Abstract
Although the sexes are united in hermaphrodites, conflict can still occur because the male and female functions have separate interests. We examined the evidence for conflict in the mating system of the terrestrial snail Cantareus aspersus (formerly Helix aspersa) where sharp, calcareous darts are ‘shot’ during courtship. We predicted that the use of the dart would either reflect or create conflict and this would be evident in either the courtship behavior or the transference of sperm. Previous studies demonstrated that the dart functions after sperm transfer to increase sperm survival. Using detailed observations of mating snails, we examined the factors that determine dart shooting order, the behavioral responses after being hit by a dart, the accuracy of dart shooting, and the allocation of sperm resources. We found that each dart was shot independently, and each animal appeared to be interested only in getting off the best possible shot, probably one that penetrates deeply near the genital pore. There is no evidence of mating conflict. Every snail transfers sperm to its partner, and the size of the donation does not depend on the success or failure of either snail’s dart shot. Although the receipt of a dart does not appear to cause harm, it may produce indirect costs due to the partial loss of control over fertilization. We conclude that mating in C. aspersus is a partnership in which independent actors demonstrate unconditional reciprocity during courtship and sperm transfer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of conflict is often the key to understanding mating behaviors and mating strategies. The different interests of males and females fundamentally cause sexual conflict, and conflict can be manifested at any stage in the reproductive process. It is most evident in cases where one sex, usually the male, harms the other sex, usually the female, in order to maximize its own reproductive success (Chapman et al. 2003). Mating conflicts arise because males are generally interested in mating frequently and promiscuously, whereas, females prefer to be selective. Conflicts occur after insemination because the male is interested in having all his sperm survive so that a maximum number can fertilize eggs. The female may however benefit from mating with other males that will cause the displacement of some of his sperm, or she may digest some of his sperm to gain energy. Sexual conflict also occurs after the arrival of offspring, when females usually invest heavily in caring for the offspring while males typically seek other sexual partners.
Sexual conflict can also occur in hermaphrodites because the male and female functions retain their separate interests even while they are united in the same individual (Michiels 1998; Koene and Schulenburg 2005). Sexual conflict in hermaphrodites is manifested in several ways, but mating conflict is especially prominent in hermaphrodites because two individuals sometimes attempt to perform the same sexual role (Leonard 1990; Michiels 1998). A striking example of unresolved mating conflict occurs in the marine flatworm, Pseudoceros bifurcus, which delivers sperm by hypodermic insemination. Here, paired animals engage in battles that have been described as ‘penis fencing’ (Michiels and Newman 1998), with each animal attempting to inseminate the other by stabbing it with its penis. In other hermaphroditic species, mating conflicts are resolved through reciprocal insemination. This may be accomplished either serially, where partners take turns in acting as the male, or simultaneously, where two individuals exchange sperm synchronously (Chase 2002).
Our investigation examined the evidence for conflict in the mating system of the garden snail, Cantareus aspersus (formerly Helix aspersa). Here, the bizarre phenomenon of dart shooting provides prima facie evidence of conflict. The snails mate with simultaneous and mutual penis insertion, and sperm exchange is synchronous. Each snail attempts to pierce the flesh of its prospective partner with a sharp, calcareous dart (Chung 1987; Adamo and Chase 1988; Landolfa 2002) toward the end of courtship. Although the delivery of the dart is commonly referred to as an act of ‘shooting’, the dart itself is not actually propelled; after being extruded it either sticks in the targeted snail or it is retracted. The dart often penetrates deeply into the body of the partner (Fig. 1), and extended courtships before the first dart shooting event (up to several hours: Adamo and Chase 1988) might indicate a mutual avoidance of the dart. However, when a snail is hit by a dart, its only apparent response is the reflexive withdrawal of the affected body part, typically for no more than a few seconds. Approximately one-half of the shot darts penetrate the body wall of the partner and remain lodged there for hours, whereas, the rest of the darts either miss the intended target altogether or they strike weakly, then fall out. Whether or not the dart hits its intended target does not influence the probability of copulation in the courting pair (Adamo and Chase 1988). There are strong grounds for identifying the dart as a male trait: The dart promotes the survival and storage of the shooter’s sperm in the recipient snail (Rogers and Chase 2001), and, in competitive mating experiments, snails that hit their partners with the dart father more offspring than snails that miss their partners with the dart (Landolfa et al. 2001; Rogers and Chase 2002).
It is important to note that the term ‘simultaneous hermaphroditism’ defines a condition ‘in which an individual reproduces through both sperm and eggs in each breeding season’ (Charnov 1979), and while all terrestrial molluscs (Stylommatophora) are, by this definition, simultaneous hermaphrodites, only some species (e.g. C. aspersus) mate with simultaneous, reciprocal sperm exchange. Only about four to nine out of approximately 60 families in the Stylommatophora use a dart (Davison et al. 2005; Koene and Schulenburg 2005). It is noteworthy that all the dart-bearing families mate in the simultaneous, reciprocal manner. Dart use is facultative in some species (Baminger et al. 2000), but in C. aspersus it is reported to be obligatory (Chung 1987).
The dart would engender mating conflict if it benefited the shooter but incurred costs in the recipient. While the benefits of dart shooting are clear at least in C. aspersus, the costs to the recipient are not well-known. The obvious possibilities for costs are physical injury, the partial loss of control over fertilization, and the risk of infection. If any of these costs were significant, one might expect a snail’s courtship behavior to be dominated by attempts to hit but not to be hit.
The dart might also create conflict by altering the reciprocity of sperm exchange. In species with simultaneous penis insertion and internal fertilization such as C. aspersus, the reciprocity of intromission would appear to indicate the absence of conflict, but the occult nature of sperm transfer hinders the confirmation of this expectation. One reason to be suspicious about the reciprocity of sperm exchange in C. aspersus comes from the recent work of Anthes and Michiels (2005) who studied the marine slug, Chelidonura sandrana. Mating in this species is reciprocal with obligate sex-role alternation, and fertilization is internal. The experimenters used an intervention that prevented one slug from donating sperm, while not affecting intromission. It was found that the intact slug transferred sperm to its manipulated partner even when it had not received any sperm from that partner. In other words, it is possible for a slug to cheat its partner by intromitting but not transferring any sperm. Similarly, the reciprocal intromissions practiced by C. aspersus do not guarantee reciprocal sperm transfers.
We looked for evidence of conflict in courtship behaviors and in spermatophore transfers. One focus was the order of dart shooting. If there is an advantage in shooting first, the snails may vie for priority, and there may be biological correlates that predict which snail will succeed in shooting first. For example, if dart shooting is a male-related activity and if male drive is a function of the autosperm store, the snail with the largest autosperm store might shoot first. We also investigated the behavioral responses elicited by the dart in the recipient snail. For example, if the snails are attempting to hit without being hit, then the accuracy and/or power of their shooting behavior should improve after they themselves had been hit because they would no longer need to avoid the dart.
While the reciprocal mating system of C. aspersus presumably evolved because it minimizes sexual conflict, the concurrent or subsequent evolution of dart shooting might have disrupted the strict reciprocity of sperm exchange. We therefore examined whether the success or failure of a dart shot affects the amount of sperm transferred by the shooter. Because successful shooters benefit from enhanced paternity, they might economize on sperm utilization by reducing the size of their spermatophores, particularly since sperm production costs are substantial in land snails (Greeff and Michiels 1999). Although one does not expect any similar compensatory response on the part of the dart recipient, nonspecific effects of the dart could nonetheless affect the size of the recipient’s spermatophore. In addition to examining absolute spermatophore sizes, we also examined spermatophore sizes relative to the total amount of available autosperm, as another measure of resource allocation.
Methods
Mating trials
Mature snails, C. aspersus, were collected from natural populations in the vicinity of Monterey Bay, California. The snails were held in individually isolated chambers for 3 weeks before being used in mating trials to allow replenishment of the allosperm and regeneration of the dart from any previous matings (Landolfa 2002; Hänggi et al. 2002). The snails were placed in mating trials at least once every 2 weeks until they mated. When a snail failed to mate, it was returned to isolation. Mating trials were conducted in a manner similar to that described by Adamo and Chase (1988) and Rogers and Chase (2002). The snails were deprived of food for 7 days, then fed for 5 days during mating trials, to increase mating frequency. A group of 20–50 snails was moistened with water, then placed in a Lucite box (36 × 36 × 8 cm). Prospective mating pairs were identified when two snails repeatedly contacted each other and both snails exhibited genital eversions ≥ stage 2 (moderate eversion of penial lobe; Adamo and Chase 1988). Selected pairs were removed to a small glass platform (85×105 mm) that could be lifted and rotated for viewing at various angles. In approximately 30% of cases, one member of the pair would cease to show interest in the other snail and it would lose its genital eversion. This snail was then removed and replaced with another snail that had a genital eversion as great, or greater, than that of its prospective mate, but no replacements were done after either snail had shot a dart. The process of removal and replacement was repeated as often as necessary (mean, approximately five times) to obtain a successful mating. Thus, while mate selection was not entirely free, the resulting pairs reflect mate choice to the extent that individual snails selected their mates from among several candidate snails. The principal consequence of our interventions was to compress the period in which snails encountered prospective mates.
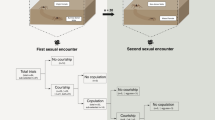
In every courtship, both snails attempt to shoot one dart. We use the abbreviations DS1 and DS2 to refer to the first and second dart shots, respectively. We accepted only those pairs in which DS1 could be categorized as either (1) a hit, in which the dart remained lodged in the recipient for at least 5.5 h after the start of copulation, at which time copulation was interrupted to retrieve the spermatophores; (2) a miss, in which the dart failed to contact the intended recipient; or (3) a no-shot, where no dart was fired despite the clear adoption of a dart shooting posture (Adamo and Chase 1988). This was done in order to enable comparisons between successful and unsuccessful shots. Thus, the recipient of DS1 was either hit or not hit. For DS2, all outcomes were accepted. These included — in addition to hits, misses and no-shots — darts that were retracted by the shooter and darts that hit the partner but fell out before the 5.5 h criterion mark. The interval between DS1 and DS2 was timed. Snails that failed to fire a dart were dissected, and their dart sacs were examined to determine if they contained a dart. All snails were used just once.
The sample of mated snails comprises 82 individuals, or 41 pairs. Another group of 12 snails, including five pairs, was designated as pre-maters. These snails were allowed to shoot their darts, and they exhibited typical courtship behaviors until just before intromission, at which time they were separated from their partners. The pre-maters were used to determine the number of autosperm in the seminal vesicle before formation of the spermatophore; also, the paired non-maters were combined with the maters for analysis of dart shooting interactions (total of 46 pairs). An additional group of 11 snails was designated non-maters. These were snails that failed to mate on multiple occasions and failed to show a genital eversion on the day of selection. The seminal vesicles of the non-maters were examined to determine if a lack of autosperm could explain their failure to mate. Not all measures were obtained from all specimens due to dissection errors or technical faults.
The entry points of the darts were measured on the recipient animal to the nearest 0.1 mm using vernier calipers. The distances from the dart to the genital pore (gonopore) and to a second landmark, e.g. the tip of the foot, were measured. The length, width, and height of each receiving snail were measured exclusive of the shell to map dart receipt locations onto a standard drawing, during maximal body extension. These whole-body measurements were then used to calculate the mean dimensions for all snails in the study population. The dart receipt locations of all snails were finally mapped onto a single drawing of a standard snail by scaling original measurements to mean dimensions.
Sperm content of the spermatophores
The spermatophores were recovered by interrupting copulation 325–335 min from the beginning of mutual intromission. After the snails were separated by slowly pulling them apart, the spermatophores were collected by pulling gently but persistently on the exposed spermatophore heads. The spermatophores were weighed and then frozen at −80°C. Sperm counts were obtained from a randomly chosen subset of 15 thawed spermatophores after disrupting the spermatophores using the method of Rogers and Chase (2001). Counting was done using an improved Neubauer hemacytometer at a magnification of 400×. The total number of sperm was estimated from two 15-μl samples, each of which was sub-sampled eight times (0.1 μl). Because the weight of a spermatophore was found to be highly predictive of the number of sperm contained therein (linear regression, R 2=0.69, F 1,13=28.88, p=0.0001), the sperm content of the remaining spermatophores was estimated by the spermatophore weight using the equation: sperm number (×106)=0.598 (spermatophore weight) − 5.000. The fractional size of the spermatophore was calculated as the sperm count of the spermatophore divided by the inferred sperm count of the seminal vesicle before filling of the spermatophore; the latter measure is described below.
Sperm content of the seminal vesicles
The sperm content of a seminal vesicle was not well predicted by its weight. Therefore, the seminal vesicle was removed by dissection within 2 days of mating or within 2 days of the last opportunity to mate in the case of non-maters to obtain a direct count for each snail. The distal ovotestis duct was stretched and cut close to the fertilization pouch–spermathecal complex, and the proximal duct was cut close to the ovotestis to avoid leakage of sperm. The liberated vesicle was placed on a glass slide and ruptured by four small incisions. Sperm was expressed by overlaying a glass cover and applying gentle pressure. The remaining sperm were removed by brushing the vesicle carcass with forceps and washing with 3.5 ml of citrate buffer (Vindelov et al. 1983). The resulting suspension was vortexed for 60 s, sonicated for 7 min, then centrifuged at 1,877×g for 3 min. After removal of the supernatant, 6 ml of 4% paraformaldehyde (in 0.1 M phosphate buffered saline) was added, followed by vortexing for 60 s, and then another 18 ml of 4% paraformaldehyde was added. The preparation was stored at 4°C for a maximum of 1 week before sperm was counted in a hemacytometer, as described above.
Using information obtained only after the animal had mated, we took the sum of the sperm in the spermatophore and the sperm remaining in the seminal vesicle after mating to estimate the quantity of autosperm that was available before mating. This measure was validated by comparing the summed quantities with the number of sperm found in pre-mating snails, that is, snails that were about to form a spermatophore (Table 1). We conclude that an insignificant number of sperm are ‘lost’ in the ducts during filling of the spermatophore since the contents of the seminal vesicle of the pre-maters did not differ significantly from the summed values derived from mated animals (t test, t=−0.192, df=72, p=0.848). Hence, we hereafter refer to the inferred content of the seminal vesicle before mating as ‘seminal vesicle sperm’.
Snail sizes
To estimate a snail’s size, the height, width, and length of the shell was measured to the nearest 0.1 mm using vernier calipers. Shell width was measured orthogonal to the long axis of the shell. These measurements were used to calculate shell volume according to the formula: shell volume (cm3)=4×10−4 (length × width × height) – 0.313.
Statistical analysis
The analyses were performed using SPSS, version 11.0. When evaluating the factors affecting sperm variables, the general linear model (GLM) included shell volume as a covariate. Shell volume was entered as an independent variable in regressions. A binomial test was used to evaluate the dependency of DS2 on the success or failure of DS1 (described in Results), rather than a multicellular test of proportionality. This was done because the constraints placed on DS1, but not DS2, precluded the latter type of test. All reported p values are two-tailed. The confidence intervals for effect sizes are reported in cases of statistically nonsignificant results.
Results
Dart shooting order
If dart shooting reflected an underlying conflict, we would expect to find biologically significant correlates of the order in which the two darts are shot. For example, if snails attempted to hit their partners without themselves being hit, larger individuals might have both offensive and defensive advantages, in which case we would expect to find that the first shooters were generally larger than the second. However, when we compared the sizes of the first and second shooters, we found that the mean difference in shell volumes was only 0.0954 cm3 (second shooter > first shooter). This is not statistically significant (paired t test, t=0.738, df=44, p=0.465; confidence limits=−0.3561, 0.1652).
Next, we reasoned that since the dart is a male trait, the order of dart shooting may be determined by male drive, which itself may depend on how much sperm is stored in the seminal vesicle at the time of courtship. Therefore, we predicted that the first snail to shoot would be the snail with the largest amount of sperm. The results (Table 2) show that neither the number of sperm available for transfer (in the seminal vesicle), nor the number actually transferred (in the spermatophore) accounts for dart shooting order. Shell volumes did not differ significantly between first and second shooters. In summary, shooting order is not explained by any of the measured variables. It may depend on the relative timing of sexual arousal in the two animals, which may be stochastic.
Table 1 shows the number of seminal vesicle sperm in mating snails, pre-mating snails and non-mating snails. The difference between mating snails and non-mating snails is not significant (GLM with shell volume included as a covariate, F 1,71=2.454, p=0.122, effect size=1.941×106, 95% confidence limits=−0.444×106, 3.698×106), but one non-mater had an empty seminal vesicle. From these results, it appears that mating is not guaranteed even when autosperm is available and the snails have had no opportunity to mate for as long as 5 months. The need for allosperm is possibly an additional requirement.
Some snails did not shoot a dart (18 of 94, or 18%), although all snails adopted the dart shooting posture at some point before intromission. We examined the dart sacs of all non-shooting snails to determine whether the use of the dart is obligatory or facultative. The sacs were empty in all cases. Hence, in agreement with Chung (1987), we conclude that dart shooting in C. aspersus is obligatory whenever the dart is present.
We determined the sperm content of the seminal vesicles to rule out recent surreptitious matings as the reason for absent darts (the dart would not yet have regenerated). The results show that non-shooters actually had slightly more sperm in their seminal vesicles than did the shooters, but the difference was not significant (GLM with shell volume included as a co-variable, F 1,61=0.476, p=0.493, marginal means difference non-shooters − shooters=0.628×106, 95% confidence limits=−1.18×106, 2.44×106). Because the contents of the seminal vesicle is reduced, on average, by 55.3% after formation of the spermatophore (see below), it is unlikely that the non-shooters had recently mated. Most probably, therefore, the non-shooters were virgins, because virgins have no darts (Chung 1986).
Interactions between dart shots
Because a snail must closely approach its partner in order to shoot accurately and land the dart deeply, a snail that is itself trying to avoid being hit, even while preparing to shoot, will likely proceed with caution. Further, because the animals have no way of knowing when the partner shoots and misses, or has no dart to shoot, a snail that is preparing to shoot will continue in a cautious mode. The snail will do so until it senses that the partner’s dart has penetrated its skin. When this happens, it has nothing more to fear. This scenario predicts that the second shooter will shoot more quickly and with greater accuracy after it has been hit than after it has been missed.
We took the null hypothesis p double=p DS1×p DS2, as an initial test of the dependency of DS2 on the success or failure of DS1, where p double is the proportion of mating pairs in which both snails hit their partners, p DS1 is the proportion of hits for all first shooters, and p DS2 is the proportion of hits for all second shooters. Our sample for this analysis included all pairs except those in which DS2 produced no dart (36 mated pairs, four pre-mating pairs). Given that p DS1=0.7 (28/40) and p DS2=0.625 (25/40), p double is predicted to be 0.438 (17.5 pairs). The observed p double was 0.475 (19/40), which is not significantly different from the null prediction (binomial test, p=0.754).
Next, we considered the locations where the darts struck. Fig. 1 shows an extreme case in which a dart entered at the right side of the head and exited through the left side; this example is not included in our data set. Generally, the locations were broadly distributed on the partner’s right flank and foot (Fig. 2), but in addition, there was a clear focus of entry points in or near the genital pore (12 of 56, or 21%). We took the distance from the dart’s entry point to the genital pore as a measure of its accuracy, since the female reproductive organs that lie just interior to the pore are known to be affected by mucus that is carried on the dart (Koene and Chase 1998). Overall, DS1 and DS2 did not differ in accuracy (GLM, F 1,47=0.160, p=0.213, effect size=3.56, 95% confidence limits=−2.11, 9.23). The accuracy of the first shot did not predict the accuracy of the second shot (linear regression, R 2=0.071, F 1,13=0.989, p=0. 338). Nor did the accuracy of DS2 differ significantly in cases where the DS1 hit compared to cases in which DS1 missed or was not fired (GLM, F 1,21=1.410, p=0.248, effect size=5.63, 95% confidence limits=−4.23, 15.48). In summary, neither the success of penetration nor the accuracy of the first shot appears to influence the outcome of the second shot.
Locations of dart entry points classified according to shooting order and shot outcomes. The designation ‘missed’ includes cases where no dart was fired. The positions of stuck darts were measured in situ, then scaled to the body dimensions of an average snail (N=93 snails). a View of right side. b View of undersurface of the foot; the shell circumference is indicated. The underlined symbols mark darts that entered at corresponding positions on the left side of the animal. Note that 12 darts penetrated the genital pore. The dart shown in Fig. 1 is not represented in this figure because it was not included in our data set
If snails are adversely affected by being hit, the second shooter might take longer to shoot its own dart when it is hit by the first shooter than when it is missed, or the second shooter might shoot quickly after being hit because it would now be safe to approach its partner. Support for the latter scenario was provided in a previous study (Adamo and Chase 1988). Here, we re-examined this issue using a larger sample and a more stringent criterion for hits (penetration≥5.5 h). Comparing cases in which DS1 hit versus those in which DS1 missed or the dart was not fired, the mean difference in the interval DS1–DS2 was nonsignificant at 2 min 59 s (GLM, F 1,43=0.172, p=0.680, 95% confidence limits=−11 min 30 s, 17 min 32 s). Thus, we failed to find that the timing of the second shot depends on the success of the first shot.
Sperm transfers
All 41 mated pairs intromitted reciprocally, and 73 of the 74 collected spermatophores contained sperm (one snail transferred an empty spermatophore). When these observations are combined with those reported by Rogers and Chase (2001), the total yields 100% reciprocal intromissions (84/84 pairs) and 98.6% sperm transfers (136/138 collected spermatophores). Thus, insemination is usually reciprocal in C. aspersus.
We asked whether dart shooting events influence sperm donations. First, do snails adjust the size of their donations depending upon whether or not they have been hit by a dart? This was proposed by Leonard (1992) who assumed that mating in C. aspersus involves sperm trading and that the dart signals the readiness of the shooter to donate sperm. Hence, snails hit by the dart should donate more sperm than if missed by the dart. Secondly, does the shooter adjust its own sperm donation depending upon the success or failure of its shot? Since a well-shot dart promotes the survival of sperm (Rogers and Chase 2001), unsuccessful shooters may increase the size of their donations to compensate for the missed shot. Indeed, Rogers and Chase (2001) reported a trend in this direction.
We examined variation in the absolute spermatophore size as well as its fractional size relative to the total amount of available autosperm for this analysis. The results (Table 3) show that neither measure of the spermatophore is significantly influenced by the success of the dart shot, whether it was the partner’s dart or the sperm donor’s dart. These results are generally consistent with the findings of Rogers and Chase (2001, 2002), except that there was no replication of the previously reported trend for unsuccessful shooters to deliver more sperm than successful shooters. In fact, the trend was in the opposite direction.
An incidental finding was that the average fractional size of the spermatophore for the entire sample was 0.553±0.150 SD (N=64). This value implies that a snail can store enough autosperm to transfer two full spermatophores in successive matings. This is consistent with the observation of Chung (1987) who reported that two ‘full’ spermatophores were delivered by one snail within a 24-h period.
As an additional and possibly more sensitive test of sperm allocation, the influence of dart receipt location, i.e. not simply hit or miss, was evaluated. No significant relationship was found between spermatophore size and the accuracy of either the donor’s dart or the partner’s dart (linear regressions, own dart: β=−0.043, t=0.302, p=0.764; partner’s dart: β=0.032, t=0.216, p=0.830). Nor was the fractional size of the spermatophore significantly affected by dart shooting accuracy (own dart: β=0.057, t=0.362, p=0.719; partner’s dart: β=0.073, t=0.448, p=0.656).
Discussion
The results provide no evidence of antagonistic behavior related to the use of the darts. Every snail that had a dart attempted to shoot it, but the outcomes were highly variable and unpredictable. We did not detect any apparent advantage in shooting first, nor any biological correlate of dart shooting order. The outcome of the first dart shooting event did not significantly influence either the timing or the accuracy of the second dart shooting event. Lastly, neither of the two dart shots affected either snail’s sperm donation. Our evidence suggests a scenario in which the two courting snails seek independently to dart their partners and exchange sperm rather than having a conflict over the dart. The interactions between the snails appear to be entirely synergistic.
Our conclusions are mainly based on negative results from statistical tests. Although the use of larger samples might have revealed some statistically significant effects, the consistency of our negative results argues against any biologically significant conflict interaction. Following current practice (Colegrave and Ruxton 2003), we did not perform post hoc power analyses but instead reported effect sizes and 95% confidence intervals. Most of these intervals are reasonably narrow around zero. We also note that our sample of 46 dart-shooting pairs is the largest of any published investigation into any aspect of dart shooting.
If snails attempted to avoid their partner’s dart, the recipient of the first dart might be expected to shoot its own dart quickly afterwards, because it no longer needed to fear being hit. Because our data fail to indicate any significant difference in the interval between dart shots depending upon whether the first shot hits or misses, it may be concluded that the dart is not an aversive stimulus. Since there is no significant delay after hits, the finding equally implies minimal physical injury from hits. These results contradict Adamo and Chase (1988) who found that the interval between shots was shortened when the first dart hit its intended target. However, we have a larger sample than Adamo and Chase (1988), and we selected for cases where the first shot was either solidly hit or completely missed (or absent).
The fact that we failed to detect any influence of the location of dart receipt on behavioral interactions does not speak to whether shooting accuracy influences the dart’s efficacy in post-copulatory processes. Earlier, it was demonstrated that snails store significantly more sperm when they are hit by the dart than when they are missed by the dart (Rogers and Chase 2001). Here we found that 21% of the darts that lodged in the partner landed in the genital pore. This could be an inconsequential result of the sensorimotor control mechanism that is responsible for the release of the dart, or it might indicate a targeting to this particular body region. The spermatophore receiving organ, the bursa tract diverticulum, is located just interior to the genital pore. Physiological experiments demonstrate an effect of dart mucus on this organ that could account for the dart’s efficacy in promoting the survival of sperm (Koene and Chase 1998). The hypothesis of a relationship between dart shooting accuracy and sperm survival is testable, requiring either direct counts of stored sperm (Rogers and Chase 2001) or competitive paternity trials (Landolfa et al. 2001; Rogers and Chase 2002).
In summary, the picture of dart shooting that emerges from this study is one in which the two dart shooting events are performed independently of one another. A snail’s sole interest is apparently in getting off the best possible shot, perhaps one that penetrates deeply near the genital pore. The real competition, or conflict, is between successive sperm donors, and that begins only after the sperm has been transferred. Also, because the snails nearly always exchange large spermatophores and neither snail is able to adjust the size of its spermatophore in response to its partner’s donation, the sperm donations themselves are likewise independent.
In contrast to C. aspersus, dart shooting in the European land snail Arianta arbustorum is facultative, with only about 40% of the snails shooting darts (Baminger et al. 2000). In A. arbustorum, each snail either shoots or fails to shoot independently of what its partner does, which is consistent with our findings regarding the accuracy and timing of the two obligatory shots in C. aspersus. Also consistent in the two species is the fact that autosperm donations are unaffected by either the occurrence of dart shots or their outcomes (Baur et al. 1998).
The absence of an obvious behavioral response to being hit by a dart, apart from a brief reflexive withdrawal, suggests that the dart does not cause significant physical injury. However, the withdrawal reflex is attenuated by a physiological mechanism within the central nervous system that acts to inhibit withdrawal responses during courtship (Balaban and Chase 1990), presumably to minimize the disruption of courtship. As for long-term direct costs, we have never observed a snail die or suffer an obvious impairment after being hit by a dart, even when it is hit through the head (Fig. 1). However, the mucus carried by the dart is toxic at high doses (unpublished results), and the long-term consequences of its transfer during dart shooting deserve examination. Long-term costs may also be more apparent under natural conditions than in the laboratory.
Our previously published work indicates a function for the dart in the survival and storage of sperm (Landolfa et al. 2001; Rogers and Chase 2001, 2002). Thus, an indirect cost might be the partial loss of control over fertilization by the female function. It follows that any defense against this cost should be expressed in the sperm-receiving organs, not in courtship behavior or sperm transfer. Recently, Koene and Schulenburg (2005) found evidence for the coevolution of the dart apparatus and the bursa tract diverticulum. From a phylogenetic analysis, these authors found that the length of the diverticulum increases as the size and complexity of the dart apparatus increases. One interpretation of these findings is that the female function is responding defensively to male-function manipulation by means of the dart.
Dart shooting may simply constitute part of the competition between sperm donors, with no cost to the female function. This would be the case if the female function benefited from being hit by a dart, which would occur if the resulting offspring either achieved higher reproductive success through a runaway genetic process or had higher viability (Pomiankowski and Reguera 2001; Landolfa 2002). Only further research will reveal exactly what manner of conflict is masked by the reciprocity, and apparent congeniality, of snail mating behavior.
References
Adamo SA, Chase R (1988) Courtship and copulation in the terrestrial snail Helix aspersa. Can J Zool 66:1446–1453
Anthes N, Michiels NK (2005) Do “sperm trading” simultaneous hermaphrodites always trade sperm? Behav Ecol 16:188–195
Balaban PM, Chase R (1990) Stimulation of mesocerebrum in Helix aspersa inhibits the neural network underlying avoidance behavior. J Comp Physiol A 166:421–427
Baminger H, Locher R, Baur A (2000) Incidence of dart shooting, sperm delivery, and sperm storage in natural populations of the simultaneously hermaphroditic land snail Arianta arbustorum. Can J Zool 78:1767–1774
Baur B, Locher R, Baur A (1998) Sperm allocation in simultaneously hermaphroditic land snail Arianta arbustorum. Anim Behav 56:839–845
Chapman T, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trends Ecol Evol 18:41–47
Charnov EL (1979) Simultaneous hermaphroditism and sexual selection. Proc Natl Acad Sci USA 76:2480–2484
Chase R (2002) Behavior and its neural control in gastropod molluscs. Oxford University Press, New York
Chung DJD (1986) Initiation of growth of the first dart in Helix aspersa Müller. J Molluscan Stud 52:253–255
Chung DJD (1987) Courtship and dart shooting behavior of the land snail Helix aspersa. Veliger 30:24–39
Colegrave N, Ruxton GD (2003) Confidence intervals are a more useful complement to nonsignificant tests than are power calculations. Behav Ecol 14:446–447
Davison A, Wade CM, Mordan PB, Chiba S (2005) Sex and darts in slugs and snails. J Zool (in press)
Greeff JM, Michiels NK (1999) Sperm digestion and reciprocal sperm transfer can drive hermaphrodite sex allocation to equality. Am Nat 153:421–430
Hänggi C, Locher R, Baur B (2002) Intermating interval and number of sperm delivered in the simultaneously hermaphroditic land snail Arianta arbustorum (Pulmonata: Helicidae). Veliger 45:224–230
Koene JM, Chase R (1998) Changes in the reproductive system of the snail Helix aspersa caused by mucus from the love dart. J Exp Biol 201:2313–2319
Koene JM, Schulenburg H (2005) Shooting darts: co-evolution and counter-adaptation in hermaphroditic snails. BMC Evol Biol DOI 10.1186/1471-2148-5-25
Landolfa MA (2002) On the adaptive function of the love dart of Helix aspersa. Veliger 45:231–249
Landolfa MA, Green DM, Chase R (2001) Dart shooting influences paternal reproductive success in the snail Helix aspersa (Pulmonata, Stylommatophora). Behav Ecol 12:773–777
Leonard JL (1990) The hermaphrodite’s dilemma. J Theor Biol 147:361–372
Leonard JL (1992) The ‘love-dart’ in helicid snails: a gift of calcium or a firm commitment? J Theor Biol 159:513–521
Michiels NK (1998) Mating conflicts and sperm competition in simultaneous hermaphrodites. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. London, Academic, pp219–254
Michiels NK, Newman LJ (1998) Sex and violence in hermaphrodites. Nature 391:647
Pomiankowski A, Reguera P (2001) The point of love. Trends Ecol Evol 16:533–534
Rogers DW, Chase R (2001) Dart receipt promotes sperm storage in the garden snail Helix aspersa. Behav Ecol Sociobiol 50:122–127
Rogers DW, Chase R (2002) Determinants of paternity in the garden snail Helix aspersa. Behav Ecol Sociobiol 52:289–295
Vindelov LL, Christensen IJ, Nissen NI (1983) A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry 3:323–327
Acknowledgements
We thank Katrina Blanchard, Robert Hutcheson, Joris Koene and Heike Reise for their comments on the manuscript. Angus Davison kindly allowed us to see unpublished work. Funded by a grant to R. Chase and an Undergraduate Student Research Award to K. Vaga, both from the Natural Sciences and Engineering Research Council of Canada. This research complies with current laws in Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P.J. Moore
Rights and permissions
About this article
Cite this article
Chase, R., Vaga, K. Independence, not conflict, characterizes dart-shooting and sperm exchange in a hermaphroditic snail. Behav Ecol Sociobiol 59, 732–739 (2006). https://doi.org/10.1007/s00265-005-0103-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0103-y