}_{1} = 8.71,\,p < 0.05 \)), but pollen foragers were not influenced by whether the flower had previously been robbed (\( \chi ^{2}_{1} = 3.43,\,p > 0.5 \)). Despite discriminating at close range against robbed flowers, 45.3% of A. mellifera foraging for nectar still landed on un-robbed flowers from which they were unable to feed.
After an approach, B. hortorum (which only collected nectar) behaved similarly to pollen-collecting A. mellifera, tending to reject flowers with damaged petals (\( \chi ^{2}_{2} = 41.8,\,p < 0.001 \)). They were not significantly more likely to land on un-robbed flowers although there was a weak trend in this direction (\( \chi ^{2}_{1} = 3.2,\,p > 0.5 \); Fig. 4). After an approach, neither B. hortorum nor A. mellifera were influenced in their decision to land by flower colour (\( \chi ^{2}_{1} = 0.0{\text{ and }}\chi ^{2}_{1} = 0.1 \), respectively).
Nectar levels varied greatly between T. majus flowers in the study area (range 0–14.9 μl) with a mean of 3.11 μl ± 0.22 (SE). However, for A. mellifera, which are unable to access flowers that have not been robbed, the average accessible reward is much lower at 1.16 μl because the flowers containing the most nectar tend to be those that have not been robbed (i.e. accessible reward is zero). By multiplying the expected reward from flowers in each of the six possible damage/robbing categories by the frequency with which bees approached each category, it is possible to calculate the average reward per flower approached. Similarly, the expected reward in flowers on which the bees actually landed can be calculated. These values can then be compared with the average reward they would obtain by randomly approaching and landing on flowers. The non-random approach of flowers exhibited by both bee species results in an improved average reward per flower approached: 3.99 μl for B. hortorum and 1.45 μl for nectar-foraging A. mellifera (improvements of 28% and 25%, respectively). The average accessible reward per flower on which the bees actually landed was still higher (4.61 μl for B. hortorum and 1.95 μl for A. mellifera), representing an increase of 48% and 68%, respectively. However, if bees were able to detect robbery at long-range and approach and visit only the optimal category of flowers (un-robbed un-blemished flowers for B. hortorum, robbed un-blemished flowers for A. mellifera), then they could obtain an average reward of 6.62 and 3.84 μl per flower, respectively.
Discussion
Our results suggest that bees exhibit both long-range and short-range selectivity among flowers when foraging and that criteria can differ between the two. All bees were more likely to approach flowers with un-blemished petals and were least likely to approach heavily damaged flowers. Because damage is strongly correlated with average nectar reward, this strategy clearly makes sense for bees collecting nectar. Damaged flowers are more likely to be old, and it seems probable that nectar secretion declines with age in T. majus, as occurs in some other plant species (see “Introduction”). For bees collecting pollen, older flowers are presumably also more likely to have had pollen stripped by a previous forager compared to young, un-blemished flowers. This long-range selectivity is almost certainly visual because damaged petals are an obvious signal of probable low reward. Scent cues associated with particular flowers are unlikely to be detectable except at short-range. Visual discrimination against damaged flowers has been described in other pollinators, including various bees and hummingbirds (Krupnick et al. 1999; Pohl et al. 2006). Honeybees and other insects show an innate preference for symmetrical shapes (Möller and Sorci 1998) and this can be reinforced by learning (Giurfa et al. 1996). Because damaged flowers are likely to be asymmetrical and less rewarding, this would readily explain our observations.
It is interesting to note that A. mellifera gathering nectar did not selectively approach flowers that had been previously robbed, even though these are the only ones from which they are able to extract rewards. It seems likely that the small hole in the nectar tube made by X. violacea is not readily detected from long-range, so that visual acuity limits the ability of bees to make optimal foraging decisions. By favouring un-blemished flowers, A. mellifera gathering nectar are increasing their chances of encountering un-robbed flowers that they cannot feed from, for un-blemished flowers are less likely to have been robbed (probably because both robbing and damage are likely to be correlated with age). Nonetheless, this strategy would appear to be worthwhile because un-blemished flowers have two to three times the reward of blemished flowers. Hence, the average available reward in flowers that A. mellifera approach was higher than a random sample.
At short-range (<2 cm), all bees were more likely to reject blemished rather than un-blemished flowers. The end result of both long-range and short-range selectivity is that un-blemished flowers receive 64.7% of all visits by B. hortorum and 73.4% of visits by A. mellifera, despite comprising only 32.1% of the floral population. A. mellifera foraging for nectar were also able to selectively avoid un-robbed flowers at close range, although there were 48 instances in which a nectar-foraging honeybee approached and landed on an un-robbed flower, demonstrating that their ability to detect and avoid un-robbed flowers without landing is far from perfect. B. hortorum and pollen-collecting A. mellifera exhibited no significant response at either long-range or short-range according to whether flowers had been robbed. When collecting pollen, there is no obvious reason why robbing should influence foraging decisions, but for B. hortorum (which were collecting nectar), robbed flowers were less rewarding and should have been avoided if possible. However, both B. hortorum and pollen-foraging A. mellifera approach flowers from the front, from which angle they would be unable to see damage to the nectar tube.
Both bee species appeared to exhibit a long-range preference for yellow rather than orange flowers, although the standing crop of nectar did not differ between the two colour morphs. This may simply be because bees have an innate preference for particular colours, notably yellow and purple, which reflects the peak sensitivity of their colour receptors (Lunau 1990; Giurfa et al. 1995; Gumbert 2000). Yellow flowers may therefore be easier for them to see in the shaded woodland in which this study was carried out. This would appear to be an example of floral discrimination driven by perceptual bias rather than being motivated by economic factors and a behaviour, which is selectively neutral to the bee. Form the plant’s perspective, this behaviour would be expected to result in long-term selection for yellow flowers over orange in the population (assuming colour has a genetic basis). However, T. majus is not a native plant but a garden escape in the study area, so current colour frequencies are likely to have been influenced by artificial selection by man in the recent past.
In addition to visual cues to floral rewards, bees are well-known to use the presence of hydrocarbons left behind by previous insect visitors and so avoid flowers that have been recently visited (Schmitt and Bertsch 1990; Stout et al. 1998; Goulson et al. 1998, 2000, 2001; Goulson and Stout 2001; Stout and Goulson 2002). In this study, we did not have information on the previous visitation history of the flowers, but it is quite probable that the bees were also using scent marks to further improve foraging efficiency. As discussed, un-blemished but previously robbed flowers were scarce, but represented the ideal food source for nectar-foraging A. mellifera because they had high average rewards. Of the 62 instances in which a nectar-foraging A. mellifera approached such a flower, 54 proceeded to land a feed, but 8 rejected the flower without landing. It seems likely that this may have been a response to the scent of a previous recent visitor.
In summary, both bee species substantially increase the average nectar reward in the flowers that they visited by using a combination of long-range and short-range cues, although their foraging efficiency was limited in part by perceptual constraints. They also exhibit floral selectivity due to innate preferences, which appear to be selectively neutral in terms of foraging efficiency. It would be valuable to investigate how the behaviour of and interactions between primary and secondary nectar robbers and legitimate foragers affects the plant, for the previous studies provide conflicting evidence as to whether nectar robbery has net positive or negative effects on plant reproductive fitness (Morris 1996; Stout et al. 2000; Irwin 2003).
References
Bertin RI (1982) Floral biology, hummingbird pollination and fruit production of trumpet creeper (Campsis radicans, Bignoniaceae). Am J Bot 69:122–134
Best LS, Bierzychudek P (1982) Pollinator foraging on foxglove (Digitalis purpurea): a test of a new model. Evolution 36:70–79
Bond HW, Brown WL (1979) The exploitation of floral nectar in Eucalyptus incrassata by honeyeaters and honeybees. Oecologia 44:105–111
Brink D, De Wet JMJ (1980) Interpopulation variation in nectar production in Aconitum columbianum (Ranunculaceae). Oecologia 47:160–163
Corbet SA, Willmer PG (1980) Pollination of the yellow passionfruit: nectar, pollen and carpenter bees. Journal of Agricultural Science 95:655–666
Corbet SA, Willmer PG, Beament JWL, Unwin DM, Prys-Jones OE (1979) Post-secretory determinants of sugar concentration in nectar. Plant Cell Environ 2:293–308
Corbet SA, Kerslake CJC, Brown D, Morland NE (1984) Can bees select nectar-rich flowers in a patch? J Apic Res 23:234–242
Crane E (1975) Honey: a comprehensive survey. Heinemann, London
Cresswell JE (1990) How and why do nectar-foraging bumblebees initiate movements between inflorescences of wild bergamot Monarda fistulosa (Lamiaceae). Oecologia 82:450–460
Cresswell JE (1999) The influence of nectar and pollen availability on pollen transfer by individual flowers of oilseed rape (Brassica napus) when pollinated by bumblebees (Bombus lapidarius). J Ecol 87:670–677
Cresswell JE, Robertson AW (1994) Discrimination by pollen-collecting bumblebees among differentially rewarding flowers of an alpine wildflower, Campanula rotundifolia (Campanulaceae). Oikos 69:304–308
Cruzan MB, Neal PR, Willson MF (1988) Floral display in Phyla incisa: consequences for male and female reproductive success. Evolution 42:505–515
Delph LF, Lively CM (1992) Pollinator visitation, floral display, and nectar production of the sexual morphs of a gynodioecious shrub. Oikos 63:161–170
Duffield GE, Gibson RC, Gilhooly PM, Hesse AJ, Inkley CR, Gilbert FS, Barnard CJ (1993) Choice of flowers by foraging honey-bees (Apis mellifera)—possible morphological cues. Ecol Entomol 18:191–197
Ellington CP, Machin KE, Casey TM (1990) Oxygen consumption of bumblebees in forward flight. Nature 347:472–473
Frost SK, Frost PGH (1981) Sunbird pollination of Strelizia nicolai. Oecologia 49:379–384
Giurfa M, Núñez JA (1992) Foraging by honeybees on Carduus acanthoides—pattern and efficiency. Ecol Entomol 17:326–330
Giurfa M, Nunez J, Chittka L, Menzel R (1995) Color preferences of flower-naive honeybees. J Comp Physiol A 177:247–259
Giurfa M, Eichmann B, Menzel R (1996) Symmetry perception in an insect. Nature 382:458–461
Gori DF (1983) Post-pollination phenomena and adaptive floral changes. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand Reinhold, New York, pp 31–49
Gori DF (1989) Floral colour change in Lupinus argenteus (Fabaceae): why should plants advertise the location of unrewarding flowers to pollinators? Evolution 43:870–881
Goulson D (1999) Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspect Plant Ecol Evol Syst 2:185–209
Goulson D (2003) Bumblebees; their behaviour and ecology. Oxford University Press, Oxford
Goulson D, Stout JC (2001) The use of conspecific and interspecific scent marks by foraging bumblebees and honeybees. Anim Behav 62:183–189
Goulson D, Hawson SA, Stout JC (1998) Foraging bumblebees avoid flowers already visited by conspecifics or by other bumblebee species. Anim Behav 55:199–206
Goulson D, Stout JC, Langley J, Hughes WOH (2000) The identity and function of scent marks deposited by foraging bumblebees. J Chem Ecol 26:2897–2911
Goulson D, Chapman JW, Hughes WOH (2001) Discrimination of unrewarding flowers by different bee species; direct detection of rewards and use of repellent scent marks. J Insect Behav 14:669–678
Gumbert A (2000) Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behav Ecol Sociobiol 48:36–43
Heinrich B (1979a) Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia 40:235–245
Heinrich B (1979b) Bumblebee economics. Harvard University Press, Cambridge, MA
Hodges CM (1981) Optimal foraging in bumblebees: hunting by expectation. Anim Behav 29:1166–1171
Irwin RE (2003) Impact of nectar robbing on estimates of pollen flow: conceptual predictions and empirical outcomes. Ecology 84:485–495
Jones CE, Buchmann SL (1974) Ultraviolet floral patterns as functional orientation cues in hymenopterous pollination systems. Anim Behav 22:481–485
Kato M (1988) Bumble bee visits to Impatiens spp.: pattern and efficiency. Oecologia 76:364–370
Kevan PG (1976) Fluorescent nectar (technical comment). Science 194:341–342
Kevan PG (1978) Floral coloration, its colorimetric analysis and significance in anthecology. In: Richards AJ (ed) The pollination of flowers by insects. Academic, London, pp 51–78
Kevan PG (1983) Floral colours through the insect eye: what they are and what they mean. In: Jones CE, Little RJ (eds) Handbook of experimental pollination ecology. Van Nostrand Reinhold, New York
Krupnick GA, Weis AE, Campbell DR (1999) The consequences of floral herbivory for pollinator service to Isomeris arborea. Ecology 80:125–134
Lunau K (1990) Colour saturation triggers innate reactions to flower signals: flower dummy experiments with bumblebees. J Comp Physiol A 166:827–834
Möller AP, Sorci G (1998) Insect preference for symmetrical artificial flowers. Oecologia 114:37–42
Morris WF (1996) Mutualism denied? Nectar-robbing bumble bees do not reduce female or male success of bluebells. Ecology 77:1451–1462
Nuttman CV, Semida FM, Zalat S, Willmer PG (2006) Visual cues and foraging choices: bee visits to floral colour phases in Alkanna orientalis (Boraginaceae). Biol J Linn Soc 87:427–435
Oberrath R, Zanke C, Bohning-Gaese K (1995) Triggering and ecological significance of floral color-change in lungwort (Pulmonaria spec.). Flora 190:155–159
Osborne JL (1994) Evaluating a pollination system: Borago officinalis and bees. Ph.D. thesis, Cambridge University, UK
Pleasants JM, Chaplin SJ (1983) Nectar production rates of Asclepias quadrifolia: causes and consequences of individual variation. Oecologia 59:232–238
Pohl N, Carvallo G, Botto-Mahan C, Medel R (2006) Nonadditive effects of flower damage and hummingbird pollination on the fecundity of Mimulus luteus. Oecologia 149:648–655
Pyke GH (1978) Optimal foraging in bumblebees and coevolution with their plants. Oecologia 36:281–293
Pyke GH (1979) Optimal foraging in bumblebees: rules of movement between flowers within inflorescences. Anim Behav 27:1167–1181
Real L, Rathcke BJ (1988) Patterns of individual variability of floral resources. Ecology 69:728–735
Robertson JL, Wyatt R (1990) Reproductive biology of the yellow-fringed orchid, Platanthera ciliaris. Am J Bot 77:388–398
Schmitt U, Bertsch A (1990) Do foraging bumblebees scent-mark food sources and does it matter? Oecologia 82:137–144
Southwick AK, Southwick EE (1983) Aging effects on nectar production in two clones of Asclepias syriaca. Oecologia 56:121–125
Stout JC, Goulson D (2002) The influence of nectar secretion rates on the responses of bumblebees (Bombus spp.) to previously visited flowers. Behav Ecol Sociobiol 52:239–246
Stout JC, Goulson D, Allen JA (1998) Repellent scent marking of flowers by a guild of foraging bumblebees (Bombus spp.). Behav Ecol Sociobiol 43:317–326
Stout JC, Allen JA, Goulson D (2000) Nectar robbing, forager efficiency and seed set: bumblebees foraging on the self incompatible plant Linaria vulgaris Mill. (Scrophulariaceae). Acta Oecol 21:277–283
Thomson JD, Maddison WP, Plowright RC (1982) Behaviour of bumblebee pollinators on Aralia hispida Vent. (Araliaceae). Oecologia 54:326–336
Thorp RN, Briggs DL, Estes JR, Erikson EH (1975) Nectar fluorescence under ultraviolet irradiation. Science 189:476–478
Thorp RN, Briggs DL, Estes JR, Erikson EH (1976) Fluorescent nectar. Science 194:342 (Reply to P.H. Kevan, pp 341–342)
Voss R, Turner M, Inouye R, Fisher M, Cort R (1980) Floral biology of Markea neurantha Hemsley (Solanaceae), a bat-pollinated epiphyte. Am Midl Nat 103:262–268
Waser NM, Mitchell RJ (1990) Nectar standing crops in Delphinium nelsonii flowers: spatial autocorrelation among plants? Ecology 71:116–123
Weiss MR (1995a) Associative color learning in a nymphalid butterfly. Ecol Entomol 20:298–301
Weiss MR (1995b) Floral color-change—a widespread functional convergence. Am J Bot 82:167–185
Weiss MR, Lamont BB (1997) Floral color change and insect pollination: a dynamic relationship. Isr J Plant Sci 45:185–199
Wetherwax PB (1986) Why do honeybees reject certain flowers? Oecologia 69:567–570
Williams AA, Hollands TA, Tucknott OG (1981) The gas chromatographic–mass spectrometric examination of the volatiles produced by the fermentation of a sucrose solution. Z Lebensm-Unters Forsch 172:377–381
Zimmerman ML (1982) Optimal foraging: random movement by pollen collecting bumblebees. Oecologia 53:394–398
Acknowledgements
We wish to thank the staff of the Quinta de Sao Pedro Field Study Centre, Lisbon, Portugal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Giurfa
Rights and permissions
About this article
Cite this article
Goulson, D., Cruise, J.L., Sparrow, K.R. et al. Choosing rewarding flowers; perceptual limitations and innate preferences influence decision making in bumblebees and honeybees. Behav Ecol Sociobiol 61, 1523–1529 (2007). https://doi.org/10.1007/s00265-007-0384-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-007-0384-4