Abstract
Bumblebee colonies experience daily and seasonal fluctuations in ambient temperature, but proper brood development requires a stable nest temperature. This study examined how adaptive colony responses to changing ambient temperature are achieved through the in-nest workers’ behavioral plasticity. We studied three Bombus huntii colonies in the laboratory. In the first experiment, we manipulated ambient temperature and recorded brood cell incubation and wing fanning by individually marked, known-age bees. The colonies maintained their nests closer to appropriate brood development temperatures (28 to 32°C) when exposed to a range of ambient temperatures from 10.3 to 38.6°C. Incubation activity was greater in cooler treatment conditions, whereas in the highest temperature treatment, some bees fanned and others moved off the brood. As the ambient temperature dropped, workers increased the duration of their incubating bouts, but, except at the highest temperature, the number of workers that incubated did not differ significantly among treatments. A subset of the bees incubated significantly more than their nest mates, some of which never incubated. Worker body size, but not age, was a good predictor of incubation rates, and smaller bees incubated at higher rates. In the second experiment, we removed the most actively incubating workers. Immediately after removals, the total colony incubation effort was lower than pre-removal levels, but incubation effort rebounded toward pre-removal levels after 24 h. The increased thermoregulatory demand after removals was met primarily by bees increasing their rates of incubation rather than by bees switching from a different task to incubation. We conclude that some B. huntii workers specialize on nest thermoregulation, and that changes in work rates are more important than task switching in meeting thermal challenges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermoregulation presents a special challenge for sessile animals because they cannot move to escape unfavorable temperatures. Social insect nests—and the brood they contain—are a good example of spatially constrained organisms that must thermoregulate in response to ambient temperature fluctuations. The larvae and pupae in colonies of eusocial Hymenoptera can experience developmental delay, disruption, or termination if nest temperatures fluctuate above or below a relatively narrow range of temperature optima (Barrow and Pickard 1985; Yoon et al. 2002; Tautz et al. 2003; Jones et al. 2005; McMullan and Brown 2005). Therefore, nest temperature homeostasis is under strong selective pressure, and eusocial Hymenoptera exhibit an array of adaptive physiological and behavioral responses to changes in ambient temperature (Seeley and Heinrich 1981; Starks and Gilley 1999; Weidenmueller et al. 2002; Stabentheiner et al. 2003; Klingner et al. 2005). Protection against ambient temperature fluctuations depends in part on nest location and design (Richards 1978; Foster 1992; Jeanne and Morgan 1992; Engels et al. 1995), but adult eusocial insect workers often play an important behavioral role in nest temperature homeostasis (Kronenberg and Heller 1982; Starks and Gilley 1999; Kleinhenz et al. 2003; Bujok et al. 2004).
We focused on nest thermoregulation in temperate bumblebees (Bombus) whose nests are often exposed to a wide range of temperatures during brood development. Bumblebees are eusocial insects, but new nests are initiated by a lone queen, often in a pre-existing cavity (Mueller et al. 1992; Heinrich 2004). Mature bumblebee colonies house modest worker populations, in many species fewer than 100 workers (Husband 1977; Foster 1992; Heinrich 2004). Bumblebees must maintain the internal nest temperature at approximately 28–32°C for optimum brood growth and development (Pomeroy and Plowright 1980; Vogt 1986a,b). Bumblebee colonies actively control their nest temperature (Heinrich and Heinrich 1983; Vogt 1986b). Behavioral mechanisms of nest temperature regulation in worker bumblebees include brood incubation and wing fanning. Incubation involves transferring heat produced by contractions of the gaster while the bee presses her venter to the brood comb (Heinrich 1974; O’Donnell and Foster 2001; Bujok et al. 2004). Fanning, seen in many winged eusocial insects, is a mechanism by which bumblebees cool the nest via evaporation and convection (Southwick and Moritz 1987; O’Donnell and Foster 2001; Weidenmueller 2004). When fanning, a Bombus worker standing on the brood comb raises her body and fans her wings as in flight, creating an air current in the nest. Bumblebee foragers rarely collect water for nest cooling (Ferry and Corbet 1996), which is frequently done by other eusocial insects with flying workers (Visscher et al. 1996; Kühnholz and Seeley 1997; O’Donnell 1998).
The extent to which workers specialize on tasks and, conversely, the conditions that favor worker task switching are fundamental aspects of worker division of labor (Beshers and Fewell 2001). Social insect workers’ responses to changes in colony needs can involve tradeoffs among competing tasks. For example, a colony’s ability to thermoregulate may come at the expense of other tasks such as foraging for food (Vogt 1986b; O’Donnell and Foster 2001; Foster et al. 2004). The goals of this study were to quantify bumblebee (Bombus huntii) colony responses to short-term changes in ambient temperatures and to assess how adaptive (homeostatic) responses to temperature change are achieved by worker behavioral plasticity.
We performed two experimental manipulations. First, we altered the nests’ ambient temperatures then recorded brood comb temperatures and workers’ behavioral responses. Second, we removed the most actively thermoregulating workers then quantified behavioral responses by the remaining workers. The purpose of the study was twofold. First, to quantify worker specialization on thermoregulation and to test for worker age and morphology effects on thermoregulatory behavior. To determine whether individual workers specialized in thermoregulation, we observed the incubation and wing-fanning behavior of uniquely marked B. huntii workers from three colonies under four temperature conditions. Thermoregulatory specialists were defined as bees that engaged in incubation and/or fanning at higher rates than expected by chance. Second, to assess how the workers’ behavior changes in response to changing colony need for thermoregulation. Workers can respond adaptively to changes in colony need by switching among tasks (van Doorn 1987; Cartar 1992) or by changing their rate of performing tasks (O’Donnell 1998; O’Donnell and Foster 2001). We assessed the relative importance of task switching vs rate change mechanisms in the thermoregulatory response of bumblebee colonies. We challenged the workers by experimentally removing the most active incubators from two B. huntii colonies, and we quantified the immediate and delayed (24 h) response to the resulting increased demand for thermoregulation. A task switching mechanism would predict that workers that had not incubated before removals would be observed incubating after removals, whereas a rate change mechanism would predict that workers that had incubated before removals would continue to do so at an increased rate after removals.
Materials and methods
Queen collection and colony establishment
We collected B. huntii queens near Cle Elum, Washington, USA (47°12′N, 120°58′W) in April and May 1999. These queens had emerged from winter hibernation and were foraging for food or searching for a nesting site. The queens established colonies in nest boxes supplied with cotton nesting material in a laboratory at the University of Puget Sound in Tacoma, WA. The nest boxes were connected via vinyl tubing to separate plywood feeder boxes. The queens were fed 50% by volume sugar water ad libitum in the feeder box and received 1–2 cm3 of packed honeybee pollen placed directly in the nest box daily. We checked the nests daily for adult worker emergence. Approximately 2 weeks after first worker emergence, we transferred three colonies from the nest boxes to glass-covered, 20-cm diameter concrete/perlite observation hives connected to separate feeder boxes as above (Pomeroy and Plowright 1980). During observation trials, the number of workers present was 70 on colony 1, 16 on colony 2, and 27 on colony 3. Colony adult populations stayed constant during the experiments. The subject colonies were in the size range typically attained by B. huntii in captivity over the course of 5 years of research (RLF, unpublished data).
Bee marking, age determination, and morphological measurements
All workers were uniquely marked with numbered plastic bee tags glued to their dorsal thoraces within 4 days after adult emergence. Date of emergence was assigned to the midpoint of each 4-day marking interval, such that the adult age of each bee is known to be within ±2 days. At the end of observations, tagged bees were collected and fixed in 99% ethyl alcohol. We dissected the forewings from the body of each worker, and we mounted the wings flat on microscope slides and covered them with transparent tape. We scanned the slides into computer files (JPEG format) at 600 dpi and measured the images to the nearest 0.1 mm using the ruler tool of Adobe Photoshop software. We measured the length of the stiff anterior (costa) vein of each wing from the base of the vein to the proximal end of the pterostigma. Mean costa vein length correlated highly with bumblebee worker body weight in a previous study (Foster et al. 2004). We measured both wings whenever possible. Left and right wing measurements were highly correlated (Colony 1: n = 64, r = 0.97, p < 0.001; Colony 2: n = 14, r = 0.93, p < 0.001; Colony 3: n = 27, r = 0.96, p < 0.001), so we used the mean of the two wing length measurements as an index of body size for each worker. In cases where one of the wings was damaged (n = 7 workers), we used the measurement of the undamaged wing as the index of body size.
Recording thermoregulatory behavior at different temperatures
We observed incubation and wing fanning by individual workers in four temperature conditions: cold (X = 12.1°C; range 10.3–13.9°C); moderate (X = 21.4°C; range 17.8–23.3°C); warm (X = 27.4°C; range 25.8–28.1°C); and hot (X = 37.7°C; range 37.0–38.6°C). These temperatures fall within the range of ambient temperatures naturally experienced by Bombus colonies in the Puget Sound area in summer (O’Donnell and Foster 2001). Except during observations, the focal colonies were housed at temperatures of 20–24°C. Before each observation trial began, the nest and feeder boxes were exposed to the experimental temperature for 15 min. The 15-min acclimation time was chosen on the basis of preliminary observations, suggesting that this was sufficient to achieve stable temperature conditions in the nest box and to elicit the maximum worker response to the change in colony thermoregulatory need (KEG, personal observation). Each trial lasted 30 min, with three to six trials per colony at each temperature treatment. The order of temperature treatments was randomized within colonies. We performed one or two trials per day, and we allowed at least 1 h and up to 24 h to elapse between trials.
We recorded ambient and nest surface temperatures at the beginning and halfway point of each trial, and we used the mean of these two measurements in all analyses. A digital thermometer was placed in the enclosure, 10–15 cm from the brood comb surface to record ambient air temperature, and a probe thermometer was placed on the brood comb surface to record nest temperature. For trials in the cold condition, we placed the observation hive in a refrigerated cold storage room. For trials in the moderate, warm, and hot conditions, we placed the observation hive in an insulated glass enclosure. The ambient temperature in the warm and hot conditions was adjusted using a ceramic heat lamp modulated by a dimmer switch.
To evaluate the extent to which the adult bees were responsible for regulating the nest surface temperature, we exposed a control nest with a brood comb but with all adult bees removed to ten ambient temperature conditions ranging from 17.8 to 36.5°C (i.e., overlapping with the temperature ranges in the moderate, warm, and hot experimental conditions). We measured brood surface and ambient temperatures using the same procedures as in the observation trials.
We filmed all trials using a Sony High-8 video camera to record queen and worker behavior. A low-heat 60-W pink bulb provided light on the nest during filming. Videotapes were scored continuously with an observer noting the identity of thermoregulating bees (i.e., those performing incubation or wing fanning) and the duration of thermoregulation behavior for each bee to the nearest second. We calculated the mean duration of incubation and wing fanning by each individual bee at each temperature condition by summing her total duration of incubation or fanning during a trial and then averaging across all trials at a given temperature. We also calculated the colony performance of incubation and wing fanning during each trial, expressed as total bee-minutes of activity. We did so by summing the time spent thermoregulating across individuals during each trial.
Worker removal experiments
To test worker responses to changes in social structure, we increased the colony need for thermoregulation by removing those workers whose average incubation rates in the moderate and warm conditions were in the top quartile for all workers on a given colony. We removed 13 of 70 workers (18.9%) from colony 1, and 4 of 27 workers (14.8%) from colony 3. Colony 2 was not included in the removal experiments because males emerged on the nest before the removal manipulations, indicating the onset of the competition phase of colony development (van Doorn 1987). Because incubation was rare in the hot condition, post-removal observations were conducted in cold, moderate, and warm conditions. To quantify the immediate response to the removals, we videotaped one 30-min trial in the moderate condition 30 min immediately after the removals. To quantify the delayed response to the removals, we subjected the colonies to 30 min each of moderate, warm, and cold conditions (in that order) beginning 24 h after the removals. We recorded behavior on videotapes after the same procedures as described above.
Statistical analyses
We analyzed the relationships of colony identity, worker age, and worker body size with incubation rates (averaged across the four temperature conditions) using least-squares multiple regression. To control for differences among colonies, the predictor variables were entered in blocks, first colony, then age, and body size. To test for worker specialization on thermoregulation, we tested the distribution of worker activity rates against a random probability (Poisson) distribution generated using the null expectation of equal rates of performance. We divided the 30-min observation period into 1-min blocks and scored the number of blocks in which each worker incubated. We used each colony’s mean rate (incubation bouts/1-min block) as the expected value (Visscher et al. 1999). We collapsed classes such that all expected values were greater than 1 to satisfy the criteria of a chi-square goodness of fit test (Ott and Longnecker 2001). To test for consistency of worker behavior across temperature conditions, we calculated partial correlations between each individual worker’s average incubation rate (minutes/session) under each condition. The partial correlations were controlled for colony differences. To test for consistency of worker behavior after the removal experiments, we calculated the Pearson correlation between average pre-removal incubation rates and post-removal incubation rates (minutes/session) for each worker. We used paired t-tests to assess the direction of workers changes in rates of incubation in response to nest mate removals. We used nonparametric Spearman rank correlations of individual worker’s pre- and post-removal incubation rates to test for consistency in thermoregulatory behavior, separately for each colony, because of unequal variances.
Results
Nest temperature regulation
In a control nest without adult bees, the brood comb temperature was not significantly different from the ambient temperature (Fig. 1; paired t-test, t = −1.46, n = 10, p = 0.18). The colonies with adult bees present regulated their internal nest temperatures significantly nearer the brood rearing optimal range, relative to ambient, in all temperature conditions except the warm condition, where temperatures approximated the optimal brood rearing range (Fig. 1; repeated measures analysis of variance (ANOVA) comparing nest and ambient temperatures with colony as a covariate; cold treatment: F 1, 8 = 35.8, p < 0.0005; moderate treatment: F 1, 10 = 11.0, p = 0.008; warm treatment: F 1, 9 = 0.88, p = 0.37; hot treatment: F 1, 8 = 8.8, p = 0.018).
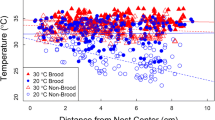
Mean±SE brood comb temperature plotted against ambient temperature for three B. huntii colonies. The shaded regions represent the approximate ideal brood comb temperature range for Bombus immature development (28 to 32°C). The solid line represents temperature unity (nest = ambient). In the plot for Colony 1, the four temperature treatments are labeled and overall significance levels for nest temperature vs ambient are indicated (double asterisk p < 0.01; single asterisk p < 0.05), and the open circles represent temperature data collected from a B. huntii brood comb with all adult bees removed
Workers used wing fanning to cool the nest only in the hot conditions when ambient temperatures exceeded 37°C (Fig. 2). Some workers removed themselves from the brood as they fanned. Queens never fanned but stood off and to the side of the brood, a behavior that was performed by all queens and only in the hot condition. The average rate of incubation per worker decreased as temperature increased (Fig. 2; ANOVA, overall R 2 = 0.80, effect of temperature treatment after accounting for colony effects F 5, 30 = 100.0, p < 0.0001). Because incubation was rarely performed in the hot treatment, we tested the relationship between incubation rate and ambient temperature for the lowest three temperature treatments. Incubation rates declined significantly with temperature across the three lowest temperature treatments (F 5, 21 = 18.4, p < 0.0005). The number of workers incubating decreased with temperature across treatments (Fig. 3; ANOVA, overall R 2 = 0.48, effect of temperature treatment after accounting for colony effects F 3, 32 = 9.65, p < 0.0001). Post hoc comparisons (Tukey honestly significant difference test, critical alpha = 0.05) showed that significantly fewer workers incubated in the hot treatment relative to all other treatments; the number of workers incubating did not differ among the other three treatments.
Relationship of worker thermoregulatory activity (solid symbols incubation; open symbols fanning) with ambient temperature for three B. huntii colonies. Values plotted are means (±SE) across trials for each colony of workers’ average duration (seconds) of fanning and incubating during 30-min observation sessions. Temperature values are mean±SE for each treatment. In the plot for Colony 1, the four temperature treatments are labeled
Relationship of worker incubation activity with ambient temperature for three B. huntii colonies. Values plotted are means (±SE) across trials for each colony of the number of worker observed incubating during 30-min observation sessions. Temperature values are mean±SE for each treatment. In the plot for Colony 1, the four temperature treatments are labeled
Worker specialization
Summed across all temperature conditions, the number of worker bees that were observed incubating was Colony 1: 49 (70% of all workers); Colony 2: 16 (100%); and Colony 3: 24 (88%). In colonies 1 and 3, the observed distribution of incubation rates was significantly different from the random Poisson distribution in cold, moderate, and warm conditions, indicating that some bees incubated at higher and lower rates than expected (Table 1). The results for colony 2 are not as clear, with a statistically significant deviation from random only in the moderate condition. Using partial correlations to control for colony differences, the average incubation rates of individual bees in the cold, moderate, warm, and hot conditions were significantly correlated for all bivariate associations (Table 2). Worker body size was inversely associated with incubation rate (multiple regression, after accounting for colony effects: β = −0.245, t = −2.69, p < 0.01), but worker age was not associated with incubation rate (β = 0.06, t = 0.62, p = 0.54).
Effects of worker removals
Both colonies were affected by the removal of the most active incubators and later compensated for their loss (Fig. 4). Immediately after removals, the bees’ minutes of incubation decreased in both colonies (paired t-test, Colony 1: t 57 = −2.26; p = 0.028; Colony 3: t 23 = −3.83, p < 0.001). Workers significantly increased their incubation rates from the immediate to the 24-h post-removal sessions in both colonies (paired t-test, Colony 1: t 57 = 4.73, p < 0.001; Colony 3: t 23 = 4.31, p < 0.001). At 24 h post removal, the bees’ rate of incubation (averaged over cold, moderate, and warm conditions) rebounded to significantly higher levels than pre-treatment in both colonies (paired t-test, Colony 1: t 57 = 3.22, p = 0.002; Colony 3: t 23 = 2.83, p = 0.01). The workers increased their rates of incubation in both colonies, from X = 1.15 to 2.02 min/30 min (176% increase) in colony 1 and from X = 4.13 to 6.34 min/30 min (153% increase) in colony 3. Workers’ incubation rates before removals were positively correlated with their incubation rates after recovery 24 h post removal (Fig. 5; Spearman correlations, Colony 1: n = 57, r = 0.39, p = 0.003; Colony 3: n = 23, r = 0.72, p < 0.001).
Discussion
Evidence for an adaptive colony-level thermoregulatory response
Experimentally increasing the deviation of ambient temperature from the optimum brood nest temperature range increased the demand for thermoregulation. The B. huntii colonies showed homeostatic responses to changes in ambient temperature. Brood combs with workers present were maintained closer to ideal brood rearing temperatures than an unoccupied brood comb. Furthermore, the workers both cooled the nest surface at elevated temperatures and warmed it at lower temperatures.
Although the bees in our study thermoregulated their nests, they did not always achieve optimal brood comb temperatures. Bumblebees live in a wide range of climates, including the arctic, where they display prolonged hibernation and increased body pubescence to reduce heat loss as adaptations to the cold temperatures (Richards 1973; Soemme 1989; Danks 2004). Bumblebees may also compensate for lower temperatures by choosing a sheltered nest site, often underground (Richards 1978; Foster 1992), and by altering their nest architecture, for example, by constructing a wax canopy that envelopes the brood (Vogt 1986b; Heinrich 2004). These thermoregulatory mechanisms were not available to our laboratory colonies. In a study of B. impatiens and B. affinus, Vogt (1986b) found that insulated laboratory nests housed at 15°C produced as many offspring as did nests housed at ideal brood rearing temperatures, whereas uninsulated nests housed at 15°C produced fewer offspring. Colonies in nature may require a combination of thermoregulatory behavior with other mechanisms to maintain an ideal temperature under severe ambient conditions.
Behavioral responses to changing temperatures
Workers and queens modified their behavior in response to changing ambient temperatures. Colony-wide performance of incubation increased as ambient temperatures dropped. As the temperature increased to above approximately 30°C, incubation nearly ceased and wing fanning was performed. Queens never fanned, but their response to elevated temperatures of standing off and to the side of the brood may contribute to nest cooling by reducing the amount of convective heat flow from the queen’s body to the brood. Workers also removed themselves from the brood, but this behavior was usually coupled with wing fanning.
Evidence for thermoregulatory specialization
Bumblebee workers are often characterized as plastic in their task performance (O’Donnell and Foster 2001; Heinrich 2004). However, in an earlier correlational study, we documented differences in bumblebee workers’ thresholds of response to temperature changes (Foster et al. 2004). The experiments in this study provide additional evidence for worker thermoregulatory specialization. Although overall incubation activity corresponded to temperature, individuals were highly variable in their performance of this task. Some workers incubated even when ambient temperature was at or above the optimal brood rearing range, suggesting strong individual differences in sensitivity to temperature. As the temperature decreased, the total amount of worker effort in incubation increased, but the number of workers that incubated did not change (but see Vogt (1986a) on B. impatiens). This result suggests that incubating workers did not switch from other tasks (or idleness). Rather, a subgroup of bees in the colony adjusted the proportion of time spent incubating in response to changes in colony need. Workers’ incubation rates were correlated across temperatures, providing further evidence for a subgroup of thermoregulatory specialists. This represents a potential cost to the colony because incubating bees are stationary and therefore unable to perform other tasks (Vogt 1986a). The bees in our experiments had to leave the nest box to collect sugar water from the attached feeding box. Under natural or free-flying conditions, the workers would experience stronger constraints on their time and energy allocation to different tasks. We predict in particular that individuals would face a strong tradeoff between in-nest tasks, such as thermoregulation and food foraging (Foster et al. 2004).
Some workers incubated at significantly higher rates than their nest mates, and some workers were never observed to incubate. In addition, a high incubation rate by a worker at one temperature predicted a high incubation rate at other temperatures. These findings provide further evidence for a subgroup of thermoregulatory specialists. Worker age was not a significant predictor of B. huntii incubation rates, but worker body size appears to play a role in setting workers’ thermoregulatory propensities. Smaller workers incubated at higher rates. Body size effects on bumblebee worker polyethism are pervasive (O’Donnell and Foster 2001; Foster et al. 2004). Previous studies have shown that larger bumblebee workers are more likely to forage (Free 1955; Cameron 1989; Goulson et al. 2002; Spaethe and Weidenmüller 2002), suggesting a tradeoff with thermoregulatory behavior. Alternatively, because of their large surface area to volume ratio compared to larger bees, smaller bees may be able to lose heat via convection more readily, making them more efficient incubators.
Effects of worker removals
Removal of part of the worker force, a manipulation known as “sociotomy,” is a powerful tool for inducing and quantifying worker responses to changing colony conditions (Kolmes and Winston 1988; Backen et al. 2000; Breed et al. 2002; Donahoe et al. 2003). In our study, removal of the most active incubators increased the level of need within the colony for incubation. At the colony level, the net effect was a decrease in total incubation effort immediately after removals with an increase to approximately pre-removal baseline levels 24 h after removals. When the top incubators were removed, the remaining workers significantly increased their rates. The response was not uniform among workers however, and incubation activity level before removals predicted incubation activity after removals. Bombus huntii workers exhibited specialization on thermoregulatory tasks in the face of changes in abiotic and social conditions.
Our data suggest that a heavy investment of worker labor into brood thermoregulation is an important adaptive element of bumblebee colony organization. We propose that behavioral brood thermoregulation provides a flexible mechanism for maintaining a nest temperature ideal for brood development, and that it, along with body thermoregulation during foraging (Roberts and Harrison 1998; Bishop and Armbruster 1999), has promoted the global ecological success of bumblebees at high elevations and high latitudes (Cameron and Williams 2003; Kawakita et al. 2004).
References
Backen SJ, Sendova-Franks AB, Franks NR (2000) Testing the limits of social resilience in ant colonies. Behav Ecol Sociobiol 48:125–131
Barrow DA, Pickard RS (1985) Larval temperature in brood clumps of Bombus pascuorum. J Apic Res 24:69–75
Beshers SN, Fewell JH (2001) Models of the division of labor in social insects. Annu Rev Entomol 46:413–440
Bishop JA, Armbruster WS (1999) Thermoregulatory abilities of Alaskan bees: effects of size, phylogeny and ecology. Funct Ecol 13:711–724
Breed MD, Williams DB, Queral A (2002) Demand for task performance and workforce replacement: undertakers in honeybee, Apis mellifera, colonies. J Insect Behav 15:319–329
Bujok B, Kleinhenz M, Fuchs S, Tautz J (2004) Hot spots in the bee hive. Naturwissenschaften 89:299–301
Cameron SA (1989) Temporal patterns of division of labor among workers in the primitively eusocial bumble bee Bombus griseocollis (Hymenoptera: Apidae). Ethology 80:137–151
Cameron SA, Williams PH (2003) Phylogeny of bumble bees in the New World subgenus Fervidobombus (Hymenoptera: Apidae): congruence of molecular and morphological data. Mol Phylogenet Evol 28:552–563
Cartar RV (1992) Adjustment of foraging efforts and task switching in energy manipulated wild bumblebee colonies. Anim Behav 44:75–87
Danks HV (2004) Seasonal adaptations in Arctic insects. Integr Comp Biol 44:85–94
Donahoe K, Lewis LA, Schneider SS (2003) The role of the vibration signal in the house-hunting process of honey bee (Apis mellifera) swarms. Behav Ecol Sociobiol 54:593–600
Engels W, Rosenkranz P, Engels E (1995) Thermoregulation in the nest of the neotropical stingless bee Scaptotrigona postica and a hypothesis on the evolution of temperature homeostasis in highly eusocial bees. Stud Neotrop Fauna Environ 30:193–205
Ferry C, Corbet SA (1996) Water collection by bumble bees. J Apic Res 35:120–122
Foster RL (1992) Intraspecific recognition functions in bumble bees. Ph.D. Dissertation, University of Washington
Foster RL, Brunskill A, Verdirame D, O’Donnell S (2004) Reproductive physiology, dominance interactions, and division of labor among bumble bee workers. Physiol Entomol 29:327–334
Free JB (1955) The division of labor within bumblebee colonies. Insectes Soc 2:195–212
Goulson D, Peat J, Stout, JC, Tucker J, Darvill B, Derwent LC, Hughes WOH (2002) Can alloethism in workers of the bumblebee, Bombus terrestris be explained in terms of foraging efficiency? Anim Behav 64:123–130
Heinrich B (1974) Thermoregulation in endothermic insects. Science 135:747–756
Heinrich B (2004) Bumblebee economics. Harvard University Press
Heinrich B, Heinrich MJE (1983) Size and caste in temperature regulation by bumblebees. Physiol Zool 56:552–562
Husband RW (1977) Observations on colony size in bumble bees (Bombus spp.). Great Lakes Entomol 10:83–85
Jeanne RL, Morgan RC (1992) The influence of temperature on nest site choice and reproductive strategies in Polistes wasps. Ecol Entomol 17:135–141
Jones JC, Helliwell P, Beekman M, Maleszka R, Oldroyd BP (2005) The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J Comp Physiol A 191:1121–1129
Kawakita A, Sota T, Ito M, Ascher JS, Tanaka H, Kato M, Roubik DW (2004) Phylogeny, historical biogeography, and character evolution in bumble bees (Bombus: Apidae) based on simultaneous analysis of three nuclear gene sequences. Mol Phylogenet Evol 31:799–804
Kleinhenz M, Bujok B, Fuchs S, Tautz J (2003) Hot bees in empty broodnest cells: heating from within. J Exp Biol 206:4217–4231
Klingner R, Richter K, Schmolz E, Keller B (2005) The role of moisture in the nest thermoregulation of social wasps. Naturwissenschaften 92:427–430
Kolmes SA, Winston ML (1988) Division of labor among worker honey bees in demographically manipulated colonies. Insectes Soc 35:262–270
Kronenberg F, Heller HC (1982) Colonial thermoregulation in honey bees (Apis mellifera). J Comp Physiol B 148:65–76
Kühnholz S, Seeley TD (1997) The control of water collection in honey bee colonies. Behav Ecol Sociobiol 41:407–422
McMullan JB, Brown MJF (2005) Brood pupation temperature affects the susceptibility of honeybees (Apis mellifera) to infestation by tracheal mites (Acarapis woodi). Apidologie 36:97–105
Mueller CB, Shykoff JA, Sutcliffe GH (1992) Life history patterns and opportunities for queen-worker conflict in bumblebees (Hymenoptera: Apidae). Oikos 65:242–248
O’Donnell S (1998) Effects of experimental forager removals on division of labor in the primitively eusocial wasp Polistes instabilis (Hymenoptera: Vespidae). Behavior 135:173–193
O’Donnell S, Foster RL (2001) Thresholds of response in nest thermoregulation by worker bumble bees, Bombus bifarius nearcticus (Hymenoptera: Apidae). Ethology 107:387–399
Ott RL, Longnecker M (2001) Statistical methods and data analysis. Duxbury, Pacific Grove, CA
Pomeroy N, Plowright RC (1980) Maintenance of bumblebee colonies in observation hives (Hymenoptera: Apidae). Can Entomol 112:321–326
Richards KW (1973) Biology of Bombus polaris Curtis and B. hyperboreus Schönherr at Lake Hazen, Northwest Territories (Hymenoptera: Bombini). Quaest Entomol 9:115–157
Richards KW (1978) Nest site selection by bumble bees (Hymenoptera: Apidae) in southern Alberta. Can Entomol 110:301–318
Roberts SP, Harrison JF (1998) Mechanisms of thermoregulation in flying bees. Am Zool 38:492–502
Seeley TD, Heinrich B (1981) Regulation of temperature in the nests of social insects. In: Heinrich B (ed) Insect thermoregulation. Wiley, New York, p 159–234
Soemme L (1989) Adaptations in insects and other terrestrial arthropods to the alpine environment. Fauna Norv B:1–10
Southwick EE, Moritz RFA (1987) Social control of air ventilation in colonies of honey bees (Apis mellifera). J Insect Physiol 33:623–626
Spaethe J, Weidenmüller A (2002) Size variation and foraging rate in bumblebees (Bombus terrestris). Insectes Soc 49:142–146
Stabentheiner A, Pressl H, Papst T, Hrassnigg N, Crailsheim K (2003) Endothermic heat production in honeybee winter clusters. J Exp Biol 206:353–358
Starks PT, Gilley DC (1999) Heat shielding: a novel method of colonial thermoregulation in honey bees. Naturwissenschaften 86:438–440
Tautz J, Maier S, Groh C, Rossler W, Brockmann A (2003) Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc Natl Acad Sci USA 100:7343–7347
van Doorn A (1987) Investigation into the regulation of dominance behavior and of the division of labour in bumblebee colonies (Bombus terrestris). Neth J Zool 37:255–276
Visscher PK, Crailsheim K, Sherman G (1996) How do honey bees (Apis mellifera) fuel their water foraging flights? J Insect Physiol 42:1089–1094
Visscher PK, Shepardson J, McCart L, Camazine S (1999) Vibration signal modulates the behavior of house-hunting honey bees (Apis mellifera). Ethology 105:759–769
Vogt FD (1986a) Thermoregulation in bumblebee colonies. I. Thermoregulatory versus brood-maintenance behaviors during acute changes in ambient temperatures. Physiol Zool 59:55–59
Vogt FD (1986b) Thermoregulation in bumblebee colonies. II. Demographic variation throughout the colony cycle. Physiol Zool 59:60–68
Weidenmueller A (2004) The control of nest climate in bumblebee (Bombus terrestris) colonies: interindividual variability and self reinforcement in fanning response. Behav Ecol 15:120–128
Weidenmueller A, Kleineidam C, Tautz J (2002) Collective control of nest climate parameters in bumblebee colonies. Anim Behav 63:1065–1071
Yoon HJ, Kim SE, Kim YS (2002) Temperature and humidity favorable for colony development of the indoor-reared bumblebee, Bombus ignitus. Appl Entomol Zool 37:419–423
Acknowledgment
Financial support was provided by NSF (IBN-9904885 and IBN-0347315 to S.O’D., and ROA-0119690 to R.L.F. and S.O’D.), the UEC-University of Puget Sound (to R.L.F); and Phi Sigma and the Murdock Charitable Trust (to K.E.G.). Special thanks to Tom Seeley for the assistance and advice on statistical analyses and thanks to Terry Mace, Tom Seeley, and two anonymous reviewers for the helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.F.A. Moritz
Rights and permissions
About this article
Cite this article
Gardner, K.E., Foster, R.L. & O’Donnell, S. Experimental analysis of worker division of labor in bumblebee nest thermoregulation (Bombus huntii, Hymenoptera: Apidae). Behav Ecol Sociobiol 61, 783–792 (2007). https://doi.org/10.1007/s00265-006-0309-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-006-0309-7