Abstract
Female birds deposit in the yolks of eggs substantial amounts of androgens, such as testosterone and androstenedione. These androgens have been shown to speed up nestling development, induce a fast development of ornaments and increase dominance in adults. Experiments in several species have reported that females invest greater amounts of androgens in the eggs fathered by attractive males, suggesting that yolk androgen is a costly investment for either the offspring or the mother. There is some evidence that nestling immunocompetence may be partially suppressed by high levels of yolk androgens, but it is not known whether this is also the case for females. We tested this hypothesis in the house martin by inducing an immune challenge through an injection of sheep red blood cells, a standard challenge of the humoral immune system. Experimental birds laid eggs with lower amounts of yolk androstenedione than controls, and there was a similar non-significant trend for testosterone. Furthermore, the probability of laying a replacement clutch was higher for birds that had laid a first clutch with relatively high levels of yolk testosterone. These results suggest that yolk androgen deposition is limited by immune costs in the female, and that only females in good condition may afford to invest high levels of androgen in eggs in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maternal effects are receiving increasing attention as powerful mechanisms that parents can use to promote adaptation to complex and changing environments (Mousseau and Fox 1998). Since the discovery of egg androgens in avian eggs (Schwabl 1993), a growing body of research has found that minute differences in the amount of androgens that a bird receives in the yolk can have important effects on its future development. It is important to note that these effects start right at the beginning of development, and high levels of egg androgens have been found to result in short incubation periods and improved embryo growth (Schwabl 1996b; Lipar and Ketterson 2000; Eising et al. 2001). This leads to a cascade of effects that include increased begging rates, fast development of sexual ornaments and higher social dominance (Schwabl 1993; Eising and Groothuis 2003; Strasser and Schwabl 2004).
Despite these obvious benefits, not all females lay eggs with high levels of androgens (see for instance, Pilz et al. 2003). Furthermore, experimental work in three different bird species shows differential allocation of egg androgen to the eggs of attractive males (Gil et al. 1999; Gil et al. 2004; Tanvez et al. 2004; Gil et al. 2006). This pattern suggests that high levels of yolk androgens must carry some type of costs, either for the offspring or the female (Gil 2003). Recently, several studies have indeed found that nestlings hatching from testosterone injected eggs have a reduced cellular immune response (Groothuis et al. 2005; Müller et al. 2005; Navara et al. 2005). However, no study so far has addressed the possibility that the cost may be paid by the female that lays those eggs. This is highly probable because high androgen titres can be immunosuppressive for females (Duffy et al. 2000). In the present study, we tested this hypothesis in the house martin Delichon urbica, by challenging experimental females with an injection of sheep red blood cells (SRBC) and comparing the levels of androgens laid in the eggs of these females with those laid by control birds. SRBC injection is a standard test developed to challenge the humoral immune system, causing a peak in specific antibodies 10–15 days post-injection (Roitt et al. 1996).
Materials and methods
The study was done in a large colony of house martins in the University of Extremadura (Badajoz) during February–May 2001. The experiment was designed to use each female as its own control, comparing androgen levels between first and replacement clutches, depending on whether females had been injected with SRBC or saline in the interim. Nests were visited every 2 days from early February and new eggs found were marked daily with an indelible marker. The females were captured in their nests at dawn when they had been incubating their first clutch for 4 days (mean=3.77, SD=1.13) and they were injected subcutaneously with either 0.1 ml of a 5% solution of SRBC or the same volume of phosphate buffered saline (N=16 control and 19 experimental broods). The females were haphazardly allocated to experimental groups and did not differ in biometry or age (caught unringed vs ringed in previous years). At the same time of the injection, we collected the eggs from the first clutch (mean=4.11 eggs, SD=1.55). We subsequently followed these nests and collected the replacement broods (mean=4.15 eggs, SD=0.94) after a similar period of incubation (mean=3.96, SD=0.71). Average time elapsing between removal of the first and replacement clutches was 22.4 days (SD=6.4). We managed to obtain eggs from replacement clutches from a total of 16 females out of the 38 that had been originally injected.
The eggs were frozen at −20°C until dissection. We dissected the yolk from the embryo while the egg was still frozen to allow a good separation. The yolk was weighed and the steroids were extracted by adding 3 ml of diethyl ether to the sample, vortexing, centrifuging and decanting the ether phase after separation by snap-freezing in a bath of ethanol at −20°C. The ether phase was evaporated under a stream of nitrogen and dissolved in a fixed volume of PBS. We used the commercial I125 RIA kits for assaying the samples (DSL Labs, USA). These kits have highly specific (100%) antibodies for their target hormone and low-cross reactivity with other hormones. Reported cross-reactivity of the androstenedione kit was less than 1% for all hormones tested, and less than 5% in the case of the testosterone kit (except 5a-DHT which was 5.8%). The intra-assay coefficient of variation was 5.4 for testosterone and 6.8 for androstenedione. The inter-assay coefficient of variation was 13.3 for testosterone and 8.85 for androstenedione.
Sex was determined by amplification of a sex-specific bird product following the procedures reported by Griffiths et al. (1998). DNA was extracted using Chelex 100 resin (Bio-Rad Laboratories, CA). The sex identification test employs the P8 and P2 primers (Griffiths et al. 1998) and polymerase chain reaction (PCR) amplification was carried out in a total volume of 25 μl. The final reaction conditions were as follows: 20 μl ddH20 (Sigma, St Louis, MO); 2.5 μl of Taq DNA polymerase 10× buffer (Promega); 50 mM of each dNTP; 100 ng of each primer; and 0.5 U of Taq polymerase (Promega). Between 50 and 250 ng of genomic DNA was used as template. A layer of 2 μl of mineral oil was added to avoid evaporation. PCR was performed in an Intelligent Heating Block (Cherlyn Electronics). An initial denaturing step at 94°C for 5 min, 55°C for 2 min and 72°C for 5 min was followed by 35 cycles of 94°C for 1 min, 55°C for 2 min and 72°C for 5 min. A final run of 72°C for 7 min completed the program. PCR products were separated by electrophoresis for 105 min at 50 V in a 3% agarose gel stained with ethidium bromide.
Results
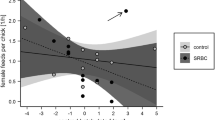
We ran some exploratory models to assess uncontrolled sources of variance in egg androgen concentration, declaring brood a random factor, and including embryo sex and laying order, as well as its interaction as fixed factors. Neither sex nor its interaction with laying order were significant: male and female eggs contained similar amounts of androgens (log T F 1,64.9=0.54, P=0.46; log A4 F 1,65.1=1.49, P=0.22; Fig. 1). Both androgens increased with increasing laying order [log T F 1,63.3=11.7, P<0.001, estimate (SE)=0.11 (0.03); log A4 F 1,63.4=7.26, P<0.01, estimate (SE)=0.06 (0.02)]. Laying order is confounded by differential incubation time because females typically start incubating before the last egg is laid, and it has been shown that androgen concentration decreases with incubation time, probably because of yolk and albumen mixing (Elf and Fivizzani 2002; Pilz et al. 2005). Therefore, in the following analyses, we used female average log yolk androgen concentrations estimated as residuals from the regressions on laying order as a way of controlling for differential incubation time. Results do not change if we use uncorrected data instead.
Females injected with SRBC were as likely as control females to lay a replacement clutch (PBS 31% vs SRBC 57%; χ 2=2.52, df=1, P=0.11). A stepwise logistic regression using the two androgen concentrations as predictors of the likelihood of laying a replacement clutch showed that females that laid replacement clutches had significantly higher levels of yolk T in their first clutch than females that did not lay a replacement clutch [χ 2=6.38, df=1, P<0.05; mean uncorrected concentrations (SE) 5.1 (0.5) pg/mg vs 3.4 (0.4) pg/mg]. Yolk androstenedione was not retained in the final model. This result was not confounded by female age because first clutch androgen concentration was not different between young and older females (testosterone F 1,33=1.18, P=0.28; androstenedione F 1,33=0.07, P=0.79).
We calculated repeatabilities between first and replacement clutches for control females. There was a strong yolk T concentration repeatability (r p=0.93, n=5, P<0.05) and a moderate repeatability for yolk A4 (r p=0.52, n=5, P=0.36).
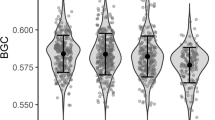
Our main prediction was that the concentration of yolk androgen in replacement broods would be lower for females injected with SRBC than for control females. We ran GLMs for yolk T and A4 with treatment as a main factor. In the case of yolk T, we added first clutch yolk T concentration as a covariate to correct the differences in relaying probability. We found that SRBC-injected females laid eggs with lower levels of yolk A4 than control females (F 1,12=6.57, P<0.05). Although the result was in the same direction, there were no statistically significant differences in yolk T levels (F 1,12=2.55, P=0.13; Fig. 2).
Discussion
We found that females that had been challenged with a foreign antigen (SRBC) laid eggs that had a lower androstenedione content than control females. A similar pattern, although not statistically significant, was found for yolk testosterone. Because SRBC injection induces a costly immune response than sequesters resources from other body functions, increases metabolic rates and reduces body condition (Ots et al. 2001), our results suggest that egg androgen allocation is costly and that it constitutes a condition-dependent investment. Furthermore, in agreement with this conclusion, we found that the probability of laying a replacement clutch was positively directed with testosterone levels in the first brood, as expected, if yolk androgen deposition was a condition-dependent trait.
Yolk androgens are passed from the female bird to the egg (Schwabl 1993) and modulate the development of the embryo (Lipar and Ketterson 2000), having long-lasting consequences in behaviour at the nestling and adult stages. Nestlings hatching from eggs experimentally injected with high androgen levels beg more intensively and have shorter incubation periods (Schwabl 1996b; Eising and Groothuis 2003), although the beneficial effects at this stage may be more relevant in situations of strong competition for food (Pilz et al. 2004). In adulthood, these birds are more likely to be dominant in flocks (Schwabl 1993), and develop larger ornaments than control birds (Strasser and Schwabl 2004).
In agreement with life history theory (Burley 1988), females have been shown to invest higher levels of egg androgens when mated to attractive males (Gil et al. 1999; 2004; Tanvez et al. 2004; Gil et al. 2006). This pattern of differential allocation suggests that females accrue indirect benefits from these offspring (Sheldon 2000), but also that a high yolk androgen investment is somehow costly (Gil 2003). At least three possibilities can be identified:
-
1.
High levels of yolk androgens can be costly to the offspring, for instance impairing their immune system. Evidence towards this point has recently been identified by reduced T-cell immune response in androgen-treated eggs (Groothuis et al. 2005; Müller et al. 2005; Navara et al. 2005).
-
2.
Female and male offspring may have different yolk androgen optima in some species, thus limiting the scope of optimal sex ratio determination (Saino et al. 2006; von Engelhardt et al. 2006).
-
3.
High androgen yolk allocation may be costly for the mother. Our study gives support to this third source of costs.
A puzzling result is that although androstenedione decreased with SRBC injection, testosterone was the hormone that predicted the probability of laying a replacement clutch. It is difficult to know to what extent the effects of these hormones are interchangeable because specific research on this area is still wanting (Groothuis and von Engelhardt 2005). Androstenedione can be converted to T by 17β-hydroxysteroid dehydrogenase (Horton and Tate 1966), an enzyme that is present and functional in the developing avian embryo (Bruggeman et al. 2002). However, A4 can also be converted to other hormones (estrogen or 5α-dihydrotestosterone) if the necessary enzymes are present. Because we do not have information on the differential availability of these enzymes on developing embryos, we cannot rule out any possibility. Although the most parsimonious assumption at the present time is that the effects of yolk A4 and T are similar, the patterns that we found in this study would suggest that there are major differences between them that deserve specific research.
We failed to find any significant effect of age in androgen levels, as found before in the European starling Sturnus vulgaris (Pilz et al. 2003). However, because not all birds are caught every year at the colony, it is likely that our measurement of age (current year vs older) is too unreliable to provide an appropriate test of this prediction.
Androgen levels in females increase around egg laying (Schwabl et al. 2005), as a consequence of the high androgen production that takes place in the growing follicles (Staub and De Beer 1997; Johnson 1999). An experimental study in the canary Serinus canaria has shown that plasma testosterone levels in the female are a reflection of yolk androgen production (Schwabl 1996a), suggesting that females that lay eggs with high levels of testosterone are also exposed to high testosterone levels in plasma. In agreement with this point, comparative evidence shows that females in colonial species lay eggs with higher amount of androgens (Gil et al. unpublished manuscript) and also have higher levels of circulating testosterone (Møller et al. 2005). Because high androgen levels have been shown to reduce immune responsiveness in males as well as females (Folstad and Karter 1992; Verhulst et al. 1999; Duffy et al. 2000), our results suggest that the immune response against SRBC injection interfered with androgen production in our experimental birds. This could have resulted in the reduced amount of androgens that we found in the yolks laid by these females.
However, evidence from other studies suggest that there is a complex relationship between costs, benefits and yolk androgen investment. Yolk androgen injections can reduce growth and increase nestling mortality in some species (Sockman and Schwabl 2000; Navara et al. 2005) or simply shift the covariance between several morphological traits (Rubolini et al. 2006), suggesting that the benefits of high yolk androgen levels may depend on the species, or in the phenotypic or genotypic quality of the offspring. In line with this idea, recent experiments have shown that the two sexes can have different optima for yolk androgen levels (Saino et al. 2006; von Engelhardt et al. 2006). Also, a food supplementation study has shown that food-supplemented females do not increase yolk androgen levels as it would be expected if this allocation would be limited by resources (Verboven et al. 2003). Taken together, all this evidence suggest that yolk androgens have a complex matrix of costs and benefits, and that the simple rule of “the more the better” cannot be applied to this particular maternal effect. In other words, it is not correct to consider yolk androgens as another egg resource, but rather as a phenotypic modifier subject to costs and benefits that shows complex transgenerational effects.
References
Bruggeman V, Van As P, Decuypere E (2002) Developmental endocrinology of the reproductive axis in the chicken embryo. Comp Biochem Physiol A: Physiol 131:839–846
Burley N (1988) The differential–allocation hypothesis: an experimental test. Am Nat 132:611–628
Duffy DL, Bentley GE, Drazen DL, Ball GF (2000) Effects of testosterone on cell mediated and humoral immunity in non-breeding adult European starlings. Behav Ecol 11:654–662
Eising CM, Groothuis TGG (2003) Yolk androgens and begging behaviour in black-headed gull chicks: an experimental field study. Anim Behav 66:1027–1034
Eising CM, Eikenaar C, Schwabl H, Groothuis TGG (2001) Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc R Soc Lond B Biol Sci 268:839–846
Elf PK, Fivizzani AJ (2002) Changes in sex steroid levels in yolks of the Leghorn chicken, Gallus domesticus, during embryonic development. J Exp Zool 293:594–600
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
Gil D (2003) Golden eggs: maternal manipulation of offspring phenotype by egg androgen in birds. Ardeola 50:281–294
Gil D, Graves JA, Hazon N, Wells A (1999) Male attractiveness and differential testosterone investment in zebra finch eggs. Science 286:126–128
Gil D, Leboucher G, Lacroix A, Cue R, Kreutzer M (2004) Female canaries produce eggs with greater amounts of testosterone when exposed to preferred male song. Horm Behav 45:64–70
Gil D, Ninni P, Lacroix A, De Lope F, Tirard C, Marzal A, Møller AP (2006) Yolk androgens in the barn swallow (Hirundo rustica): a test of some adaptive hypotheses. J Evol Biol 19:159–169
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Groothuis TGG, Eising CM, Dijkstra C, Müller W (2005) Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol Lett 1:78–81
Groothuis TGG, von Engelhardt N (2005) Investigating maternal hormones in avian eggs: measurement, manipulation and interpretation. Ann NY Acad Sci 1046:168–180
Horton R, Tate JF (1966) Androstenedione production and interconversion rates measured in peripheral blood and studies on the possible site of conversion to testosterone. J Clin Invest 45:301–313
Johnson AL (1999) Reproduction in the female. In: Whittow GC (ed) Avian physiology. Academic, New York, pp 569–596
Lipar JL, Ketterson ED (2000) Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Agelaius phoeniceus. Proc R Soc Lond B Biol Sci 267:2005–2010
Møller AP, Garamszegi LZ, Gil D, Hurtrez-Bousses S, Eens M (2005) Correlated evolution of male and female testosterone profiles in birds and its consequences. Behav Ecol Sociobiol 58:534–544
Mousseau TA, Fox CW (1998) Maternal effects as adaptations. Oxford University Press, New York
Müller W, Groothuis TGG, Kasprzik A, Dijkstra C, Alatalo RV, Siitari H (2005) Prenatal androgen exposure modulates cellular and humoral immune function of black-headed gull chicks. Proc R Soc Lond B Biol Sci 272:1971–1977
Navara KJ, Hill GE, Mendonca MT (2005) Variable effects of yolk androgens on growth, survival, and immunity in eastern bluebird nestlings. Physiol Biochem Zool 78:570–578
Ots I, Kerimov AB, Ivankina EV, Ilyina TA, Horak P (2001) Immune challenge affects basal metabolic activity in wintering great tits. Proc R Soc Lond B Biol Sci 268:1175–1181
Pilz KM, Smith HG, Sandell MI, Schwabl H (2003) Interfemale variation in egg yolk androgen allocation in the European starling: do high-quality females invest more? Anim Behav 65:841–850
Pilz KM, Quiroga M, Schwabl H, Adkins-Regan E (2004) European starling chicks benefit from high yolk testosterone levels during a drought year. Horm Behav 46:179–192
Pilz KM, Adkins-Regan E, Schwabl H (2005) No sex difference in yolk steroid concentrations of avian eggs at laying. Biol Lett 1:318–321
Roitt I, Brostoff J, Male D (1996) Immunology. Mosby, London
Rubolini D, Romano M, Martinelli R, Leoni B, Saino N (2006) Effects of prenatal yolk androgens on armaments and ornaments of the ring-necked pheasant. Behav Ecol Sociobiol 59:549–560
Saino N, Ferrari RP, Romano M, Martinelli R, Lacroix A, Gil D, Møller AP (2006) Maternal allocation of androgens and antagonistic effects of yolk androgens on sons and daughters. Behav Ecol 17:172–181
Schwabl H (1993) Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci U S Am 90:11446–11450
Schwabl H (1996a) Environment modifies the testosterone levels of a female bird and its eggs. J Exp Zool 276:157–163
Schwabl H (1996b) Maternal testosterone in the avian egg enhances postnatal growth. Comp Biochem Physiol 114A:271–276
Schwabl H, Flinks H, Gwinner E (2005) Testosterone, reproductive stage, and territorial behavior of male and female European stonechats Saxicola torquata. Horm Behav 47:503–512
Sheldon BC (2000) Differential allocation: tests, mechanisms and implications. Trends Ecol Evol 15:397–402
Sockman KW, Schwabl H (2000) Yolk androgens reduce offspring survival. Proc R Soc Lond B 267:1451–1456
Staub NL, De Beer M (1997) The role of androgens in female vertebrates. Gen Comp Endocrinol 108:1–24
Strasser R, Schwabl H (2004) Yolk testosterone organises behavior and male plumage coloration in house sparrows (Passer domesticus). Behav Ecol Sociobiol 56:491–497
Tanvez A, Béguin N, Chastel O, Lacroix A, Leboucher G (2004) Sexually attractive phrases increase yolk androgen deposition in canaries (Serinus canaria). Gen Comp Endocrinol 138:113–120
Verboven N, Monaghan P, Evans DM, Schwabl H, Evans N, Whitelaw C, Nager RG (2003) Maternal condition, yolk androgens and offspring performance: a supplemental feeding experiment in the lesser black-backed gull (Larus fuscus). Proc R Soc Lond B Biol Sci 270:2223–2232
Verhulst S, Dieleman SJ, Parmentier HK (1999) A tradeoff between immunocompetence and sexual ornamentation in domestic fowl. Proc Natl Acad Sci U S Am 96:4478–4481
von Engelhardt N, Carere C, Dijkstra C, Groothuis TGG (2006) Sex-specific effects of yolk testosterone on survival, begging and growth of zebra finches. Proc R Soc Lond B Biol Sci 273:65–70
Acknowledgements
We thank Maribel Reviriego for her help during fieldwork, and Elena Bulmer for laboratory assistance. Two anonymous referees provided helpful comments of the first version of the MS. Research was funded by two research projects of the Spanish Ministerio de Ciencia y Tecnología (BOS 2002-00105 to DG and BOS 2003-01713 to FDL). DG was supported by a Marie Curie fellowship from the EU. AMR was supported by a Ph.D. studentship from the Junta de Extremadura (FIC01A043).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Graves
Rights and permissions
About this article
Cite this article
Gil, D., Marzal, A., de Lope, F. et al. Female house martins (Delichon urbica) reduce egg androgen deposition in response to a challenge of their immune system. Behav Ecol Sociobiol 60, 96–100 (2006). https://doi.org/10.1007/s00265-005-0145-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0145-1