Abstract
Parasites are ubiquitous in populations of free-ranging animals and impact host fitness, but virtually nothing is known about the factors that influence patterns of disease risk across species and the effectiveness of behavioral defenses to reduce this risk. We investigated the correlates of malaria infection (prevalence) in Neotropical primates using data from the literature, focusing on host traits involving group size, body mass, and sleeping behavior. Malaria is spread to these monkeys through anopheline mosquitoes that search for hosts at night using olfactory cues. In comparative tests that used two different phylogenetic trees, we confirmed that malaria prevalence increases with group size in Neotropical primates, as suggested by a previous non-phylogenetic analysis. These results are consistent with the hypothesis that larger groups experience increased risk of attack by mosquitoes, and counter to the hypothesis that primates benefit from the encounter-dilution effect of avoiding actively-seeking insects by living in larger groups. In contrast to non-phylogenetic tests, body mass was significant in fewer phylogeny-based analyses, and primarily when group size was included as a covariate. We also found statistical support for the hypothesis that sleeping in closed microhabitats, such as tree holes or tangles of vegetation, reduces the risk of malaria infection by containing the host cues used by mosquitoes to locate hosts. Due to the small number of evolutionary transitions in sleeping behavior in this group of primates, however, this result is considered preliminary until repeated with a larger sample size. In summary, risk of infection with malaria and other vector-borne diseases are likely to act as a cost of living in groups, rather than a benefit, and sleeping site selection may provide benefits by reducing rates of attack by malaria vectors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Living in groups can be beneficial in terms of increased protection from predators and increased foraging efficiency (Alexander 1974; Blumstein et al. 1999), but social animals may also incur costs in terms of increased feeding competition (e.g., Janson 1988; Hoare et al. 2004) and a higher risk of acquiring infectious disease (Brown and Brown 1986; Møller et al. 1993; Brown et al. 2001; Tella 2002; Altizer et al. 2003; for general review, see Krause and Ruxton 2002). Costs involving infectious disease and sociality have generally focused on directly transmitted parasites, but sociality may also impact risks of attack by arthropod vectors (Davies et al. 1991; Mooring and Hart 1992; Côté and Poulin 1995), and some authors have proposed that vector-borne diseases are more virulent (Ewald 1983). In this paper, we explore the links between group size, disease risk and host behavioral defenses to vector-borne disease in a comparative study of primate behavior and the prevalence of malaria infection.
Given that infection with malaria requires ecological overlap between host and appropriate arthropod vectors, what is the expected relationship between group size and risk of infection with vector-borne diseases? Competing hypotheses have been proposed. In one hypothesis, living in a group lessens the probability of attack by biting arthropods through the encounter-dilution effect (Mooring and Hart 1992), based on mechanisms involved in reducing predation risk by living in a group. This hypothesis therefore predicts a negative association between group size and prevalence of infection with vector-transmitted parasites. In a meta-analysis of vertebrates and their parasites, Côté & Poulin (1995) showed that group size was negatively correlated with the intensity of infection by mobile parasites. Similarly, in a study of African primates, Freeland (1977) found that the occurrence of polyspecific associations (multi-species groups) correlated positively with the activity of biting flies, suggesting that animals seek conspecifics as risk of attack increases. Neither of these studies directly investigated the prevalence of vector-borne infections.
Alternatively, when mobile parasites seek hosts by using cues that become stronger in larger groups, rates of attack by flying arthropods are expected to increase with group size, leading to increased prevalence of vector-borne parasites. This hypothesis may apply to risk of infection with malaria, since it is transmitted through nocturnally active anopheline mosquitoes that are attracted to hosts through emission of body odorants and carbon dioxide (Bock and Cardew 1996; Hallem et al. 2004). In support of this hypothesis, Tella (2002) showed that evolutionary transitions to coloniality in birds were associated with increased prevalence and species richness of vector-borne parasites, and Brown and Sethi (2002) established that mosquito abundance increased with colony size in cliff swallows. In primates, Davies et al. (1991) found that malaria infection rates increased with sleeping group size in a comparative study of Amazonian primates. Body mass also was statistically significant, which they interpreted as indicating increased production of attractants by larger-bodied hosts. Because phylogeny was not taken into account, however, it is possible that some other host traits or environmental variables, shared through common descent, account for these associations (Felsenstein 1985; Harvey and Pagel 1991).
To address the links between risk of infection by vector borne parasites and group size, we conducted two sets of analyses using primates and their malaria parasites. First, we re-investigated the host traits examined by Davies et al. (1991) after controlling for host phylogeny. These analyses allowed us to rule out the possibility that patterns documented in this previous study were driven by traits shared through common descent. Second, we investigated an additional host behavioral trait that may influence levels of malaria infection across species. Among the primate species used in Davies et al.' (1991) comparative study, some species tend to sleep in closed microhabitats, such as tree hollows or dense tangles of epiphytes, while other species sleep in the open, for example on branches of trees. By sleeping in a closed environment, primates and other mammals may effectively contain the cues used by nocturnally-active mosquitoes to search for hosts (Heymann 1995; 2001), while also gaining other benefits related to protection from predators, colder night time temperatures, or inclement weather (Kappeler 1998). Because smaller-bodied species tend to live in smaller groups and sleep in closed environments, Heymann (1995; 2001) suggested that sleeping behavior might be the causal variable that accounts for the risk of malaria infection in Amazonian primates.
Methods
Agents and vectors of malaria infections in Neotropical primates
Plasmodium brasilianum is the primary infectious organism that causes malaria in Neotropical primates (Coatney et al. 1971; Davies et al. 1991; Deane 1992; Collins 1994). This protozoan is morphologically and developmentally very similar to Plasmodium malaria, an agent of human malaria (Cochrane et al. 1988). Some Neotropical primate species have also been reported to be infected with Plasmodium simium and Plasmodium falciparum (Deane et al. 1969; de Arruda et al. 1989; Fandeur et al. 2000). No information is available on the course of infection in wild Neotropical primates. Experimental infections result in a 72-h quartan type periodicity and can be fatal (Taliaferro and Taliaferro 1934; Coatney et al. 1971).
Several arthropod vectors play a role in the transmission of P. brasilianum. Sporozoites of P. brasilianum have been detected in Anopheles neivai (Davies et al. 1991), and P. brasilianum/P. malariae has been found in Anopheles darling and Anopheles nuneztovari by immunological assays and PCR (de Arruda et al. 1989; Fandeur et al. 2000). Other potential vectors include Anopheles oswaldoi and Anopheles triannulatus (Davies et al. 1991). Anopheles mosquitoes are usually nocturnal or crepuscular (Rubiopalis and Curtis 1992; Voorham 2002). Differences among these vector species could conceivably impact patterns of infection geographically and among primate hosts, but little is known about this variation. Similarly, implementation of behavioral defenses could differ among host species if a parasite exerts different effects on different hosts, but quantitative information is lacking on these effects for the host species in our dataset.
Comparative data
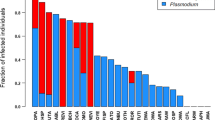
We used the data set on the prevalence of infection with Plasmodium brasilianum provided by Davies et al. (1991) for Amazonian primates and added data from Deane (1992) for primate taxa from the Atlantic coastal forests of eastern and south-eastern Brazil (Table 1). Body mass and sleeping group size data were taken from Davies et al. (1991) for the Amazonian primates. For the seven additional primates, we obtained data on body mass (as the midpoint of male and female mass for species with data on prevalence) from Smith and Jungers (1997) and group size from the primary literature, thus following generally the same procedure used by Davies et al. (1991) for collating data on host traits.
We categorized a species as using “open” microhabitats if individuals of that species typically sleep while sitting on branches or tree-forks within canopies, and as “closed” when animals retire into tree hollows or dense tangles formed by epiphytes and lianas. Most callitrichines studied in the wild preferentially use closed shelters for sleeping, e.g. tree holes, the base of palm fronds, or dense tangles of epiphytes (Coimbra-Filho 1977; Izawa 1979; Rylands 1981; Soini 1988; Stevenson and Rylands 1988; Peres 1991; Heymann 1995; Smith 1997). Several species of titi monkeys (Callicebus spp.) also tend to sleep in closed shelters (Callicebus cupreus: Kinzey 1981, E.W. Heymann, pers. obs.; Callicebus personatus: S. Heiduck, pers. comm.; but not Callicebus torquatus: Kinzey 1981). All other Neotropical primates use open sleeping sites, such as horizontal branches (see genus accounts in Coimbra-Filho and Mittermeier 1981; Mittermeier et al. 1988). Species for which quantitative data on sleeping habits indicated the use of both closed and open microhabitats were categorized according to which sleeping behavior was more frequent (e.g. the tamarins Saguinus mystax and Saguinus fuscicollis, Heymann 1995).
In most cases, we combined data for species within genera because sleeping behavior was generally consistent among species within a genus, and species exhibited variation in how many individuals had been sampled for malaria (range 1-1428, mean=91). After species were combined, the sample sizes for prevalence estimates per genus ranged from 5 to 1521 individuals, with a mean of 230 (standard deviation=372). In only one case did we split a genus for analysis: we analyzed Callicebus torquatus separately because it sleeps in the open, while other members of this genus sleep in closed microhabitats (see above). We repeated analyses with and without inclusion of night monkeys from the genus Aotus, which were excluded by Davies et al. (1991). Members of Aotus are nocturnal and may be subject to different selective pressures with regard to malaria transmission because the vectors are mainly nocturnal or crepuscular (e.g., Rubiopalis and Curtis 1992; Voorham 2002). Aotus does rest during its nighttime activity period (e.g., Garcia and Braza 1987), but animals that are generally active while vectors are active may be able to implement behavioral strategies to reduce attacks by flying insects (Day and Edman 1984; Dudley and Milton 1990). Previous researchers have documented differences among host species in behavioral responsiveness and tolerance to mosquitoes (Webber and Edman 1972; Edman et al. 1984). Currently, no quantifiable information is available on interspecific differences in mosquito tolerance and anti-mosquito behavior among species of Neotropical primates.
Comparative methods and statistical tests
To determine whether the variables used in this study are correlated with phylogeny, we implemented the test for serial independence and the runs test using the computer program Phylogenetic Independence (Abouheif 1999; Reeve and Abouheif 2003). The variable common to all analyses (prevalence of infection) was more similar among closely related species than expected by chance (P<0.01 in all tests). We therefore based our primary conclusions on results from phylogenetically independent contrasts (Felsenstein 1985; Harvey and Pagel 1991), although for comparison we also provide results using species values without correcting for phylogeny.
Contrasts were calculated using the computer program CAIC (Purvis and Rambaut 1995). We used two phylogenies in these tests (see electronic supplements S1 and S2 for phylogenetic trees). First, we used the phylogeny provided by Purvis (1995) after updating the taxonomy to reflect the division of Callithrix into Callithrix and Mico (Amazonian marmosets). Second, we used more recent phylogenetic information on Neotropical primates that combined results from Porter et al. (1997), Schneider (2000) and Goodman et al. (1998). We tested whether the contrasts were standardized correctly under three different sets of branch lengths: equal, absolute time (gradual), and log10-transformed absolute time (log-gradual). We found that the assumptions were best met when continuous traits were log10-transformed (after adding 1 to deal with values of zero prevalence). This was true regardless of the branch lengths, inclusion of Aotus, or the phylogenetic topology. We therefore present results from log10-transformed data using independent contrasts with two phylogenetic topologies, three sets of branch lengths for each topology, and repeated with and without Aotus. To investigate the association between sleeping behavior (a discrete trait) and prevalence of malaria, we examined evolutionary transitions in sleeping habits using the BRUNCH algorithm in CAIC (Purvis and Rambaut 1995).
We conducted bivariate and multivariate analyses. Analyses were conducted with the significance level α<0.05. In terms of our predictions, group size could correlate positively with prevalence of infection if vectors are better able to locate larger groups; conversely, a negative association is expected if individuals in larger groups benefit from the encounter-dilution effect (Mooring and Hart 1992). Thus, for analyses of group size we used two-tailed tests. For analyses of sleeping habit and body mass, directional predictions were possible – we expected prevalence of infection to be greater in animals that sleep in the open or are larger in body mass. We therefore used directed tests (Rice and Gaines 1994) for investigating these predictions. Directed tests allocate a disproportionate probability under the null hypothesis to the tail of the distribution in the predicted direction (γ), while retaining a smaller probability in the other tail to detect unexpected deviations opposite to predictions (δ<γ). Directed tests are subject to the constraint that δ + γ = α. We followed the guidelines in Rice and Gaines (1994) by setting γ/α to 0.8, giving values of γ=0.04 and δ=0.01. Prior to running the multivariate analyses, we checked whether collinearity was a potential problem by using variance inflation factors (VIF). For a full model with body mass, group size and sleeping behavior, VIF was less than ten in independent contrasts analysis that included Aotus (range 0.49–0.98) and when using species values (range 1.3–3.1). Thus, we used standard multiple regression methods (Petraitis et al. 1996).
Results
Group size, body mass and sleeping behavior in bivariate tests
In bivariate tests using phylogenetically independent contrasts, group size was a significant predictor of malaria prevalence, while body mass was largely non-significant (Table 2). This pattern was remarkably consistent across six different topology-branch length combinations and when analyses were repeated after excluding Aotus. We found a different pattern in non-phylogenetic tests, with body mass emerging as a significant predictor of malaria prevalence while group size was non-significant.
The phylogenetic distribution of sleeping behavior produced either two or three contrasts for analysis, depending on the phylogeny that was used. Evolutionary transitions to closed sleeping habits were correlated with a statistically significant reduction in the prevalence of malaria for the majority (8/12) of phylogeny-based bivariate (BRUNCH) tests that we conducted (Table 2). This pattern was significant in all analyses that included Aotus. However, results were non-significant (but with all contrasts in the predicted direction) when using untransformed data (as noted above, this also resulted in violation of more assumptions in the contrasts analyses). When the analysis was conducted using species values, we found a significant difference in ANOVA (Table 2), with species that were scored as using closed sleeping sites exhibiting lower prevalence of malaria infection.
Multivariate tests
Results from the multivariate model largely mirrored those from bivariate tests (Table 3). When using independent contrasts, group size was consistently entered and statistically significant, along with sleeping behavior and body mass in a smaller number of analyses (e.g., for the Porter-Schneider-Goodman tree with log-transformed branches and Aotus, mass: t 12=2.05, P=0.04, group size: t 12=4.90, P=0.0004, sleeping behavior transitions: t 12=3.43, P=0.003). When Aotus was excluded, sleeping behavior was no longer statistically significant in any of the multivariate tests. In non-phylogenetic tests, only sleeping behavior was statistically significant, but only in analyses that included Aotus.
To further ascertain whether group size was a causal factor independent of transitions in sleeping behavior, we repeated phylogenetic analyses after excluding contrasts that involved a corresponding transition in use of closed versus open sleeping habits. For the analyses shown in Table 3, group size was statistically significant in all analyses at P<0.05, and body mass was non-significant in the majority of tests (e.g., for the analysis reported above, group size: t11=2.49, P=0.03, mass: t11=1.31, P=0.14). In non-phylogenetic tests, however, no variables were statistically significant when looking within either of the two sleeping categories (e.g., open habitat: mass: t7=1.36, P=0.13, group size: t7=1.05, P=0.33; closed habitats, including Aotus, mass: t4=0.25, P=0.51, group size: t4=-0.56, P=0.61). Thus, malaria prevalence is associated most generally with group size in primates, but only when analyzing the set of species that also include variation in sleeping behavior, possibly due to larger sample sizes or to a correlated increase in group size over transitions in sleeping behavior. These results therefore emphasize the importance of group size as a predictor of malaria prevalence and the potential role of sleeping behavior in producing spurious associations in analyses that ignore phylogeny.
Discussion
The results presented here indicate that group size is the primary host behavioral trait that influences malaria prevalence in Neotropical primates. Previous studies of birds have found similar support for an association between group size and prevalence of arthropod-borne infections (e.g., Buggy Creek virus in cliff swallows, Brown et al. 2001; comparative study, Tella 2002), and our results confirm the previous non-phylogenetic analyses of group size conducted by Davies et al. (1991) in primates. Similarly, the abundance of mosquitoes inside human dwellings in the tropics increases with sleeping group size in humans (Haddow 1942; Ribbands 1949; Gillies 1955; Pålsson et al. 2004), although other ecological and behavioral characteristics of human populations, including use of window screens, modify overall patterns of infection. In contrast to the non-phylogenetic analyses conducted by Davies et al. (1991) and research showing that larger animals attract more mosquitoes (Port et al. 1980), most of our phylogeny-based analyses found that body mass was not statistically significant. By examining evolutionary transitions in host traits and prevalence of malaria, we were better able to control for correlations that arise through common ancestry (Felsenstein 1985; Harvey and Pagel 1991). This turns out to be crucial for the variables under investigation, because only evolutionary changes in group size showed a consistent association with changes in malaria prevalence.
We also found that use of closed sleeping sites is associated with a reduction in risk from vector-borne disease in some tests, particularly when Aotus was included in the analysis. Again, similar results have been found in humans, with individuals that sleep in houses with open eaves experiencing an increased abundance of mosquitoes in bedrooms (e.g., Pålsson et al. 2004). Closed sleeping sites may provide additional benefits for primates, including reduced predation risk, increased retention of body heat, and shelter from inclement weather (e.g., Anderson 1998; Kappeler 1998; Di Bitetti et al. 2000). In addition, use of closed sleeping sites is correlated with other traits examined here, with smaller-bodied primates and those living in smaller groups tending to sleep in concealed sites (e.g., Anderson 1984).
The small number of contrasts in tests of sleeping behavior reduces statistical power and also limits our ability to draw general conclusions. The statistically significant results involving sleeping site preference reflect both a large mean difference in prevalence between genera with different sleeping behaviors and a low variance among the few contrasts that are available. Moreover, the results were sensitive to the dataset used, particularly with regard to inclusion of Aotus. Future studies could increase the power of the tests by examining a larger clade of mammals, or by using continuous measures of the percentage of time that species spend sleeping in different microhabitats. In the field, spatial and seasonal variation in arthropod activity should correlate with use of closed sleeping sites by primates. An additional factor that we have not been able to include in our analyses due to the lack of data involves variation in sleeping height. Mosquitoes often show preferences for foraging at different heights in the forest (Lourenço de Oliveira and Luz 1996). Based on the prediction that sleeping site selection is a behavior that reduces contact with arthropod vectors, sleeping sites of the hosts should be found at heights that avoid overlap with preferred heights of the vectors.
Our analyses provide further support to the view that host behavioral traits are directly linked to inter-specific variation in vector abundance or the prevalence of vector-borne parasites (Brown et al. 2001; Brown and Sethi 2002; Tella 2002). Interestingly, the importance of host behavior in this case is intertwined with the behavior of the vector. Thus, mosquitoes appear to be better able to find primate hosts that live in larger groups. This might result from larger groups of animals producing more olfactory cues (Davies et al. 1991), but other stimuli (body heat) could also play a role. Similarly, cues emitted from animals sleeping in open microhabitats – be they olfactory or others – are perhaps more readily detected by mosquito vectors, which results in higher prevalence of malaria. In addition, it could be that larger groups of primates act to increase local density, reducing the distance between hosts and tending to increase measures of prevalence, although this would require that vectors bite multiple hosts while visiting a group and are capable of spreading the disease among these individuals during a visit to the group. Because the production of infectious Plasmodium sporozoites through asexual replication in the mosquito gut epithelium takes at least 48 h (Coatney et al. 1971), however, it is unlikely that a mosquito can spread the disease among primate hosts in a single visit to a social group.
The lack of an effect of body mass is surprising, given that studies in humans have shown that the proportional surface area or mass of a person in a group is positively associated with the number of bites by Anopheles gambiae (Port et al. 1980). If emission of olfactory or other cues is the mechanism that accounts for the association between sociality and malaria prevalence, and if larger bodied hosts produce more of these cues, then why did we find few significant effects of body mass in our phylogeny-based tests? It may be that the area typically covered by a group at any time (group spread) is more important than the body sizes of individuals in the group, with greater spread potentially attracting more mosquitoes by producing olfactory cues over a wider area. Suitable data on group spread are not yet available for all primates in our sample, but in future research, a larger proportion of the variance in malaria prevalence might be accounted for by a metric that takes into account body surface area, group size and group spread. In addition, previous researchers have identified a number of behavioral counterstrategies to flying arthropods in Neotropical primates, including fly-swatting to reduce exposure to bot flies (Dudley and Milton 1990) and application of millipede secretions to reduce attacks by biting insects (Valderrama et al. 2000). If these behaviors are implemented to greater effect or at increased rates in large-bodied hosts, this could obscure associations between body mass and disease risk.
Finally, immune defenses may play a role in accounting for the absence of an association with body mass, with larger bodied hosts exhibiting higher leukocyte (neutrophil) counts in primates (Nunn et al. 2000; Nunn 2002). Other immune cell types may account for additional variation in prevalence of infection across species. For example, previous comparative studies of leukocyte counts found that Aotus exhibits extremely high eosinophil counts (Hawkey 1977; Nunn 2002), perhaps providing an additional explanation for the low levels of malaria infection (0%) in wild members of this species. Additionally, Aotus may experience less risk at night because it is generally more active at this time; mosquitoes are less likely to feed successfully on active hosts than on sleeping hosts (Day and Edman 1984), which in primates could be due to active avoidance of mosquitoes while hosts are awake (Dudley and Milton 1990).
Our results have implications for conservation of biodiversity and human health. Many endangered primates are susceptible to malaria (e.g. Brachyteles arachnoides, Pan troglodytes, Garnham 1966; Deane et al. 1969; Coatney et al. 1971). In addition, mosquitoes and other arthropods carry a wide variety of parasites and pathogens that infect wild primates, including viruses (e.g., yellow fever), helminths (e.g., microfilaria) and other protozoa (e.g., trypanosomes). If selective logging tends to remove older trees that offer opportunities for shelter in the form of tree holes or tangles of vines, then smaller-bodied wild primates that are currently at lower risk of malaria infection may lose their protection from these often-virulent pathogens (Ewald 1983). Moreover, ecological disturbances, including selective logging and road building, have the potential to increase mosquito abundance, or to shift the composition of the mosquito community to species in which host behaviors have not yet adapted, in both cases leading to higher prevalence of infection (e.g., Patz et al. 2000). In terms of human health, it is already well known that sleeping in a closed dwelling reduces the risk of acquiring malaria in humans. Our results suggest that our primate relatives discovered the importance of sheltered sleeping sites for reducing the risk of vector-borne diseases well in advance of humans. Thus, increased understanding of primate behavioral defenses may provide unforeseen benefits for reducing disease risk in humans, particularly in developing countries where easy-to-use prophylactics could provide greatest benefits (Edman 1988).
References
Abouheif E (1999) A method for testing the assumption of phylogenetic independence in comparative data. Evol Ecol Res 1:895–909
Alexander RD (1974) The evolution of social behavior. Ann Rev Ecol Syst 5:325–383
Anderson JR (1984) Ethology and ecology of sleep in monkeys and apes. Adv Study Behav 14:165–229
Anderson JR (1998) Sleep, sleeping sites, and sleep–related activities: awakening to their significance. Am J Primatol 46:63–75
Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, Dobson AP, Ezenwa V, Pedersen AB, Poss M, Pulliam JRC (2003) Social organization and parasite risk in mammals: integrating theory and empirical studies. Ann Rev Ecol Evol Syst 34:517–47
Blumstein DT, Evans CS, Daniel JC (1999) An experimental study of behavioural group size effects in tammar wallabies, Macropus eugenii. Anim Behav 58:351–360
Bock G, Cardew G (1996) Olfaction in mosquito–host interactions. In: Ciba Foundation symposium. Wiley, Chichester, New York.
Brown CR, Brown MB (1986) Ectoparasitism as a cost of coloniality in cliff swallows (Hirundo pyrrhonota). Ecology 67:1206–1218
Brown CR, Sethi RA (2002) Mosquito abundance is correlated with cliff swallow (Petrochelidon pyrrhonota) colony size. J Med Entomol 39:115–120
Brown CR, Komar N, Quick SB, Sethi RA, Panella NA, Brown MB, Pfeffer M (2001) Arbovirus infection increases with group size. Proc Roy Soc Lond B 268:1833–1840
Coatney GR, Collins WE, McWilson W (1971) The primate malarias. National Insitiute of Allergy and Infectious Diseases, Bethesda.
Cochrane AH, Matsumoto Y, Kamboj KK, Maracic M, Nussenzweig RS, Aikawa M (1988) Membrane–associated antigens of blood stages of Plasmodium brasilianum, a quartan malaria parasite. Infec Immunity 56:2080–2088
Coimbra-Filho AF (1977) Natural shelters of Leontopithecus rosalia and some ecological implications (Callitrichidae: Primates). In: Kleiman D (ed) The biology and conservation of the Callitrichidae. Smithsonian Institution Press, Washington, pp 79–89
Coimbra–Filho AF, Mittermeier R (1981) Ecology and behavior of neotropical primates 1. Academia Brasileira de Ciencias, Rio de Janeiro.
Collins WE (1994) The owl monkey as a model for malaria. In: Baer JF, Weller RE, Kakoma I (eds) Aotus: The owl monkey. Academic Press, San Diego, CA, pp 217–244
Côté IM, Poulin R (1995) Parasitism and group size in social animals: a meta-analysis. Behav Ecol 6:159–165
Davies CR, Ayres JM, Dye C, Deane LM (1991) Malaria infection rate of Amazonian primates increases with body weight and group size. Funct Ecol 5:655–662
Day JF, Edman JD (1984) Mosquito engorgement on normally defensive hosts depends on host activity patterns. J Med Entomol 21:732–740
de Arruda M, Nardin EH, Nussenzweig RS, Cochrane AH (1989) Sero-epidemiological studies of malaria in Indian tribes and monkeys of the Amazon Basin of Brazil. Am J Trop Med Hyg 41:379–385
Deane LM, Ferreira Neto JA, Okumura M, Ferreira MO (1969) Malaria parasites of Brazilian monkeys. Rev Inst Med Trop Sao Paulo 11:71–86
Deane LM (1992) Simian malaria in Brazil. Mem Inst Oswaldo Cruz 87:1–20
Di Bitetti MS, Vidal EML, Baldovino MC, Benesovsky V (2000) Sleeping site preferences in tufted capuchin monkeys (Cebus apella nigritus). Am J Primatol 50:257–274
Dudley R, Milton K (1990) Parasite deterrence and the energetic costs of slapping in howler monkeys, Alouatta palliata. J Mammal 71:463–465
Edman JD (1988) Disease control through manipulation of vector-host interaction: some historical and evolutionary perspectives. In: Scott TW, Grumstrup-Scott J (eds) Proceedings of a symposium: the role of vector-host interactions in disease transmission, vol 68 Entomological Society of America, Lanham, MD, pp 43–50
Edman JD, Day JF, Walker ED (1984) Field confirmation of laboratory observations on the differential antimosquito behavior of herons. Condor 86:91–92
Ewald PW (1983) Host-parasite relations, vectors, and the evolution of disease severity. Ann Rev Ecol Syst 14:465–485
Fandeur T, Volney B, Peneau C, De Thoisy B (2000) Monkeys of the rainforest in French Guiana are natural reservoirs for P. brasilianum/P. malariae malaria. Parasitology 120:11–21
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Freeland WJ (1977) Blood-sucking flies and primate polyspecific associations. Nature 269:801–802
Garcia J, Braza F (1987) Activity rhythms and use of space of a group of Aotus azarae in Bolivia during the rainy season. Primates 28:337–342
Garnham PCC (1966) Malaria parasites and other Haemosporidia. Blackwell Scientific, Oxford.
Gillies MT (1955) The Density of adult anopheles in the neighbourhood of an East African village. Am J Trop Med Hyg 4:1103–1113
Goodman M, Porter CA, Czelusniak J, Page SL, Schneider H, Shoshani J, Gunnell G, Groves CP (1998) Toward a phylogenetic classification of primates based on DNA evidence complemented by fossil evidence. Mol Phylog Evol 9:585–598
Haddow AJ (1942) The mosquito fauna and climate of native huts at Kisumu, Kenya. Bull Entomol Res 33:91–142
Hallem EA, Fox AN, Zwiebel LJ, Carlson JR (2004) Olfaction-mosquito receptor for human-sweat odorant. Nature 427:212–213
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, Oxford
Hawkey CM (1977) The haematology of exotic mammals. In: Archer RK, Jeffcott LB, Lehmann H (eds) Comparative clinical haematology. Blackwell Scientific Publications, Oxford
Heymann EW (1995) Sleeping habits of tamarins, Saguinus mystax and Saguinus fuscicollis (Mammalia: Primates; Callitrichidae), in north-eastern Peru. J Zool Lond 237:211–226
Heymann EW (2001) Malaria infection rate of Amazonian primates: the role of sleeping habits. Folia Primatol 72:153
Hoare DJ, Couzin ID, Godin JGJ, Krause J (2004) Context-dependent group size choice in fish. Anim Behav 67:155–164
Izawa K (1979) Studies on peculiar distribution pattern of Callimico. Kyoto University Overseas Research Reports of New World Monkeys 1:1–19
Janson CH (1988) Food competition in brown capuchin monkeys (Cebus apella): quantitative effects of group size and tree productivity. Behaviour 105:53–76
Kappeler PM (1998) Nests, tree holes and the evolution of primate life histories. Am J Primatol 46:7–33
Kinzey WG (1981) The titi monkeys, genus Callicebus. In: Coimbra-Filho A, Mittermeier R (eds) Ecology and behavior of Neotropical primates. Academia Brasileira de Ciencias, Rio de Janeiro, pp 241–276
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Lourenço de Oliveira R, Luz SLB (1996) Simian malaria at two sites in the Brazilian Amazon. 2. vertical distribution and frequency of anopheline species inside and outside the forest. Mem Inst Oswaldo Cruz 91:687–694
Mittermeier RA, Rylands AB, Coimbra-Filho AF, Fonseca GAB (1988) Ecology and behavior of Neotropical primates 2. World Wildlife Fund, Washington
Møller AP, Dufva R, Allander K (1993) Parasites and the evolution of host social behavior. Adv Study Behav 22:65–102
Mooring MS, Hart BL (1992) Animal grouping for protection from parasites: Selfish herd and encounter-dilution effects. Behaviour 123:173–193
Nunn CL (2002) A comparative study of leukocyte counts and disease risk in primates. Evolution 56:177–190
Nunn CL, Gittleman JL, Antonovics J (2000) Promiscuity and the primate immune system. Science 290:1168–1170
Pålsson K, Jaenson TGT, Dias F, Laugen T, Bjorkman A (2004) Endophilic Anopheles mosquitoes in Guinea Bissau, West Africa, in relation to human housing conditions. J Med Entomol 41:746–752
Patz JA, Graczyk TK, Geller N, Vittor AY (2000) Effects of environmental change on emerging parasitic diseases. Int J Parasitol 30:1395–1405
Peres CA (1991) Ecology of mixed-species groups of tamarins in Amazonian terra firme forests. Ph.D. thesis, University of Cambridge
Petraitis PS, Dunham AE, Niewlarowski PH (1996) Inferring multiple causality: the limitations of path analysis. Funct Ecol 10:421–431
Port GR, Boreham PFL, Bryan JH (1980) The relationship of host size to feeding by mosquitos of the Anopheles gambiae Giles complex (Diptera, Culicidae). Bull Entomol Res 70:133–144
Porter CA, Page SL, Czelusniak J, Schneider H, Schneider MPC, SAmpaio I, Goodman M (1997) Phylogeny and evolution of selected primates as determined by sequences of the epsilon-globin locus and 5' flanking regions. Int J Primatol 18:261–295
Purvis A (1995) A composite estimate of primate phylogeny. Phil Tran Roy Soc Lond B 348:405–421
Purvis A, Rambaut A (1995) Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. Comp Applic Biosci 11:247–251
Reeve J, Abouheif E (2003) Phylogenetic Independence. 2.0 edn. McGill University, Montreal
Ribbands CR (1949) Studies on the attractiveness of human populations to anophelines. Bull Entomol Res 40:227–238
Rice WR, Gaines SD (1994) Heads I win, tails you lose - testing directional alternative hypotheses in ecological and evolutionary research. Trends Ecol Evol 9:235–237
Rubiopalis Y, Curtis CF (1992) Biting and resting behavior of anophelines in western Venezuela and implications for control of malaria transmission. Med Vet Entomol 6:325–334
Rylands AB (1981) Preliminary field observations on the marmoset, Callithrix humeralifer intermedius (Hershkovitz, 1977), at Dardanelos, Rio Aripuana, Mato Grosso. Primates 22:46–59
Schneider H (2000) The current status of the New World monkey phylogeny. Anais Da Academia Brasileira De Ciencias 72:165–172
Smith AC (1997) Comparative ecology of saddleback (Saguinus fuscicollis) and moustached (Saguinus mystax) tamarins. Thesis, University of Reading
Smith RJ, Jungers WL (1997) Body mass in comparative primatology. J Hum Evol 32:523–559
Soini P (1988) The pygmy marmoset, Cebuella. In: Mittermeier RA, Rylands AB, Coimbra-Filho AF, Fonseca GAB (eds), vol 2. World Wildlife Fund, Washington, pp 79–129
Stevenson MF, Rylands AB (1988) The marmosets, genus Callithrix. In: Mittermeier RA, Coimbra-Filho AF, da Fonseca GAB (eds) Ecology and behavior of Neotropical primates, vol 2. World Wildlife Fund, Washington, DC, pp 131–222
Taliaferro WH, Taliaferro LG (1934) Morphology, periodicity and course of infection in Panamanian monkeys. Am J Hyg 20:2–49
Tella JL (2002) The evolutionary transition to coloniality promotes higher blood parasitism in birds. J Evol Biol 15:32–41
Valderrama X, Robinson JG, Attygalle AB, Eisner T (2000) Seasonal anointment with millipedes in a wild primate: A chemical defense against insects? J Chem Ecol 26:2781–2790
Voorham J (2002) Intra–population plasticity of Anopheles darlingi's (Diptera, Culicidae) biting activity patterns in the state of Amapa, Brazil. Revista De Saude Publica 36:75–80
Webber LA, Edman JD (1972) Anti-mosquito behavior of ciconiiform birds. Anim Behav 20:228–232
Acknowledgments
We thank V. Ezenwa, P. M. Kappeler, C. Brown and three anonymous reviewers for helpful suggestions. This project was supported through funding from the NSF (Grant #DEB-0212096 to CN).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Brown
Electronic supplementary material
Tree 2
Phylogenetic tree of New World primates based on Porter et al. (1997), Schneider (2000) and Goodman et al. (1998). This tree is referred to as PSG in Table 2. Numbers indicate branch length in Ma. In addition to branch length differences, which were based on information in the references, the major differences to the Purvis tree involve the position of Aotus, the internal arrangement of the marmosets (Callithrix, Cebuella, Mico), and arrangement of species in the clade containing Ateles.
Rights and permissions
About this article
Cite this article
Nunn, C.L., Heymann, E.W. Malaria infection and host behavior: a comparative study of Neotropical primates. Behav Ecol Sociobiol 59, 30–37 (2005). https://doi.org/10.1007/s00265-005-0005-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0005-z