Abstract
Female Polistes paper wasps are capable of independent nesting, yet many populations demonstrate a mixture of solitary and cooperative nest foundation. Previous studies of Polistes have found survival and/or productivity advantages of cooperative nest foundation compared to solitary nesting, and reproductive skew models have been designed to predict the dynamics of such flexible cooperation. In this paper, we examine the success of different nesting strategies in a previously unstudied population of Polistes aurifer in southern California. The colony cycle of this population is less synchronous than that of other temperate species, and the frequency of solitary nesting averages 86.2%. Our data suggest that this low rate of cooperative nest founding is adaptive, as demonstrated by the lack of survival or productivity advantages for cooperative foundress associations. Due to foundress turnover and nest foundation later in the season, many nests produce only one set of offspring. This results in a loss of the eusocial nature of some nests in the population. Data from a small sample of multifoundress nests show significant positive reproductive skew, despite concession model predictions that skew should be low in populations with low ecological constraints on independent nesting. This lack of support for the concessions skew model reflects a diminished incentive for cooperation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major challenge for the study of social evolution is explaining why some individuals accept subordinate roles in cooperative groups instead of breeding independently. Polistes paper wasps have provided fertile ground for investigation of this subject, mainly because of the flexibility in nest founding behavior by mated females called foundresses. In the temperate Polistes colony cycle, foundresses emerging after winter diapause have three main nesting options: (1) initiating a nest solitarily, (2) joining a cooperative nesting association, or (3) usurping or adopting an existing nest. Usurpers usually take over a nest before offspring emergence, where they tend to destroy eggs and early stage larvae but spare late stage larvae and pupae of the original foundress (Makino 1985; Klahn 1988). Multiple potential egg-layers may therefore be present on the nest either simultaneously (in foundress associations) or sequentially (via usurpation or adoption).
Many studies have investigated possible advantages to cooperative nest foundation in Polistes wasps (reviewed in Reeve 1991; Queller 1996). For example, solitary foundress nests may face higher rates of usurpation (Gamboa 1978; Klahn 1988), primarily because the nest is left unattended more often while the foundress forages (Metcalf and Whitt 1977; Gamboa et al. 1992). Although evidence suggests that predation and parasitism rates do not differ between solitary and multiple foundress nests (Gamboa 1978; Gibo 1978; Strassmann et al. 1988), foundress associations may be more likely to rebuild their nests after a predation event (Gibo 1978; Strassmann et al. 1988). This advantage may be attributed in part to the lower probability that two or more foundresses will die before offspring emergence (Reeve 1991). Indeed, several studies have shown that solitary nests are more likely to fail due to loss of the foundress (Metcalf and Whitt 1977; Gibo 1978; Tibbetts and Reeve 2003). Studies have also reported higher productivity of multiple foundress nests relative to solitary nests, although this advantage is not usually realized per capita (Gibo 1978; Gamboa 1980; Shreeves et al. 2003; Tibbetts and Reeve 2003). Cooperating foundresses are often closely related, thus providing potential inclusive fitness benefits to subordinates that sacrifice direct reproduction (reviewed in Reeve 1991). However, nesting associations in some populations do include unrelated foundresses (e.g., Queller et al. 2000; Reeve et al. 2000). Fitness benefits for subordinates in such arrangements would require direct reproduction, either shared with the resident dominant or via inheritance of the dominant position (Kokko and Johnstone 1999; Ragsdale 1999). Delayed inheritance benefits have been suggested as a possible adaptive explanation for unrelated, nonreproducing subordinates in an Italian population of P. dominulus (Queller et al. 2000).
Reproductive skew models attempt to integrate the potential survivorship and productivity benefits of cooperation to predict when groups should form, and under what level of skew subordinates with the option to breed solitarily should stay in a group (reviewed in Johnstone 2000; Reeve 2000). The level of skew indicates the degree of sharing of reproduction among group members, with lower skew values indicating a more equitable distribution. Skew models have three main parameters: x, the expected reproductive success a potential subordinate would have as a solitary breeder relative to a dominant group member on its own; k, the advantage to being in a group as measured by the relative productivity of a group compared to a solitary individual; and r, the genetic relatedness between dominant and subordinate group members.
The skew model with assumptions most appropriate for Polistes wasp societies is known as the “concessions” model, in which dominants control group membership and allocate reproduction by yielding some egg-laying to subordinates as incentives to stay peacefully in the group (Reeve and Ratnieks 1993; Johnstone 2000; Reeve 2000). The model predicts that the minimum amount of direct reproduction a subordinate requires to cooperate will increase with decreasing values of k and r, and increasing values of x. When a population has weak ecological constraints on independent nesting (i.e. x=1), the productivity of two-foundress nests must be more than twice that of solitary foundress nests for cooperative associations to be stable, assuming equal sharing of reproduction (Nonacs 2002; calculated according to Reeve and Ratnieks 1993, and Johnstone 2000). The minimum k required for cooperation when the subordinate receives less reproduction is even higher. Nonacs (2002) incorporated the possibility of split sex ratios into the skew model framework, such that solitary foundresses produce an optimal 3:1 female biased sex ratio while multiple foundress brood ratios are 1:1. If foundresses perfectly manipulated brood sex ratios to fit this model, k would only need to exceed 1.167 to favor a subordinate joining a full sister with no direct reproduction. Thus, even with split sex ratios, the model predicts that there must be some minimum value of k to promote cooperation in a population of Polistes wasps.
Adequate testing of skew model predictions within a given population requires accurate information about constraints on independent breeding (x), productivity advantages to cooperation (k), and relatedness among group members (r). Accurate assessment of these parameters can only be accomplished by intensive studies of natural populations. The numerous species of Polistes wasps are ideal for such studies because they are found in diverse habitats that may coincide with variation in life history traits. Much of the work examining advantages of cooperative nest founding has been conducted in cool temperate Northeastern or Midwestern USA, or subtropical Texas, USA, both of which have rainy summers. Studies of warm temperate European field populations of P. dominulus (Queller et al. 2000, Shreeves et al. 2003) have provided interesting information about cooperative groups where summer drought is common, but these have not explicitly reported on the success of solitary and usurper/adopter founding strategies and only Queller et al. (2000) used genetic techniques to examine relatedness and offspring production. Studies comparing solitary and cooperative founding are impossible in other warm temperate species from Japan that have 100% solitary founding (e.g., P. chinensis, P. jadwigae; reviewed in Queller 1996; Yamane 1996).
Here we combine behavioral, demographic, and genetic data to examine the relative success of solitary and cooperative nesting strategies in a previously unstudied population of P. aurifer in southern California. The climate in this region is very mild, and warm temperatures can make nesting activity possible from late February through early October. However, the nesting season in this region also coincides with the driest months of the year, so often no rain falls at all during the entire colony cycle and thus prey availability probably decreases as the season progresses. We first describe the colony cycle of this population, and then we specifically examine the relative success of solitary, adoption/usurpation, and multiple foundress nesting strategies in terms of colony survival and individual offspring production. On multiple foundress nests, we also measure reproductive skew and relate the results to concession model predictions.
Methods
Study site
We conducted this study in grassland areas of the University of California, Los Angeles (UCLA) Stunt Ranch Reserve in the Santa Monica Mountains (34°6′N–118°39′W, elevation 244–488 m) during spring and summer 1999–2001. This area, which also includes chaparral, coastal sage scrub, oak and riparian woodland habitats, receives an average of 61 cm annual rainfall, but this amount varies greatly across years. For the study period of 1999–2001, rainfall in the Santa Monica Mountains averaged 36.6 cm and ranged from 20.8 to 52.6 cm (Table 1). No rain fell during the study period, although the ground was often blanketed with a layer of fog until just after sunrise. Temperatures during the nesting season ranged from 10 to 41°C.
Monitoring nest activity
In this P. aurifer population, nests are naturally initiated inside cracks in the soil that form anew each spring when the ground hardens after the winter rainy season (Liebert 2004). These ground nests are very difficult to observe, as the cracks are covered with a thick mat of mostly dried grasses; thus we used wooden nest boxes to attract nesting wasps. For the 1999 season, we mounted 50 boxes on 1-m wooden stakes or metal fence posts on a south-facing slope. The boxes had removable lids, a 3.5-cm-diameter hole in the front, and a 5.5-cm hole on the bottom to allow videotaping of the nest without opening the box. We began monitoring wasp activity during late March and continued to census the boxes every 2–4 days throughout the spring and summer until nesting activity ceased in early fall.
Censuses were conducted in the early morning while temperatures remained below 21°C; at this time, the wasps were sluggish and all nest residents were likely to be present. At each census we attempted to record the identity of each wasp in the box as well as the size and brood composition of the nest. After immobilizing unmarked wasps by briefly chilling them in an icepack, we applied a unique mark on the thorax using Testors enamel paint and measured head width and wing length with dial calipers. The wasp was allowed to recover from being chilled, then placed back in the box. We repeated this procedure for two more nesting seasons, adding 50 more boxes in another grassland area of the reserve for a total of 110 boxes for 2000 and 2001. While marking wasps in 2000 and 2001, we also clipped a small (~5 mm) piece of the tarsus of one middle leg and immediately placed the leg tissue into a 1.5-ml tube filled with 1 ml of acetone for later DNA extraction (Fukatsu 1999; Starks and Peters 2002).
Definition of terms
Because Polistes wasps can assume different roles and nests progress through multiple stages during a typical colony cycle, it is necessary to define the terms we will use to categorize individuals and nests. A female that initiates or joins a nest in the period before offspring emergence is referred to as a foundress, and this period is called the pre-emergence phase. To be classified as a multiple foundress nest, at least two foundresses must be present at the majority of censuses. Nests initiated within a week of the first observed offspring emergence in a given season will be referred to as late-season nests. Due to the rarity of witnessing actual foundress replacement events, females that usurp the original foundress by force and those that take over an orphaned nest are both referred to as adopters. A nest on which offspring have emerged is considered to be in the post-emergence phase, and we will refer to adult females that have emerged within a given season as “offspring” to distinguish them from foundresses. Early female offspring are those that emerge within 10 days after the first offspring emergence on that nest.
Videotaping
To confirm identities of nest residents and to observe egg-laying and foraging behavior, we videotaped as many nests as possible during both pre-emergence and post-emergence stages. Pre-emergence nests were taped for an average of 9.9 h and post-emergence nests averaged 7.3 h. We videotaped nests between 1000 hours and 1600 hours on days with an average temperature above 23°C, because wasp activity levels were likely to be highest under these conditions.
Experimental nest adoptions
Naturally occurring usurpations and adoptions are often difficult to locate and observe. In order to more closely monitor the success of adopters, we experimentally simulated this condition using experimental enclosures. Just before offspring emergence, we removed the resident foundress on 14 nests, and transplanted the nests to the enclosures to be adopted by “floater” foundresses that had been captured while foraging on the UCLA campus. The enclosures were built of cement and chain-link fencing, which were covered with aluminum and fiberglass insect screening to prevent escape but allow exposure to ambient light and temperatures. Empty nest boxes were attached around the perimeter of the enclosures at a height of 1.8 m, spaced 0.6–0.8 m apart. Naturally initiated P. aurifer nests at Stunt Ranch Reserve have been observed as close as 0.6 m (Liebert 2004), so this type of aggregation can be found in natural habitat. Wasps were supplied with fresh water, flowering potted plants, honey or sugar water, and wax moth larvae (Galleria mellonella), ad libitum. Moth larvae were placed in separate containers as well as sprinkled on the plants in an attempt to maximize successful prey discovery by foraging wasps. We marked and genotyped all emerging offspring from these transplanted nests as well as two nests initiated by floater foundresses in the enclosures using the methods described above. These genetic data were included in analyses of offspring production and reproductive skew.
Identification codes used in this paper indicate the origin and location of nests. Each code contains two letters that indicate whether the nest was left in the field (F), transplanted to the enclosure (T), or initiated in the enclosure (EN); additional letters indicate whether the nest was originally initiated in a nest box (B), shed (S), kiosk (K), or underground in soil cracks (G) at Stunt Ranch Reserve.
Genetic analysis
To ensure accurate measurement of individual productivity, we conducted genetic analyses of all foundresses and emerging offspring. We extracted genomic DNA from leg tissue using the procedure of Starks and Peters (2002), modified from Peters et al. (1995). We then genotyped wasps at six polymorphic microsatellite DNA loci using primers developed in the lab of J. Strassmann and D. Queller at Rice University (Table 2). Three primers were derived from P. bellicosus (Strassmann et al. 1997) and three from P. dominulus (Henshaw 2000). Polymerase chain reaction (PCR) protocols were slightly modified from Strassmann et al. (1996; see Table 2 for PCR conditions). Allele sizes were visualized using the CEQ 2000 fragment analysis system (Beckman Coulter, Fullerton, Calif.). Samples were included in subsequent data analysis only if genotypes were available for at least three loci. Genotyping of the original foundresses on two nests (FB1 and FS1) was unsuccessful, but in both cases we were able to reconstruct the probable foundress genotype based on groups of full sister offspring that were produced at the expected time after egg-laying was observed. Ninety-six percent of foundresses (N=77) and 92.6% of offspring (N=230) were successfully genotyped at a minimum of three loci.
Offspring assignment
Since wasps are haplodiploid, females have two alleles per locus while males have only one. If a foundress is singly mated, as is the case for all published studies of mate number in polistine wasp species (Strassmann 2001), female offspring will share the same paternal allele and will have one of the two maternal alleles at each locus. This makes assigning maternity a generally simple process even without access to the paternal DNA. To assign female offspring to a particular foundress, we first manually inspected the data and excluded candidate foundresses whose genotypes did not contain alleles present in the offspring. We then manually grouped offspring whose genotypes were consistent with haplodiploid full sisters; that is, they all contained one of the same allele and one of only two possible maternal alleles at each locus. To account for the possibility that the offspring could share this set of genotypes by chance rather than by descent, we used Kinship 1.3.1 (Goodnight et al. 1999) to calculate the likelihood ratio that each pair of individuals were haplodiploid full sisters versus non-relatives or maternal cousins. Significance levels for the likelihood ratio were based on simulations of 5,000 pairs of individuals with genotypes determined according to given population allele frequencies. We used the program Relatedness 5.0.8 (Goodnight and Queller 1999) to calculate relatedness values based on the genotype data. Since relatedness is calculated relative to background population allele frequencies, the inclusion of sibling groups that share the same alleles can artificially decrease relatedness values. We therefore used the bias correction feature of Relatedness to weight nests equally, thus excluding nestmates of each focal individual during calculation of population allele frequencies. These allele frequencies were then used for the calculation of pairwise and within-nest relatedness values (based on Queller and Goodnight 1989). Standard errors were calculated by jackknifing over loci.
Maternity assignment was mostly straightforward. However, one potential complication in this study is that some of the latest emerging offspring on productive nests could have been produced by early female offspring, rather than the nest foundress. For late female offspring, this would require that the early female’s mate had the same genotype as her father. A measure of the probability that the mate and father have identical haploid genotypes is given by dp (Boomsma and Ratnieks 1996), and is calculated according to the following equation:
where qi2 is the squared frequency of the ith allele at one locus, summed over all alleles per locus and multiplied across loci. This is a conservative measure of the probability that an early female offspring produced a late female offspring (Paxton et al. 2002), because it does not account for the added requirement that the late offspring genotype must be a heterozygote. (A homozygote would unambiguously be assigned to the early female, because it would have alleles from both the early offspring’s mate and the paternal allele from the foundress’ mate). For both 2000 and 2001 population allele frequencies, dp<0.00003. This very low value of dp suggests that late female offspring production by early female offspring is extremely unlikely. We therefore assigned late female offspring to original foundresses even if there were matching early female offspring genotypes. Since males receive only a maternal allele, the presence of any alleles belonging to the foundress’ mate is sufficient to exclude the foundress as a candidate mother. However, if the males have the maternal alleles present in both the foundress and the early female offspring at all loci, the male’s origin cannot be determined with certainty. This situation arose only for three late emerging males on one nest (FB9). Because the probability that an early female-produced male would have only the maternal allele at all six loci is (0.5)6, or 0.016, we have assigned the males in question to the foundress rather than the early female offspring.
Measuring reproductive skew
Many indices are available to calculate reproductive skew, but most do not account for expected skew due to random processes or differences in length of group membership, or are too sensitive to per capita group productivity (Tsuji and Tsuji 1998; Nonacs 2000, 2003a; Tsuji and Kasuya 2001). To minimize these problems, we used the binomial skew index (B), which has the fewest problems such as numerical discontinuities, and is sensitive to group productivity and differential presence within the group. It is calculated with the formula:
where: pi = proportion of total reproduction for ith member; ni = time in the group of ith member; \(N_{{\text{t}}} = {\sum\limits_{i = 1}^N {n_{{\text{i}}} } }\); (N=group size; if all ni are equal, ni=1 and Nt=N); and K=total offspring by the entire group.
The index is bounded by a minimum value of −1 and a maximum value of 2. When group members share reproduction equally, the index value is equivalent to (1/N̄−1)/K, where N̄ is a weighted mean group size equal to N t /nmax, and nmax is the maximum time any individual could be present. Random distribution gives an index value of zero, and complete monopoly of reproduction equals:
where reproduction is monopolized by an individual i=1.
As the measure of time spent within the group, we used the number of days each group member was present during the offspring production period. Significance levels for the B index were calculated from 1,000 simulations. For ease of comparison with other studies, we calculated two additional indices: the standardized Morisita Ip index (Tsuji and Tsuji 1998), which ranges from −1 (no skew), to 0 (random distribution), to +1 (maximum skew); and the Sc index (Keller and Krieger 1996), which ranges from 0 (no skew) to 1 (maximum skew). All indices were calculated using Skew Calculator 2003, version 1.2 (Nonacs 2003b; http://www.obee.ucla.edu/Faculty/Nonacs/).
Statistical analysis
Data were analyzed using either JMP 4.0.4 (SAS Institute) or StatView 4.5. Proportions were arcsine-transformed before use in parametric tests. Because of the highly skewed nature of the offspring production data, we used non-parametric tests for analyses where data could not be transformed to meet the assumptions of parametric tests. All tests are two-tailed and means are reported with standard errors unless otherwise stated.
Results
Nest foundation
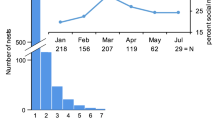
From 1999 to 2001, 47 P. aurifer nests were initiated in nest boxes. Nine additional nests were located in a storage shed and two were in a small wooden kiosk, for a total of 58 nests monitored over the 3-year field study. The first nests of the season appeared in March or April, but nests were also initiated in May, June, and as late as July (Fig. 1). For all 3 years of the study, the majority of nests (86.2%) were attended by a solitary foundress. The infrequent foundress associations ranged in size from two to six, and this number often changed throughout the founding stage as females joined or left the group. During the early stages of nest initiation, changes in identity of the resident foundresses on solitary nests were also common due to adoption of abandoned nests or usurpation of an active nest by a new female. We observed a foundress turnover event on 19% of nests over 3 years (including only nests with at least ten cells; 1999:11.1%, N=9; 2000:29.4%, N=17; 2001:13.6%, N=22).
Because nests initiated later in the season sometimes occurred after the first offspring emergence, it is possible that some nests were initiated by early female offspring rather than foundresses that had lost their nests or left foundress groups. The dark eyes and low wing wear of some late nest foundresses and floater females found resting in nest boxes suggests this possibility because both are indicators of recent eclosion. Consequently, to document early female reproduction, we compared head widths of foundresses that initiated mid- or late-season nests to measurements from known spring foundresses and early female offspring. Although there is some overlap, the distribution of early spring foundress head widths (3.88±0.034, N=57) is significantly larger than both the early female offspring distribution (3.49±0.027, N=61) and the unassigned foundresses (3.60±0.055, N=14); in contrast, the head width distributions of early females and unassigned foundresses do not differ significantly (Tukey-Kramer HSD test, P<0.05). This suggests that rather than working, some early P. aurifer females pursue opportunities for independent nesting soon after emergence.
Nest success
Most nests did not succeed in producing offspring. Mean nest survival to offspring emergence was 43.1% over 3 years (Table 1), and this rate was similar across years (χ2=0.440, N=58, P=0.8025). Although nests with a solitary foundress were left unattended more of the time than multiple foundress nests (one-tailed t-test: t=2.03, df=35, P<0.001), multiple foundress nests had no survival advantage (one-tailed Fisher’s exact test: N=58, P=0.6042). There also was no difference in nest success based on timing of nest initiation (early vs late, with “late” defined as nest initiation within 1 week of the first offspring emergence of the season; Fisher’s exact test: N=58, P=0.4898). This result does not differ if the data are broken down by nest initiation date or if early and late categories are used; nest survival is unrelated to the date of nest initiation (Wilcoxon 2-sample test: N=57; S=609.5, Z=−1.435, P=0.151).
Nests failed for a variety of reasons, but most common was foundress disappearance (63.6% of failed nests; N=33). In the field, it is often impossible to know whether the foundress died while off the nest or if she chose to leave, which might occur if, for example, the brood became infected by a parasitoid. In a few cases, the missing foundress was observed to join or visit other nests, so this abandonment may sometimes be voluntary. However, there are also orb-weaving spiders and insectivorous birds at the site that could prey on foraging wasps. A nest is considered to have failed due to predation if it was found completely missing, shredded, or perforated (usually by ants), and the foundress had been present at the previous census. This occurred on only 5 (15.2%) of 33 nests. We did not observe any nest rebuilding after predation, although we would have missed any rebuilding attempts outside of the boxes. Nests that were joined or adopted by immigrant females were almost equally likely to fail (N=7) or succeed (N=6) in producing offspring following this change in foundress status.
Offspring production period
The offspring production period was relatively short for most nests. On 60.7% of nests (N=28), all offspring were produced within 30 days. The production period ranged from 40 to 102 days on the remaining nests, although on only two nests was it longer than 90 days. The first offspring emerged 61.9±5.14 (X±SE) days after nest initiation, so total development time from egg to adult falls within the development range observed in other Polistes species (Reeve 1991). Also, as reported for other species (West-Eberhard 1969), the time from nest initiation to offspring emergence decreased significantly as the season progressed (Spearman rank correlation: N=24, ρ=−0.585, P=0.003).
Compared to most Polistes species, P. aurifer nests are small (44.5±23 cells) and produce relatively few offspring. The total offspring produced per nest ranged from 1 to 28 and was highly variable. Surprisingly, multiple foundress nests produced no more offspring on average than solitary nests (10.5±3.28 vs 10.8±2.70, respectively; includes nests producing at least one offspring and excludes transplanted nests; t-test: t=0.2719, df=13, P=0.6596). Therefore, members of multiple foundress groups generally had lower per capita productivity than solitary foundresses. On average, solitary foundresses foraged more often for prey than groups on multiple foundress nests, even though the latter had more potential foragers (0.0060±0.00114 vs 0.0028±0.00127 items/h; median 2-sample test, S=1, Z=−2.089, P=0.037).
The first emerging offspring were always female and all but one nest had a female-biased sex ratio (this nest was initiated by uninseminated females in the enclosure and therefore produced all males). Male production varied across the remaining nests, with many producing only females and others producing up to 43% males. The two successful late nests initiated after offspring emergence at Stunt Ranch produced only two to three female offspring, but many nests initiated early in the season also produced only a few offspring in one bout due to loss of the nest or foundress. In almost all cases, nests were visited by many unrelated males and some females after mid-July, even while nest residents were still actively rearing brood. These males were usually chased away at first, but eventually many males and sometimes females would gather on the nest, at which point brood care ceased.
Solitary foundress offspring production
Genetic data confirmed the expectation that solitary foundresses were singly mated and most produced all the offspring on their nests. Overall mean relatedness of female offspring on 22 solitary foundress nests was 0.66, with individual nest values ranging from 0.26 to 0.82 (Fig. 2). On 15 nests, the genotype of the original foundress was consistent with having produced all of the offspring. This is supported by Kinship pairwise relatedness values for female offspring on these nests, all of which are significant at P<0.01 for full siblings (vs null hypothesis of zero relatedness; after simulation of 5,000 pairs). Although nests TB1 and FB2 have relatively low average offspring relatedness (r=0.55 and 0.53 respectively; Fig. 2), visual inspection of the genotypes and Kinship results are consistent with a relationship of full sisters produced by the original nest foundress. The relatively low r-value is apparently generated by the fact that the offspring on these nests have different maternal alleles at five of six loci. Thus, all offspring from these 15 nests were most likely to have been produced by one singly mated foundress.
Mean relatedness among female offspring on solitary foundress nests with and without foundress replacement. Bars represent 95% confidence intervals and numbers under x-axis show number of female offspring per nest. Black line represents expected 0.75 relatedness value for haplodiploid full sisters. Asterisk indicates Kinship significance of all offspring as full sisters at P<0.01 for nests with this symbol next to the name on the x-axis. Key to nest identification codes: The letters indicate whether the nest was left in the field (F), transplanted to the enclosure (T), or initiated in the enclosure (EN); additional letters indicate whether the nest was originally initiated in a nest box (B), shed (S), kiosk (K), or underground in soil cracks (G) at Stunt Ranch Reserve. Numbers simply allow for individual nest identification within location categories
Overall mean relatedness among female offspring did not differ between nests with foundress replacement and those where no replacement was observed; Wilcoxon 2-sample test: S=69, Z=−0.775, P=0.438. On nests where the original foundress was replaced by an adopter, the mean proportion of offspring produced by the original foundresses (0.78±0.06) was significantly higher than the mean for the adopters (0.03±0.05); Wilcoxon 2-sample test: S=537, Z=4.66, P<0.0001. Two of the adopted nests were natural occurrences on unmanipulated nests at Stunt Ranch, and the others were experimental enclosure nests. The Stunt Ranch sample size (N=2) is too small for a separate statistical analysis, but the pattern is the same as for the enclosure nests. Adopter females also produced significantly fewer offspring on average (0.4±0.45; N=18) than late season nest foundresses (1.5±0.53, N=13); Wilcoxon 2-sample test: S=257.5, Z=2.45, P=0.014. Overall, adopter females successfully reproduced on only 2 of the 14 nests where such a replacement was observed and were therefore less successful than original foundresses.
On eight solitary nests, genetic data revealed offspring with genotypes that lacked alleles from either the original or adopter foundress at a minimum of two loci (hereafter referred to as “foreign offspring”). Two of these nests (FB6 and TB8) had been adopted, but the foreign offspring genotypes did not match the original or the adopter foundress. Three solitary nests with no observed foundress turnover (FS1, FB1, and TG4) also contained lone foreign offspring from different matrilines among the early females (Fig. 3). The relatedness of these foreign offspring to the other offspring on the nest does not differ significantly from zero, according to Kinship simulations. All wasps were marked as adults after emergence, so it is possible that the unassigned foreign offspring were immigrant joiners rather than the progeny of an early foundress that disappeared. Care was taken at censuses to identify newly eclosed offspring by their low wing wear and dark eyes, as well as to match the number of new unmarked adults with the number of pupal caps opened since the previous census. However, because censuses were not conducted daily, we cannot rule out the possibility of immigration as the source of these foreign offspring.
Division of reproduction on solitary nests with no observed foundress turnover and multiple foundress nests. Open areas show the number of offspring attributed to the original (observed) foundress; shaded areas are offspring produced by additional foundresses, with each shade representing a different matriline. Numbers at base of bars indicate the number of foundresses observed on each nest. Bar length indicates the total number of offspring genotyped for each nest. The lone offspring attributed to a second foundress on EN1 could belong to the dominant egg-layer (see Results). One offspring each on nests FB8 and FB11 did not match genotypes of any candidate foundresses; these could be immigrant joiners rather than natal offspring
On two nests with no observed foundress replacement (FB1 and FS1), large foreign sibling groups were among the early emerging offspring (Fig. 3); this suggests that the original foundress had disappeared soon after laying eggs. The foundress observed upon discovery of the nest may therefore have been an adopter, usurper, or the only remaining member of an early cooperative foundress group that disassociated. In any case, this foundress cared for many larvae that were not her own offspring. These data indicate that changes in foundress status can occur in the early stages of nest foundation, but that disappearance or death at this stage does not necessarily result in total loss of offspring.
Multiple foundress nests and reproductive skew
All six foundress associations contained non-relatives among the group members. This is shown by the fact that none of the foundress groups had an average relatedness at the level of full sisters (overall mean r: 0.131±0.08; range: −0.043 to 0.395), although four nests contained subsets of apparent sisters. The foundresses on nest EN1 comprised three sets of early female sisters that had left their natal nests in the enclosure. Two of the three foundresses genotyped on nests EN2 and FB8 were related at a level equivalent to full sisters but unrelated to the third foundress, and one pair of putative sisters was among the seven non-reproducing foundresses on nest FB9. None of the foundresses on nests FB10 or FB11 were related at a level significantly different from zero. On all nests except EN1, the foundress with the most offspring did not have any co-foundress sisters.
The number of foundresses contributing to offspring production varied across nests, as demonstrated by the wide range of mean female offspring relatedness (0.14–0.74; X±SD: 0.51±0.24). On two nests (FB10 and FB9), Kinship simulation results supported a full sibling relationship for the female offspring (vs zero relatedness; P<0.001). In addition, all male offspring were assigned to the same foundress that produced the female offspring. These two nests therefore had only one reproducing foundress, despite the presence of three and eight potential egg-layers, respectively. Two additional nests may also have had only one reproducing foundress. On nest EN1, seven of nine male offspring can be attributed only to one of the eight foundresses. This same foundress could have also produced the other two males, but one other candidate foundress is also a match. To be conservative, we assigned each foundress one of the two males in question. Nest FB11 contained one foreign offspring whose genotype did not match any of the collected foundresses. If this individual were an immigrant, then all offspring would be attributed to one foundress. This leaves only two of six multiple foundress nests (EN2 and FB8) where more than one reproducing foundress were clearly present (Fig. 3).
The three skew indices all produce a wide range of skew values among the six multifoundress nests (Table 3). On five of six nests, B index values were significant at P<0.05, indicating positive skew relative to a random distribution (Table 3). Skew values did not correlate significantly with average foundress relatedness (Spearman rank correlation, ρ=−0.429, P=0.396). Of the three nests that produced both early and late offspring, two had higher skew values for early offspring and one nest had a higher value for late offspring (Table 3). The timing of nest initiation also did not relate to skew; although the sample size was small, the two late-initiated nests (EN1 and EN2) represented the extreme high and low B index values of all six nests (Table 3). Thus, there was significant positive reproductive skew on five of six nests, but no clear relationship between skew and relatedness, timing of offspring production, or timing of nest initiation.
Relative success of different nesting strategies for individual fitness
Of three possible nesting strategies (solitary founding, adoption/usurpation, and multiple founding), solitary foundresses produced significantly more offspring on average (3.9±0.94) than both multiple foundresses (1.3±0.52) and adopters (0.4±0.38). Multiple foundresses had higher average offspring production than adopters, but the difference was not significant (Wilcoxon 2-sample test, solitary vs multiple: S=284, Z=−1.972, P=0.0486; solitary vs adopter: S=177, Z=3.004, P=0.0024). These values include foundresses on failed nests (zero productivity), so survivorship is incorporated into the analysis.
Discussion
Ecology of colony cycle
The colony cycle of P. aurifer is less synchronous than other temperate species. It is more similar to the “short-term” life cycle described by Yamane (1996) for subtropical and tropical species such as P. japonicus of Taiwan, in which the colony cycle lasts less than 6–7 months, nests are generally small (40–100 cells) and have low (10–30) numbers of workers. Because the dry season always coincides with the period of offspring production, limited prey availability may set a limit to the length of the nesting season. The 3 years of this study had below average rainfall, so it is possible that nesting behavior and offspring production could differ in years with high rainfall or explosive growth in Lepidopteran populations. However, drought years occur regularly enough that this might be a driving selective force shaping life history characteristics. The long period of warm temperatures combined with low nest productivity leads to the “blinking on and off” of nests throughout the colony cycle, with nests at different stages existing simultaneously. Solitary founding, usurping/adopting, and multiple founding strategies all coexist within this population. However, adopters/usurpers have very low fitness compared to those that start their own nests. Because of our use of enclosures, the adopter fitness data may be biased; however, the reduced predation risk and easy access to resources in the enclosure is likely to have overestimated adopter success. Since nests can be initiated and produce offspring 3–4 months after the onset of nest building, and most nests produce fewer than ten offspring anyway, the optimal choice for individual females capable of building a nest is to pursue independent reproduction.
Reduction of eusocial characteristics
When nests are destroyed by predators or usurped by new foundresses, a pool of floater females is created that may join or usurp existing nests, or start their own. Thus, the change in foundress status cascades throughout the population. Because most predation and usurpation events are likely to occur when nests are filled with late stage brood, early female offspring may also soon join this pool of females seeking opportunities for independent nesting late in the season. Our data support this idea, as we observed one offspring-initiated nest in the enclosure and we detected several possible immigrant joiners among offspring groups. In addition, the size distribution of late nest foundresses in the field suggests this phenomenon occurs under natural conditions. The eusocial character of the colony cycle is therefore lost for some nests that produce one short “burst” of offspring rather than two sets of “worker” and “reproductive” brood. If foundresses are better off nesting alone, and nesting opportunities exist late in the season, then it makes sense that offspring should pursue options for independent nesting where possible. This is not a complete loss of eusociality, but is the coexistence of eusocial and solitary nests within the same population, which until now has only been observed in halictine, augochlorine and some allodapine bees. The sweat bee species Halictus rubicundus and Lasioglossum (Evylaeus) calceatum both nest solitarily in high elevation habitat but are social nesters in lower elevation sites (Wcislo and Danforth 1997). Gadagkar (1997) has also suggested that there are some conditions where solitary nesting should be favored over worker behavior in the polistine wasp Ropalidia marginata, and found a close approximation of this prediction in empirical studies of that species. These authors contend that eusociality may reverse itself if selection favors solitary behavior in formerly eusocial species.
Such a flexible strategy for female offspring makes adaptive sense for this P. aurifer population. Where the chance of nest loss or foundress replacement is high, offspring capable of flexibility in their expression of worker behavior have an advantage over those unable to take advantage of a shortened nest cycle. The production of an all-female early brood also follows from this idea, because only females have the flexibility to take on both worker and reproductive roles. The idea of caste flexibility as an adaptation for species that experience high risk of nest loss is not new. A study of P. gallicus in an environment with an unpredictable end to the nesting cycle also found mixing of workers and reproductives within the early brood (Gervet et al. 1986). Solís and Strassmann (1990) found that P. exclamans, which also builds small colonies and experiences high rates of foundress turnover, responded to the absence of brood on a nest by producing more reproductive female offspring (as measured by the ability to tolerate cold overwintering temperatures). Strassmann (1989) also suggested increased competition for prey in drought years as a possible cause of an early end to worker behavior in P. annularis. All of these species share an uncertain length of the nesting season. The Mediterranean climate of southern California may contribute to this unpredictability for P. aurifer. Because of the lack of summer rainfall, the availability of prey almost certainly decreases along with the decline of green vegetation later in the nesting season. The end of the colony cycle is likely to depend on the previous winter’s rainfall, rather than the onset of cold winter temperatures as in cool temperate environments. Variation in resource availability from one year to the next may also increase the value of reproduction within a season, rather than overwintering to attempt reproduction the following year.
The observation of unrelated males gathering on active nests in mid-July may be another example of behavioral flexibility helping to ensure reproduction in a colony cycle with an unpredictable end. The significance of this male-gathering behavior is unknown, but it is possible that these males were pursuing an alternative mating strategy of seeking out reproductive females at their nests rather than the more typical patrolling of territories or gathering at landmarks found in other species (Beani and Turillazzi 1988, 1990) and in our study population. A more thorough examination of this phenomenon would help determine whether mating occurs in these gatherings.
No advantage to cooperative nest founding
The rate of cooperative nest founding in this population is lower than all published reports of Polistes species that have a non-zero level of multiple founding (summarized in Queller 1996), and the average nest size is the second lowest of those reported by Reeve (1991). We found no survival or productivity advantage to cooperation among foundresses. The small number of multiple foundress nests makes it difficult to pinpoint the reason(s) for these results. However, one possible explanation for reduced productivity is that frequent changes to group membership on these nests may disrupt the dominance hierarchy, leading to reduced efficiency in the coordination of foraging activities among subordinate foundresses. Indeed, the multiple foundress nests in this study did forage for prey at lower rates than the solitary nests. Additional data regarding the failure of group nests are needed for further explanation of their surprisingly low success rate.
In this population, there is no reason to expect that a potential subordinate would have less success as a solitary foundress than a lone dominant; therefore, the x parameter of reproductive skew models can be considered equal to one. As mentioned previously, when x=1, transactional skew models require that the productivity (k) of multifoundress nests be more than twice that of solitary foundress nests to favor stable cooperative associations. (Nonacs 2002; calculated according to Reeve and Ratnieks 1993, and Johnstone 2000). However, the number of offspring produced by solitary foundress nests in this study did not differ from the number produced by multifoundress nests, even when survival rates are taken into account, so k is also equivalent to one. If foundresses perfectly manipulated split sex ratios as discussed by Nonacs (2002), this value of k still does not favor subordinate joining behavior. Given the lack of incentive for individuals to attempt to join groups or accept joiners during the founding stage, the prevalence of solitary founding appears to be adaptive for this population of P. aurifer.
If cooperative nest foundation has no advantages, then why would a low rate of multiple founding persist in this population, especially with high skew among nonrelatives? It is possible that constraints on solitary nesting may vary over time and between individuals. For example, joining might be favored over initiating a nest for foundresses who lose their nests late in the season and do not have the energy to rebuild and lay eggs. These females may opt to help sisters on nearby nests, or they may simply join nests with unrelated females and attempt to lay a few eggs after the original foundress dies. Since there is a 29% chance that the original foundress on early-initiated nests will not survive to offspring emergence, this may be a chance for foundresses to produce as many offspring as they would by initiating their own late-season nests.
However, it remains unclear why individuals would join a group as a subordinate at the beginning of the season when direct reproduction is an option, especially when the co-foundresses are unrelated. The potential for inheritance of the dominant position, as suggested for P. dominulus in a similar situation (Queller et al. 2000), may increase the benefit of this strategy for both related and unrelated subordinates. However, given the equivalent failure rate of solitary and multiple foundress nests, solitary nesting at the start of the season still appears to be a better strategy. Co-founding by nonrelatives may also be due to recognition errors, which could occur because kin recognition cues are based on a shared colony odor learned after emergence (reviewed in Gamboa 1996). If unrelated individuals emerged from the same nest due to shared reproduction by unrelated foundresses, they may erroneously perceive each other as relatives. Another possibility is that some foundresses may be less able to nest successfully on their own after overwintering and therefore may be forced to join a group. West-Eberhard (1969) first suggested that cooperating foundresses assumed subordinate or dominant roles because of pre-existing differences in fertility. This “subfertility” hypothesis has been generally dismissed as studies have shown that ovarian regression is a reversible consequence, not a cause, of subordinate behavior (reviewed in Reeve 1991); however, fertility differences may still be important under certain circumstances. Variation in age or work history of foundresses resulting from asynchrony in the colony cycle (West-Eberhard 1969), genetic differences (Liebert et al. 2004), or dispersal and overwintering of early female offspring (Mead et al. 1995; Starks 2001), might produce foundresses of varying fecundity. As this study population of P. aurifer is characterized both by a relatively asynchronous colony cycle and caste flexibility of early female offspring, the subfertility hypothesis may deserve closer examination.
No support for transactional skew models
Our results suggest that P. aurifer wasps from this population do not behave according to transactional models of reproductive skew. The small sample size of multiple foundress nests does not provide a definitive test of skew model predictions. However, the existing data do not even show trends in the expected direction for concession models (Johnstone 2000; Reeve 2000). For example, because of the low ecological constraints on solitary nesting and lack of productivity advantages for cooperation, concession models would predict low skew for multifoundress groups in this population, especially for early season nests. This is because dominant foundresses would need to offer large “staying incentives” (Reeve and Ratnieks 1993) in order for subordinates to remain in the group. However, five of six multifoundress nests in this study had positive skew values indicating unequal sharing differing from a random distribution. Moreover, on at least two of the early season nests, one female monopolized all of the reproduction. Skew also did not vary with relatedness in any consistent way, despite concession model predictions of increasing skew with higher relatedness. These results also do not support a version of the concession model called “the bidding game” (Reeve 1998), in which subordinates choose among different nests for the best staying incentive and generally low skews are expected.
Our lack of support for model predictions contrasts with findings for P. fuscatus (Reeve and Nonacs 1992, 1997; Reeve et al. 2000), and introduced P. dominulus (Tibbetts and Reeve 2000). However, our results are consistent with those of P. bellicosus (Field et al. 1998), European P. dominulus (Queller et al. 2000), P. carolina (Seppä et al. 2002) and the reinterpretation of Reeve and Nonacs (1992) data for P. fuscatus (Nonacs et al. 2004). In addition, a recent study of the Australian allodapine bee Exoneura nigrescens manipulated the three parameters (x, k, and r), and found no support for any concession model predictions (Langer et al. 2004). Testing of concession model predictions has helped generate interesting data demonstrating the fascinating variation of life history and behavioral traits within facultatively cooperative insects. However, the increasing number of Polistes studies that lack support for the concession model casts doubt upon its general application within the genus, suggesting perhaps that the two Polistes populations (both in the northeastern US) that have supported the model share some life history or behavioral characteristics not present elsewhere.
Potential effects of nest boxes
Although the timing of the colony cycle in nest boxes appears to coincide with natural nests in underground cavities at our nest site, it might be argued that artificial nest boxes might alter the natural colony cycle. One concern might be that if nest sites are normally limiting in this population, the addition of boxes might influence nesting behavior. However, there appear to be many unused soil cracks in apparently similar microhabitat where other nests are found, so saturation does not seem to be a problem. These natural cavities are also transient; the winter rains turn the soil into mud before cracking again each spring. Thus, defense of protected nest sites from year to year is not possible, and the sudden appearance of new suitable nesting sites in a given year may not be unusual.
The boxes may also have affected predation rates. Nests may have had more protection from predators such as ants or raccoons than they would have in their natural nesting sites, perhaps allowing more solitary nest success than would normally be possible. Predator protection would also reduce our ability to document re-nesting success found to be an advantage of multiple founding in other studies (Gibo 1978; Strassmann et al. 1988). Yet predation did occur in nest boxes, perhaps even at higher than natural rates if predators were able to learn that nest boxes were a potential food source. Additionally, wasps that were familiar with the boxes as nesting sites may have been able to locate nests for usurpation or adoption more easily than if the nests were hidden in vegetation. The use of nest boxes is a necessary compromise in order to follow colony development and behavior that would otherwise be impossible to observe. However, it is important to remember that the application of these results to the whole population is based on the assumption that wasps in nest boxes behave similarly to wasps nesting in natural sites.
Conclusion
In summary, the prevalence of solitary nest founding in this population of P. aurifer appears to be adaptive, as demonstrated by the lack of survival or productivity advantages for cooperative foundress associations. The majority of P. aurifer nests from this population have one reproducing foundress, even if the nest has experienced a foundress turnover such as usurpation or adoption. Occasionally, adopters and joiners may produce their own offspring later in the season, but on the whole these strategies are less successful than solitary nest foundation. Due to foundress turnover and nest foundation later in the season, many nests produce only one set of offspring. This results in a loss of the eusocial nature of some nests in the population. Data from a small sample of multifoundress nests show significant positive reproductive skew, despite concession model predictions that skew should be low in populations with low ecological constraints on independent nesting. We suggest that this lack of support for the concession skew model is a result of low levels of incentive for cooperation.
References
Beani L, Turillazzi S (1988) Alternative mating tactics in males of Polistes dominulus (Hymenoptera: Vespidae). Behav Ecol Sociobiol 22:257–264
Beani L, Turillazzi S (1990) Male swarms at landmarks and scramble competition polygyny in Polistes gallicus (Hymenoptera: Vespidae). J Insect Behav 3:545–555
Boomsma JJ, Ratnieks FLW (1996) Paternity in eusocial Hymenoptera. Philos Trans R Soc Lond B 351:947–975
Field J, Solís CR, Queller DC, Strassmann JE (1998) Social and genetic structure of paper wasp cofoundress associations: tests of reproductive skew models. Am Nat 151:545–563
Fukatsu T (1999) Acetone preservation: a practical technique for molecular analysis. Mol Ecol 8:1935–1945
Gadagkar R (1997) Social evolution—has nature ever rewound the tape? Curr Sci 72:950–956
Gamboa GJ (1978) Intraspecific defense: advantage of social cooperation among paper wasp foundresses. Science 199:1463–1465
Gamboa GJ (1980) Comparative timing of brood development between multiple- and single-foundress colonies of the paper wasp, Polistes metricus. Ecol Entomol 5:221–225
Gamboa GJ (1996) Kin recognition in social wasps. In: Turillazzi S, West-Eberhard MJ (eds) Natural history and evolution of paper-wasps. Oxford University Press, Oxford, pp 161–177
Gamboa GJ, Wacker TL, Duffy KG, Dobson SW, Fishwild TG (1992) Defence against intraspecific usurpation by paper wasp cofoundresses (Polistes fuscatus, Hymenoptera: Vespidae). Can J Zool 70:2369–2372
Gervet J, Pratte M, Semenoff S, Gabouriaut D (1986) Pattern of offspring production in colonies of paper wasps (Polistes gallicus L.). Insectes Soc 33:375–387
Gibo DL (1978) The selective advantage of foundress associations in Polistes fuscatus: a field study of the effects of predation on productivity. Can Entomol 110:519–540
Goodnight KF, Queller DC (1999) Relatedness 5.0.8. http://Gsoft.smu.edu/Gsoft.html
Goodnight KF, Queller DC, Poznansky T (1999) Kinship 1.3.1. http://Gsoft.smu.edu/Gsoft.html
Henshaw MT (2000) Microsatellite loci for the social wasp Polistes dominulus and their application in other polistine wasps. Mol Ecol 9:2155–2234
Johnstone RA (2000) Models of reproductive skew: a review and synthesis. Ethology 106:5–26
Keller L, Krieger MJB (1996) Mating success of birds. Nature 380:208–209
Klahn JE (1988) Intraspecific comb usurpation in the social wasp Polistes fuscatus. Behav Ecol Sociobiol 23:1–8
Kokko H, Johnstone RA (1999) Social queuing in animal societies: a dynamic model of reproductive skew. Proc R Soc Lond B 266:571–578
Langer P, Hogendoorn K, Keller L (2004) Tug-of-war over reproduction in a social bee. Nature 428:844–847
Liebert AE (2004) Ground nesting in the paper wasp Polistes aurifer (Hymenoptera: Vespidae). Insectes Soc 51:99–100
Liebert AE, Johnson RN, Switz GT, Starks PT (2004) Triploid females and diploid males: underreported phenomena in Polistes wasps? Insectes Soc 51:205–211
Makino S (1985) Foundress-replacement on nests of the monogynic paper wasp Polistes biglumis in Japan (Hymenoptera, Vespidae). Kontyu 53:143–149
Mead F, Gabouriaut D, Habersetzer C (1995) Nest-founding behavior induced in the first descendants of Polistes dominulus Christ (Hymenoptera: Vespidae). Insectes Soc 42:385–396
Metcalf RA, Whitt GS (1977) Relative inclusive fitness of the social wasp Polistes metricus. Behav Ecol Sociobiol 2:353–360
Nonacs P (2000) Measuring and using skew in the study of social behavior and evolution. Am Nat 156:577–589
Nonacs P (2002) Sex ratios and skew models: the special case of evolution of cooperation in Polistine wasps. Am Nat 160:103–118
Nonacs P (2003a) Measuring the reliability of skew indices: is there one best index? Anim Behav 65:615–627
Nonacs P (2003b) Skew calculator 2003 1.2. http://www.obee.ucla.edu/Faculty/Nonacs
Nonacs P, Reeve HK, Starks PT (2004) Optimal reproductive skew models fail to predict aggression in wasps. Proc R Soc Lond B 271:811–817
Paxton RJ, Ayasse M, Field J, Soro A (2002) Complex sociogenetic organization and reproductive skew in a primitively eusocial sweat bee, Lasioglossum malachurum, as revealed by microsatellites. Mol Ecol 11:2405–2416
Peters JM, Queller DC, Strassmann JE, Solís CR (1995) Maternity assignment and queen replacement in a social wasp. Proc R Soc Lond B 260:7–12
Queller DC (1996) The origin and maintenance of eusociality: the advantage of extended parental care. In: Turillazzi S, West-Eberhard MJ (eds) Natural history and evolution of paper-wasps. Oxford University Press, Oxford, pp 218–234
Queller DC, Goodnight KF (1989) Estimating relatedness using genetic markers. Evolution 43:258–275
Queller DC, Zacchi F, Cervo R, Turillazzi S, Henshaw MT, Santorelli LA, Strassmann JE (2000) Unrelated helpers in a social insect. Nature 405:784–786
Ragsdale JE (1999) Reproductive skew theory extended: The effect of resource inheritance on social organization. Evol Ecol Res 1:859–874
Reeve HK (1991) Polistes. In: Ross KG, Matthews, R.W. (ed) The social biology of wasps. Cornell University Press, Ithaca, N.Y., pp 99–148
Reeve HK (1998) Game theory, reproductive skew, and nepotism. In: Dugatkin LA, Reeve HK (ed) Game theory and animal behavior. Oxford University Press, New York, pp 118–145
Reeve HK (2000) A Transactional theory of within-group conflict. Am Nat 155:365–382
Reeve HK, Nonacs P (1992) Social contracts in wasp societies. Nature 359:823–825
Reeve HK, Nonacs P (1997) Within-group aggression and the value of group members: theory and a field test with social wasps. Behav Ecol 8:75–82
Reeve HK, Ratnieks FLW (1993) Queen–queen conflicts in polygynous societies: mutual tolerance and reproductive skew. In: Keller L (ed) Queen number and sociality in insects. Oxford University Press, Oxford, pp 45–85
Reeve HK, Starks PT, Peters JM, Nonacs P (2000) Genetic support for the evolutionary theory of reproductive transactions in social wasps. Proc R Soc Lond B 267:75–79
Seppä P, Queller DC, Strassmann JE (2002) Reproduction in foundress associations of the social wasp, Polistes carolina: conventions, competition, and skew. Behav Ecol 13:531–542
Shreeves G, Cant MA, Bolton A, Field J (2003) Insurance-based advantages for subordinate co-foundresses in a temperate paper wasp. Proc R Soc Lond B 270:1617–1622
Solís CR, Strassmann JE (1990) Presence of brood affects caste differentiation in the social wasp, Polistes exclamans Viereck (Hymenoptera: Vespidae). Funct Ecol 4:531–541
Starks PT (2001) Alternative reproductive tactics in the paper wasp Polistes dominulus with specific focus on the sit-and-wait tactic. Ann Zool Fenn 38:189–199
Starks PT, Peters JM (2002) Semi-nondestructive genetic sampling from live eusocial wasps, Polistes dominulus and Polistes fuscatus. Insectes Soc 49:20–22
Strassmann JE (1989) Group colony foundation in Polistes annularis (Hymenoptera: Vespidae). Psyche 96:223–236
Strassmann JE (2001) The rarity of multiple mating by females in the social Hymenoptera. Insectes Soc 48:1–13
Strassmann JE, Queller DC, Hughes CR (1988) Predation and the evolution of sociality in the paper wasp Polistes bellicosus. Ecology 69:1497–1505
Strassmann JE, Solís CR, Peters JM, Queller DC (1996) Strategies for finding and using highly polymorphic DNA microsatellite loci for studies of genetic relatedness and pedigrees. In: Ferraris J, Palumbi SR (ed) Molecular zoology. Wiley, New York, pp 163–180
Strassmann JE, Barefield K, Solís CR, Hughes CR, Queller DC (1997) Trinucleotide microsatellite loci for a social wasp, Polistes. Mol Ecol 6:97–100
Tibbetts EA, Reeve HK (2000) Aggression and resource sharing among foundresses in the social wasp Polistes dominulus: testing transactional theories of conflict. Behav Ecol Sociobiol 48:344–352
Tibbetts EA, Reeve HK (2003) Benefits of foundress associations in the paper wasp Polistes dominulus: increased productivity and survival, but no assurance of fitness returns. Behav Ecol 14:510–514
Tsuji K, Kasuya E (2001) What do the indices of reproductive skew measure? Am Nat 158:155–165
Tsuji K, Tsuji N (1998) Indices of reproductive skew depend on average reproductive success. Evol Ecol 12:141–152
Wcislo WT, Danforth BN (1997) Secondarily solitary: the evolutionary loss of social behavior. Trends Ecol Evol 12:468–474
West-Eberhard MJ (1969) The social biology of Polistine wasps. In: Miscellaneous publications, vol 140. Museum of Zoology, University of Michigan, Ann Arbor
Yamane S (1996) Ecological factors influencing the colony cycle of Polistes wasps. In: Turillazzi S, West-Eberhard MJ (eds) Natural history and evolution of paper-wasps. Oxford University Press, Oxford, pp 75–97
Acknowledgements
We thank Michael Donnelly, Dennis Maglinte, and Frances Johnson for help with the experimental enclosures and video transcription, Roy Snelling for identifying the species, and John Pollinger for assistance with lab techniques. Smadar Gilboa and two anonymous reviewers provided helpful comments on the manuscript. Field work was performed at the University of California Natural Reserve System, Stunt Ranch Santa Monica Mountains Reserve. This research was supported by Mildred E. Mathias Graduate Student Research Grants from the University of California Natural Reserve System to A.E.L., and by NSF grant 9808788 to P.N. The research presented complies with the current laws of the country in which it was performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Keller
Rights and permissions
About this article
Cite this article
Liebert, A.E., Nonacs, P. & Wayne, R.K. Solitary nesting and reproductive success in the paper wasp Polistes aurifer. Behav Ecol Sociobiol 57, 445–456 (2005). https://doi.org/10.1007/s00265-004-0875-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0875-5