Abstract
The essence of eusociality is a trade-off between producing one’s own offspring and helping collateral kin via such activities as defence, foraging and brood rearing. This trade-off often involves morphological differences between “helper” and “reproductive” castes, but the advantages, correlates and phylogenetic context of morphological caste differentiation have seldom been analysed. Six species of Australian gall-inducing thrips on Acacia show morphological polymorphism. One morph, referred to as a soldier, has reduced wings and antennae but greatly enlarged fore-femora, which are thought to be adaptations for gall defence. The other, dispersing morph, has fully developed wings and relatively slight fore-femora. Here, we quantify the defensive behaviour of soldier morphs, and compare soldier and foundresses, using behavioural assays designed to measure proclivity to attack kleptoparasites (specialised invaders in the genus Koptothrips) and effectiveness in killing them. In all five species investigated, soldiers were able to kill Koptothrips. Moreover, the effectiveness of soldiers was relatively high in the more-derived species in the phylogeny of the clade, Kladothrips intermedius, K. habrus and K. waterhousei. Soldiers of K. intermedius and K. habrus also killed kleptoparasites more effectively than did foundresses, and K. habrus soldiers exhibited higher proclivity to attack than did foundresses. Data from naturally invaded galls demonstrate that soldiers in the field do kill Koptothrips, and vice versa. These results show that soldiers of Australian gall thrips are motivated and effective for gall defence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eusociality is typified by the trade-off between personal reproduction and investment in helping others, usually relatives, to survive and reproduce (Wilson 1971; Crespi and Choe 1997). In some species, this trade-off involves the evolution of physical differentiation between “reproductives” and “helpers”. Helpers exhibit morphological and/or behavioural specialisations that presumably lead to increased abilities to help effectively, but such specialisations may also engender a reduction in opportunities for personal reproduction. Although morphological caste differences between reproductives and helpers have been described from many ants, termites, aphids, and some bees, wasps and thrips (Wilson 1971; Michener 1974; Hölldobler and Wilson 1990), the effects of such morphological differences on motivation for helping, and efficacy of helping behaviour, have usually been assumed rather than quantified. As a result, the role of the joint evolutionary dynamics of morphological and behavioural change in social evolution remains largely unstudied, despite their obvious importance for the evolution of reproductive skew (e.g. Crespi and Choe 1997; Chapman et al. 2002; Jeon and Choe 2003) and colony-level organisation (e.g. Bourke and Franks 1995; Bourke 1999).
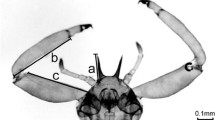
Six described species of gall-inducing thrips found on Australian Acacia species have evolved a non-dispersing morph (Crespi 1992a; Crespi and Mound 1997; Crespi et al. 1997a, 1997b; Kranz et al. 1999, 2001a, 2001b). This morph comprises a relatively small proportion of the total brood in a gall and they are characterised by reduced wings and antennae, and enlarged forelimbs with sharp pointed fore-tarsal teeth at the inner apex. These individuals have been observed in the laboratory to attack individuals of gall-invading kleptoparasites in the genus Koptothrips. The term “soldier” has been used to designate them as a distinct caste (Crespi 1992a; Mound and Crespi 1995), where caste is defined here as groups of individuals that have distinct adaptations for the performance of specialised tasks for the duration of their adult lives; these tasks enhance the reproductive output of a non-helping caste at the expense of their own reproductive output (Michener 1974; Oster and Wilson 1978; Crespi and Yanega 1995). Crespi (1992a) found that soldiers of Kladothrips habrus (formally Oncothrips habrus, Crespi et al. 2004) were more inclined than the foundress to attack an artificially presented Koptothrips. Such a behavioural difference between morphs was statistically non-significant in K. intermedius (formally O. tepperi, Crespi et al. 2004) but in this species, soldiers were also observed to attack invaders, and the more robust forelimbs of the soldiers were inferred to give them a mechanical advantage over foundresses in killing invaders. Soldiers of K. intermedius were therefore inferred to also function as defensive specialists. The observations that soldiers are normally less fecund than the foundress (Crespi 1992a; Chapman et al. 2002) mean that these soldiers may be regarded as comprising an altruistic or helping caste. The presence of a helping caste is a distinguishing trait of social, or eusocial, colonies (Michener 1974; Wilson 1975; Crespi and Yanega 1995) and these thrips species therefore provide an important addition to the comparative database available for studying the evolution of eusociality (Crespi 1992a).
The studies by Crespi (1992a) and Perry et al. (2003) both used behavioural assays that did not assess the mechanical utility of large forelimbs, because they only observed the “proclivity” (tendency) of the gall occupants to engage in attacking behaviour and did not look at the outcome of such encounters. By contrast, Mound and Crespi (1995) reported on experiments that involved enclosing soldiers and Koptothrips inside galls, but they focussed on documenting, rather than quantifying, antagonism between these taxa. As a result, their sample sizes were small. Here we present an approach inspired by methodologies successfully used to test aphid soldier behaviour (e.g. Foster 1990; Foster and Rhoden 1998) which places combatants in an arena that mimics the enclosed space of the gall and allows the combatants to interact freely until the death of one. Our efficacy assay has not previously been employed to test the defensive behaviour of soldiers or foundresses of Australian gall-inducing thrips. We address two hypotheses that are central to the analysis of caste evolution in Australian gall-inducing thrips. First, how motivated are soldiers and foundresses to attack Koptothrips? Our “proclivity” assay provides evidence on the coevolution of behaviour and caste in these insects, and in particular allows inferences regarding the altruistic tendencies of soldiers in comparison to foundresses. Second, how effective are soldiers and foundresses at killing Koptothrips? These experiments allow evaluation of the effects of morphological specialisation on caste performance. Proclivity to attack, and success of attacks, were studied in soldiers of K. intermedius, K. habrus, K. waterhousei, K. morrisi and K. hamiltoni. In addition, we compared soldiers with foundresses for proclivity and efficacy in K. habrus, and for efficacy in K. intermedius; only for these two species were individuals of both castes readily available. Note that the genus Oncothrips (Karny) was formally synonymised with the genus Kladothrips (Froggatt). This genus now includes all 23 of the described gall-inducing thrips species from Acacia, including the species used in this study (Crespi et al. 2004). We analysed these data in a comparative context, and in relation to levels of reproduction exhibited by soldiers. Thus, K. waterhousei, K. morrisi and K. hamiltoni are relatively-basal on a phylogeny of this clade (Crespi et al. 1997b; Morris et al. 2001) and exhibit relatively low reproductive skew, whereas K. intermedius and K. habrus are derived and exhibit high skew (Chapman et al. 2002).
Methods
Life-history of gall-inducing thrips on Australian Acacia
There are over 5,500 described thrips species, with widely varying life-histories (Mound 1971; Mound and Heming 1991; Ananthakrishnan 1992). All are haplodiploid. The thrips investigated here induce galls on native Australian Acacia trees and shrubs and feed on the contents of plant cells within the gall in which they are interred (Mound and Crespi 1995; Crespi and Mound 1997). Solitary foundresses, or founder female-male pairs, initiate galls on young growing phyllodes (modified leaf-like petioles), which curl, roll or evaginate into a pouch so that opposing sides of the phyllode meet or overlap to form an enclosed chamber (Crespi and Mound 1997). Once the foundress is enclosed, she deposits eggs on the inner gall surface from which larvae emerge that develop within the protected confines of the gall. In the social species, the first cohort produced by the foundress comprises a morphologically distinct “soldier” morph; these individuals remain within the gall for the duration of their lifespan (Crespi 1992a). Foundresses remain alive within the gall at least until some of the soldiers have eclosed as adults, after which they die. The second cohort to eclose is the dispersing morph which, with the exception of one species (K. intermedius), apparently leave the gall as second-instar larvae, to pupate in the soil (Morris et al. 2001). Primarily, the foundress produces the second cohort, with soldiers producing a small proportion of the dispersing brood. However, soldiers are less fecund than the foundress in all species, except in K. morrisi (Kranz et al. 2001b; Chapman et al. 2002). Moreover, there is an apparent phylogenetic trend for decreasing soldier reproduction from basal to more derived lineages (Chapman et al. 2002). Soldiers and dispersers are closely related to one another (r of about 0.5 and higher) in all social species examined thus far (K. hamiltoni, K. habrus, K. morrisi, K. intermedius and K. waterhousei; Chapman and Crespi 1998; Chapman et al. 2000).
Thrips in the genus Koptothrips are kleptoparasites of social and non-social gall-inducing thrips on Acacia (Mound 1971; Crespi and Abbot 1999). Koptothrips species invade the galls, killing the occupants and breeding within the gall (Crespi 1992a, 1992b; Crespi and Abbot 1999). Koptothrips can be quite common in natural populations and are thought to be a major selective force underlying the evolution and maintenance of soldier morphology and behaviour (Crespi 1992a; Crespi and Mound 1997; Crespi and Abbot 1999; Kranz et al. 2001a, 2001b).
Sampling regime
Five species of gall-inducing thrips species with soldiers were collected over a 9-month period, from various arid and semi-arid regions of Australia. Collection sites and sampling dates are summarised in Table 1. Galls were detached from branches, sealed within plastic bags and transported on ice to Flinders University within several days of collection. Galls were stored at 12ºC until needed for the behavioural assays.
Behavioural assays
Behavioural assays were conducted within 10 days following gall collection. Galls were bisected and examined under a stereo microscope at ×4 magnification. Female and male soldiers from bisected galls were randomly chosen for either the proclivity or efficacy assays (described below). Because there is only one foundress per gall, and foundresses tend to die before most soldiers have eclosed, it was possible to compare foundresses with soldiers for only two species, K. intermedius and K. habrus. Koptothrips flavicornis adults were found within the galls of Kladothrips intermedius, Kladothrips habrus, Kladothrips waterhousei and Kladothrips hamiltoni, and Ko. dyskritis were found within the collections of Kladothrips morrisi. We only used Koptothrips that had been collected from the same localities and host plants as their thrips hosts.
Defensive proclivity
Proclivity to defend against kleptoparasites was assayed following the methods as described by Perry et al. (2003). Using fine watchmaker forceps, Koptothrips were gently clasped between the abdomen so that the pronotum, forelimbs and head were free to move. Koptothrips were presented so that they were in line with the heads of the assayed subjects. Both soldiers and foundresses were tested within the confines of a bisected gall, and only one individual of each morph from a gall was assessed. Each assay lasted until an attack occurred or 4 min had elapsed. An attack was considered to have occurred when a soldier or foundress grasped or attempted to grapple the Koptothrips around the head or pronotum using their forelimbs. Non-aggressive responses, such as backing away, turning around from, or not showing a response to a Koptothrips within the 4-min period, were regarded as showing no proclivity for defence. Each trial therefore resulted in one of two possible outcomes.
Defensive efficacy
Our proclivity assay measures the likelihood that an individual will attack a potential enemy but does not measure how effective such an attack might be. Given the purported function of soldier morphology in gall defence, it is critical to assess whether soldiers are effective in killing Koptothrips, and also if they are more effective than foundresses, who express such apparent morphological adaptations for defence to a much lower degree. However, measuring such effectiveness is problematic. Simply introducing an invader to an otherwise intact gall and then observing an end result after some period of time does not allow the effects of soldier morphology, the number of soldiers within a gall, gall brood size, gall volume, or presence of a foundress, to be disentangled. To circumvent these potentially confounding factors, we devised the following procedure, which assays the effectiveness of a soldier or foundress in killing Koptothrips.
For these trials, we substituted galls (which vary in shape and size in relation to the thrips species; Crespi and Worobey 1998; Wills et al. 2001) with a 200-μl Eppendorf Polymerase Chain Reaction (PCR) tube. From a bisected gall, one soldier and where possible one foundress were removed and placed into a PCR tube to face a single Koptothrips. Koptothrips were collected and placed into 10-ml centrifuge tubes and stored in a foam ice box in the refrigerator at 12°C until enough soldiers and foundresses had been set up to accept the Koptothrips and begin the trials. The “cage matches” were started within 1–2 h from the time a test subject (soldier or foundress) was placed into a tube, with the assay initiated once a Koptothrips was placed into the tube. Assays were conducted over a period of 40 h with tubes contained inside a closed cardboard box stored at room temperature. The outcome of the trial was recorded as either an outright victory or defeat (i.e. Koptothrips survived or died). We note that efficacy of defence requires either defensive proclivity (i.e. a spontaneous attack on the Koptothrips), or a defensive response to an attack upon the soldier by the Koptothrips.
Controls were necessary to take account of natural deaths that might have arisen as a result of natural mortality or handling the thrips prior to placing them into the PCR tubes. Therefore, two soldiers or two Koptothrips from within the same gall (i.e. gallmates) were selected and placed into a PCR tube together. A death in the control group can be assumed to be the result of natural causes, as opposed to the effect of confrontations between individuals, as gallmates (e.g. soldiers) reside naturally within a gall together apparently without conflict (Crespi 1992a; Bejah 1997). Foundresses have been observed to fight each other for access to galling sites and incipient galls (Crespi 1992b). Therefore, foundresses were placed singly into tubes to measure mortality rates due to a combination of natural and handling-related mortality.
Field observations of host and invader deaths
Galls were collected in the field and surveyed for the presence of Koptothrips with soldiers, foundresses or both in the presence of heterospecifics that were dead. Data were collected from four of the species to evaluate if kleptoparasitic gall invasion coincides with the death of invaders or hosts, implying that lethal confrontations might occur between the host and kleptoparasitic invaders in the field.
During the process of setting up the efficacy assays, galls invaded by Koptothrips had the live kleptoparasites removed for use in the assays. The gall contents were then brushed into a petri dish and galls were further washed out with 70% ethanol to remove any thrips remains. Gall contents were examined under ×4 magnification to see if there were any intact carcasses. Where live soldiers or foundresses were found alone with remains, the galls were then examined to assess whether the remains were dead Koptothrips. If host remains were found together with live Koptothrips, it could be inferred that death was as a consequence of Koptothrips invasion. If live soldiers or foundresses were found alone with Koptothrips remains, then the inference was that soldiers or foundresses were responsible for the death of the gall invaders.
These data cannot take account of cases where Koptothrips invade a gall but leave as a result of defence by soldiers, or where soldiers or founders have died from natural causes prior to invasion by kleptoparsites. Furthermore, the field data are not a surrogate for a natural efficacy experiment. The efficacy trials we perform test an individual soldier’s defensive efficacy, whereas under natural circumstances, more than one soldier or Koptothrips may be engaged in defence or invading a gall. Therefore, as we are unable to control for these circumstances, comparing results of the efficacy trials and field data would not be appropriate.
Analytical approach
Mortality rates not due to direct interactions between gall inducers and Koptothrips might vary with both species and morph. Therefore, in order to assess how effective foundresses or soldiers were in killing Koptothrips, kill rates for each treatment were compared with the corresponding control. We contrasted the proportion of deaths that were observed in the control with those in the treatment group for soldiers, foundresses and Koptothrips. We also compared foundress and soldier control survival, such that we could account for between-morph differences in control survival with survival in the experimental tests.
Statistical analysis
To examine survival between the control and treatment groups in Kladothrips intermedius, Kladothrips habrus, Kladothrips waterhousei, Kladothrips morrisi and Kladothrips hamiltoni, 2 by 2 contingency tables were constructed and two-tailed Fisher exact tests were used to compare mortality rates in treatments with their controls. These tests were used to infer whether deaths of either gall-inducing species or their kleptoparasites were likely to be due to natural or handling-related factors, or whether mortality rates were due to treatment effects. Interpreting significance levels for these multiple tests is not straightforward since multiple unplanned comparisons can inflate type I errors. Therefore, we followed the recommendations of Rice (1989) and used a sequential Bonferroni adjustment. Following comparisons of treatments with controls, we compared defensive efficacy between soldiers and foundresses for two species (Kladothrips intermedius and Kladothrips habrus) and proclivity in one species (Kladothrips habrus), also using a Fisher Exact test. Employing a sequential Bonferroni adjustment for these later tests is problematic, since the first two tests were planned, and the third test examined a different hypothesis. Therefore, we chose to interpret significances for theses tests cautiously, but without a Bonferroni adjustment.
Employing a logit analysis of the survival odds ratios as described by Sokal and Rohlf (1995), 95% confidence limits were calculated for the mean proportion of soldier and Koptothrips survival in the control and treatment groups. Lastly, employing a Pearson’s correlation analysis, we investigated whether there was a correlation between a soldier’s defensive proclivity and efficacy within each species, and with data collected here, and previous data on soldier proclivity, average reproductive skew, and soldier numbers, we assessed the patterns of current among-species covariation in these traits.
Results
Observations of attacking behaviour
Attacks against Koptothrips were witnessed during the proclivity assays involving Kladothrips habrus soldiers (N=34 assays, n=23 attacks) and foundresses (N=26 assays, n=2 attacks). Even though Koptothrips were presented towards the heads of the assay subjects, soldiers and foundresses would attempt to attack the Koptothrips from behind by first mounting them head on but then orientating themselves so that their head and abdomen were positioned in line with that of the Koptothrips. Soldiers and foundresses would then embrace the Koptothrips around the pronotum (thorax) using their forelimbs in the manner of a bear hug, squeezing and then releasing their grasp repeatedly at first but eventually remaining locked on.
On six occasions when Koptothrips were accidentally released from the tweezers, Kladothrips habrus soldiers were seen to grip the Koptothrips head on so that the dorsal surface of the Koptothrips thorax was beneath the soldier’s ventral thorax surface. Soldiers would then arch their abdomens upwards raising the Koptothrips into the air. It appeared that by doing this, soldiers would minimise the Koptothrips’ ability to gain purchase to the side of the gall. In each case, the resistance of the Koptothrips would eventually taper off and the soldier would let go. When Koptothrips were stimulated with a fine paintbrush after such encounters, they could barely move, and within an hour were motionless and appeared to be dehydrated, as the abdomen was buckled inward. However, upon inspection at ×40 magnification no obvious physical signs of damage to the Koptothrips could be found (i.e. punctures or cuticle damage) so the precise cause of death was unclear.
Only twice during the efficacy assays were soldiers directly observed to attack Koptothrips, once each during an assay involving Kladothrips morrisi and Kladothrips habrus. Interestingly, for the Kladothrips morrisi soldier fight, the Koptothrips had been grasped from behind around the abdomen and not the thorax; in this case, the soldier died while embracing the Koptothrips. It has been suggested that Koptothrips might have the ability to introduce venom into their assailants using their fore-tarsal teeth and a gland in the tarsus (Crespi and Mound 1997). Indeed, during the proclivity assay, we observed that both soldiers and founders died quickly (within 1–3 mins) if a Koptothrips had grasped them. Soldiers during a confrontation, perhaps in the manner described above, might position themselves to avoid contact with Koptothrips forelimbs. Additionally, in several cases (Kladothrips waterhousei, n=1, Kladothrips morrisi, n=3), both soldiers and Koptothrips were found dead at the end of the trial. In each case there was no easily discernible evidence to suggest death had resulted from an interaction between individuals.
Control versus treatment survival
For each species, survival of soldiers in the efficacy assays was significantly higher in the control group than the treatment group (Fisher Exact test, Kladothrips intermedius, P=0.002, Kladothrips waterhousei, P=0.016, Kladothrips habrus, Kladothrips morrisi and Kladothrips hamiltoni, P<0.001). Similarly, survival of Koptothrips in the efficacy assays for each of the host species was significantly higher in the controls than in the treatments (Fisher Exact test, Kladothrips intermedius, Kladothrips habrus, Kladothrips waterhousei, Kladothrips morrisi and Kladothrips hamilton, P<0.001). Further, a Bonferroni adjustment did not lead to a decrease in the number of significant outcomes across our table of results. The back-transformed mean survivals and 95% confidence limits for soldiers and Koptothrips in control and treatment groups are shown in Fig. 1a,b. Survival of foundresses for both Kladothrips intermedius and Kladothrips habrus was significantly higher (both species P<0.001) in the control groups (Kladothrips intermedius 92% and Kladothrips habrus 88%) than treatment groups (Kladothrips intermedius 40% and Kladothrips habrus 8%). The fact that control deaths were relatively low in frequency, and deaths in treatment groups were common, suggests that: (1) thrips are robust to the artificial environment of the PCR tubes, (2) intraspecific fights are either very rare or ineffective, and (3) the higher death rate observed for the treatment groups arises due to confrontations between hosts and their Koptothrips enemies.
Mean proportion (with upper and lower 95% confidence limits) of individuals (soldiers) that survived in the control versus treatment groups during the efficacy assay, where for each species survival was significantly higher in the control than for the treatment groups. The total number of soldier controls for each species was (N=56) Kladothrips intermedius, (N=160) Kladothrips habrus, (N=40) Kladothrips waterhousei, (N=48) Kladothrips morrisi, and (N=96) for Kladothrips hamiltoni. b Mean proportion (with upper and lower 95% confidence limits) of Koptothrips individuals that survived in the control versus treatment groups during the efficacy assay, where for each species survival was significantly higher in the control than for the treatment groups. The total number of Koptothrips controls set up within each species was (N=31) Kladothrips intermedius, (N=44) Kladothrips habrus, (N=18) Kladothrips waterhousei, (N=24) Kladothrips morrisi, and (N=28) Kladothrips hamiltoni
Soldier versus foundress control survival
For Kladothrips intermedius and Kladothrips habrus, there was no significant effect of morph type (soldiers versus foundresses) on survival in the control groups (Fisher’s Exact test Kladothrips intermedius, P=0.44; N=56 soldiers, 96% survived and N=60 foundresses, 92% survived and for Kladothrips habrus, P=0.16; N=160 soldiers, 96% survived and N=16 foundresses, 88% survived). No significant variation in survival was found between the morphs; therefore, if differences in survival were observed between soldiers and foundresses in the experimental treatments, it would most likely reflect variation in soldier or foundress ability to survive encounters with Koptothrips, and not handling due to the experimental procedure.
Efficacy and proclivity assay for Kladothrips habrus
A total of 21 foundresses and 68 soldiers of Kladothrips habrus were tested in proclivity assays. Assays in which Koptothrips had been dropped were excluded from further analysis. The outcome of the proclivity assay resulted in 3 attacks by foundresses and 32 by soldiers. These attack rates are significantly different (Fisher Exact test, P=0.0002). For the efficacy trials, 26 foundresses and 34 soldiers were assayed, resulting in 2 Koptothrips kills by foundresses and 23 by soldiers. These rates also differ significantly (Fisher Exact test, P<0.001).
Efficacy assay for Kladothrips intermedius
Kladothrips intermedius foundresses were obtained from 2 separate collection periods (N 1 =13 and N 2 =12) and it was therefore necessary to determine if data from efficacy assays for the 2 periods could be pooled. A Fisher Exact test indicated that the relative number of successful kills by foundresses (n 1 =6 and n 2 =4) did not differ between collections (P=0.688) so these 2 data sets were pooled for subsequent analyses. Foundresses exhibited 10 kills in 25 trials, and soldiers 20 kills in 28 trials. Soldiers thus displayed a greater efficacy for killing Koptothrips (Fisher Exact test, P=0.028).
Proclivity and efficacy assays for Kladothrips waterhousei, Kladothrips hamiltoni and Kladothrips morrisi soldiers
The proclivity of soldiers to attack Koptothrips was very low in Kladothrips waterhousei (40 interactions, 0 attacks) but higher in Kladothrips hamiltoni (50 interactions, 7 attacks). However, the efficacy assays showed that soldiers of Kladothrips waterhousei, Kladothrips hamiltoni and Kladothrips morrisi were effective at killing Koptothrips: Kladothrips waterhousei (N=21 interactions, n=15 successful kills), Kladothrips hamiltoni (N=57, n=32) and Kladothrips morrisi (N=55, n=24).
Within-species correlation between efficacy and proclivity
We addressed the question of whether efficacy and proclivity are measuring the same type of defensive behaviour by combining these results with the data presented above on proclivity to attack with the results of Perry et al. (2003) (Kladothrips morrisi 243 interactions, 1 attack; Kladothrips intermedius, 87 interactions, 11 attacks,). By a Pearson Correlation analysis, there was no significant correlation between soldier efficacy and proclivity for defence (r=−0.327, n=5, P>0.4).
Observation of host or invader deaths
Koptothrips had invaded, and remained within (either live or dead), 24 (8.2%) of 294 galls in natural populations of Kladothrips intermedius, 24 (15.8%) of 152 galls of Kladothrips habrus, 10 (6.3%) of 114 galls of Kladothrips waterhousei, 13 (10.1%) of 128 galls of Kladothrips hamiltoni, and none of 147 galls of Kladothrips morrisi. The most common result of invasion by Koptothrips was death of the host thrips (Fig. 2). However, soldiers had apparently killed Koptothrips in Kladothrips intermedius, 6 (25%) of 24 cases, in Kladothrips habrus, 3 (12.5%) of 24 cases, in Kladothrips waterhousei, 1 (10%) of 10 cases and Kladothrips hamiltoni, 3 (23%) of 13 cases. Further, in Kladothrips habrus and Kladothrips intermedius, foundresses had killed Koptothrips on several occasions.
Occurrence of deaths in galls as observed in four eusocial galling-thrips taxa coinciding with either the death of the host thrips or invading Koptothrips species. Galls of Kladothrips intermedius were not observed to contain foundresses found dead in the presence of live Koptothrips while in Kladothrips waterhousei and Kladothrips hamiltoni, dead Koptothrips were never found alongside living foundresses
Among-species covariation in soldier efficacy, skew, and soldier numbers
We used the data collected here, and previous data on soldier efficacy, average reproductive skew and soldier numbers (Chapman et al. 2002; Perry et al. 2003; Crespi et al. 2004) to assess the patterns of current among-species covariation (Ricklefs and Stark 1996) in these key social traits (Table 2). There was a strong negative correlation between average number of soldiers in a colony and soldier efficacy (product-moment correlation, r=0.96, n=5, P<0.01). Moreover, soldier efficacy was negatively correlated with the extent of soldier reproduction (skew, relative to foundresses), for skew estimated from demographic data (r=0.88, n=5, P=0.0489). This association was weaker and not statistically significant for skew estimated from microsatellite data (r=0.75, n=5, P=0.147), perhaps in part due to low statistical power.
Discussion
Taken together, our results demonstrate that the soldier morphs of Australian Acacia thrips represent a true caste specialised for defence. The data from experiments and natural populations demonstrate that soldiers of all five species of gall-inducing thrips studied are effective at killing Koptothrips. The proportion of resolved efficacy trials that resulted in the soldier killing the Koptothrips ranged from 44% to 71%, with the highest values in Kladothrips intermedius, Kladothrips habrus, and Kladothrips waterhousei, the more-derived species in the phylogeny of this clade (Morris et al. 2001). Koptothrips also proved to be deadly, however: the proportion of trials with a living invader and a dead soldier ranged from 29% to 56%. These data suggest that encounters between Koptothrips and soldiers entail substantial mortality risks, and that both soldiers and Koptothrips are under strong selection for adaptations related to lethal fighting.
Soldiers were more effective than foundresses in killing Koptothips for the two species studied, Kladothrips intermedius (twice as lethal) and Kladothrips habrus (12 times more lethal). Proclivity to attack was also greater for soldiers relative to the foundress in Kladothrips habrus, which concords with the results of Crespi (1992a). By contrast, a higher proclivity to attack in soldiers compared to foundresses was not detected in two previous studies of attacking behaviour in Kladothrips intermedius (Crespi 1992a; Perry et al. 2003), the results in both of these studies were not significant (P=0.196 and P=0.086, respectively). This apparent lack of a strong caste difference in proclivity to attack in Kladothrips intermedius may be related to three factors: (1) the relatively high efficacy of Kladothrips intermedius foundresses uncovered by the experiments, (2) the relatively high success of Kladothrips intermedius foundresses in killing Koptothrips under natural circumstances (Fig. 2), and (3) the lower degree of morphological difference between foundresses and soldiers in Kladothrips intermedius compared to Kladothrips habrus (Fig. 1 in Crespi 1992a). Thus, more soldierlike morphology and behaviour in foundresses of Kladothrips intermedius may have mitigated against a caste difference in proclivity to attack.
We assumed that efficacy for defence might require prior defensive proclivity where soldiers who are willing to attack might also be effective defenders. However, there was no correlation within species between proclivity to attack and attack efficacy. Thus, although Kladothrips habrus and Kladothrips intermedius had relatively high proclivity and efficacy compared to the other species, Kladothrips waterhousei exhibited low proclivity and high efficacy; perhaps, as some of the observations suggest, soldiers prefer to attack Koptothrips from the rear, or they delay an attack until multiple soldiers have been recruited. Alternatively, Koptothrips might avoid a confrontation with soldiers; perhaps successfully invading a gall requires that Koptothrips remain undetected, or soldiers require a longer period of time to detect an intruder than the proclivity assay provides. In both instances, soldiers remain unprovoked by Koptothrips and may not be motivated to attack spontaneously. Therefore, the proclivity assay may not predict the efficacy of a soldier’s defence. Indeed, the main pattern in these data is a lack of species with soldiers exhibiting high proclivity and low efficacy (Table 2), which makes sense considering that such a strategy would not be adaptive for colony defence.
There are no qualitative behavioural differences between foundresses and soldiers in how they fight with Koptothrips (see also Crespi 1992b; Crespi and Mound 1997). In both cases, the attacker endeavours to grasp the victim around the thorax using their forelimbs. The attacker then raises the victim into the air and squeezes the victim in slow pulses until it is moribund, after which it is released. The higher success of soldiers compared to foundresses in Kladothrips habrus and Kladothrips intermedius apparently results from the morphological differences between them, most importantly, the enlarged, armed forelimbs of soldiers. These enlarged forelimbs may lead to a greater ability of the soldiers to grasp and hold the Koptothrips and subject it to a greater squeezing force, relative to the slimmer forelimbs of the foundress. This hypothesis can be investigated further via morphometric studies of intraspecific and interspecific variation in foreleg armature, in relation to success in defence.
How might have defensive behaviour, reproductive skew, and aspects of colony demographics and ecology evolved together in Australian gall thrips with soldiers? First, the strong negative correlation among species between the average number of soldiers in a colony and soldier efficacy suggests that having fewer soldiers is associated with increased soldier effectiveness. Similar findings were reported by Shingleton and Foster (2001), who compared two congeneric aphid species with soldiers and found that the species with smaller colonies had soldiers that were more motivated to attack (although their soldier and non-soldier castes were morphologically more similar to one another). In both aphids and thrips, the evolution of increased soldier efficiency may have allowed for fewer soldiers to be produced with similar defensive benefits, or strong selection for more dispersers (which trades-off with fewer soldiers) may have driven an evolutionary increase in their efficacy.
Second, soldier efficacy appears to be negatively related to the extent of soldier reproduction. This is coupled with previous work showing that species with soldiers apparently inhabit relatively small galls (Crespi and Worobey 1998), and that reproductive skew between foundresses and soldiers is higher in social species with smaller galls (Wills et al. 2001; Chapman et al. 2002). These findings suggest that relatively small galls, lower soldier numbers, higher defensive efficacy of soldiers, and higher levels of skew evolve together, reaching their most-developed states in the derived species Kladothrips habrus and Kladothrips intermedius. The causal connections between these variables may involve: (1) smaller galls selecting for higher skew through their effects on limiting total brood size to a number that can be produced relatively easily by the foundress (Wills et al. 2001); (2) smaller galls leading to lower numbers of soldiers, which could select for higher efficacy by individual soldiers or vice versa; (3) higher skew favouring the evolution of higher soldier proclivity and efficacy. Further analysis of these apparent trends requires additional data on other species or host-plant races with soldiers, especially including Kladothrips habrus from Acacia pendula and Kladothrips waterhousei from numerous Acacia species (Crespi et al. 1997a, 1997b); such a larger data set will also allow robust phylogenetically based comparative tests.
Most previous studies of morphological castes in social insects have focussed on documenting caste differences between queens and workers or soldiers, and testing for behavioural differences among workers that vary qualitatively or quantitatively in morphology (e.g. Table 8-3 of Hölldobler and Wilson 1990). In most of these cases, higher task efficacy of workers or soldiers than queens is assumed (i.e. inferred from apparent functional design) rather than demonstrated directly. Further study of the comparative proclivity and efficacy of morphological and behavioural castes of social insects will shed light on the benefits and costs involved in caste ecology and evolution.
References
Ananthakrishnan TN (1992) Unique aspects in the biology of thrips-induced galls. In: Shorthouse JD, Rohfritsch O (eds) Biology of insect induced galls. Oxford University Press, New York, pp 185–195
Bejah K (1997) The functional significance of morphological and behavioural castes in Oncothrips tepperi in galls on Acacia oswaldii. Thesis, Flinders University of South Australia
Bourke AFG (1999) Colony size, social complexity and reproductive conflict in social insects. J Evol Biol 12:245–257
Bourke AFG, Franks N (1995) Social evolution in ants. Princeton University Press, Princeton
Chapman TW, Crespi BJ (1998) High relatedness and inbreeding in two species of haplodiploid eusocial thrips (Insecta: Thysanoptera) revealed by microsatellite analysis. Behav Ecol Sociobiol 43:301–306
Chapman TW, Crespi BJ, Schwarz MP, Kranz, BD (2000) High relatedness and inbreeding at the origin of eusociality in Australian gall thrips. PNAS 97:1648–1650
Chapman TW, Kranz BD, Bejah K, Morris DC, Schwarz MP, Crespi BJ (2002) The evolution of soldier reproduction in social thrips. Behav Ecol 13:519–525
Crespi BJ (1992a) Eusociality in Australian gall thrips. Nature 359:724–726
Crespi BJ (1992b) The behavioral ecology of Australian gall thrips. J Nat Hist 26:769–809
Crespi BJ, Abbot P (1999) The behavioral ecology and evolution of kleptoparasitism in Australian gall thrips. Fla Entomol 82:147–164
Crespi BJ, Choe JC (1997) Evolution and explanation of social systems. In: Choe JC, Crespi BJ (eds) The evolution of social behaviour in insects and arachnids. University Press, Cambridge, pp 499–524
Crespi BJ, Mound LA (1997) Ecology and evolution of social behaviour among Australian gall thrips and their allies. In: Choe JC, Crespi BJ (eds) The evolution of social behaviour of insects and arachnids. Cambridge University Press, Cambridge, pp 166–180
Crespi BJ, Worobey M (1998) Comparative analysis of gall morphology in Australian gall thrips: the evolution of extended phenotypes. Evolution 52:1686–1696
Crespi BJ, Yanega D (1995) The definition of eusociality. Behav Ecol 6:109–115
Crespi BJ, Carmean D, Chapman TW (1997a) The ecology and evolution of galling thrips and their allies. Annu Rev Entomol 42:51–71
Crespi BJ, Carmean D, Mound LA, Worobey M, Morris DC (1997b) Phylogenetics of social evolution in Australian gall-forming thrips: evidence from mitochondrial DNA sequence, adult morphology, and gall morphology. Mol Phylogenet Evol 9:163–180
Crespi BJ, Morris DC, Mound LA (2004) Evolution of ecological and behavioural diversity: Australian Acacia thrips as model organisms. Australian Biological Resources Study and Australian National Insect Collection. CSIRO Entomology, Canberra
Foster WA (1990) Experimental evidence for effective and altruistic colony defence against natural predators by soldiers of the gall-forming aphid Pemphigus spyroythecae (Hemiptera: Pemphigidae). Behav Ecol Sociobiol 27:421–430
Foster WA, Rhoden PK (1998) Soldiers defend colonies against predators in the field. Anim Behav 55:761–765
Hölldobler B, Wilson EO (1990) The ants. Belknap Press of Harvard University Press, Cambridge, Mass
Jeon J, Choe JC (2003) Reproductive skew and the origin of sterile castes. Am Nat 161:206–224
Kranz BD, Schwarz MP, Mound LA, Crespi BJ (1999) Social biology and sex ratios of the eusocial gall-inducing thrips Kladothrips hamiltoni. Ecol Entomol 24:432–442
Kranz BD, Chapman TW, Crespi BJ, Schwarz MP (2001a) Social biology and sex ratios in the gall-inducing thrips, Oncothrips waterhousei and Oncothrips habrus. Insectes Soc 48:315–323
Kranz BD, Schwarz MP, Wills TE, Chapman TW, Morris DC, Crespi BJ (2001b) A fully reproductive fighting morph in a soldier clade of gall-inducing thrips. Behav Ecol Sociobiol 50:151–161
Krawac CA (2001) The evolution of galls size in Australian gall-inducing thrips on Acacia. Thesis, Flinders University of South Australia
Michener CD (1974) The social behaviour of the bees: a comparative study. Harvard University Press, Cambridge, Mass
Morris DC, Schwarz MP, Crespi BJ, Cooper JB (2001) Phylogenetics of gall-inducing thrips on Australian Acacia. Biol J Linn Soc 74:73–86
Mound LA (1971) Gall-forming thrips and allied species (Thysanoptera: Phlaeothripinae) from Acacia trees in Australia. Bull Br Mus Nat Hist Entomol 25:389–466
Mound LA, Crespi BJ (1995) Biosystematics of two new species of Australia Acacia gall-thrips with soldiers (Insecta; Thysanoptera). J Nat Hist 29:147–157
Mound LA, Heming BS (1991) Thysanoptera. Insects of Australia, vol 1. Commonwealth Scientific and Industrial Research Organisation, division of Entomology. Melbourne University Press, Melbourne
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton
Perry SP, McLeish SJ, Schwarz MP, Boyette AH, Zammit J, Chapman TW (2003) Variation in propensity to defend by reproductive gall morphs in two species of gall-forming thrips. Insectes Soc 49:1–5
Rice WR (1989) Analysing tables of statistical tests. Evolution 43:223–225
Ricklefs RE, Stark JM (1996) Applications of phylogenetically independent contrasts: a mixed progress report. Oikos 77:167–172
Shingleton AW, Foster WA (2001) Behaviour, morphology and the division of labour in two soldier-producing aphids. Anim Behav 62:671–679
Sokal RR, Rohlf JF (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. Freeman, New York
Wills TE, Chapman TW, Kranz BD, Schwarz MP (2001) Reproductive division of labour coevolves with gall size in Australian thrips with soldiers. Naturwissenschaften 88:526–529
Wilson EO (1971) The insect societies. Belknap Press of Harvard University Press, Cambridge, Mass
Wilson EO (1975) Sociobiology. Belknap Press of Harvard University Press, Cambridge, Mass
Acknowledgements
This work was supported by an Australian Research Council Grant to M.P. Schwarz, J.B. Cooper, B.J. Crespi and T.W. Chapman (DP0346322) and an ARC postdoctoral fellowship to T.W. Chapman. We are grateful to Dr. Laurence Mound, Dr. Laurence Packer and anonymous reviewers for their thoughtful contributions on the drafts of the manuscript, and to Michael McLeish, John Zammit and Dr. Adam L. Cronin for their assistance during field collections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Gwynne
Rights and permissions
About this article
Cite this article
Perry, S.P., Chapman, T.W., Schwarz, M.P. et al. Proclivity and effectiveness in gall defence by soldiers in five species of gall-inducing thrips: benefits of morphological caste dimorphism in two species (Kladothrips intermedius and K. habrus). Behav Ecol Sociobiol 56, 602–610 (2004). https://doi.org/10.1007/s00265-004-0811-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0811-8