Abstract
Free-ranging adult male baboons give loud two-syllable ‘wahoo’ calls during dawn choruses, interactions between groups, when chasing females, and in aggressive interactions with other males. Previous research has shown that the rate and duration of these contest wahoos are correlated with a male’s competitive ability: high-ranking males call more often, call at faster rates, and call for longer bouts than do low-ranking males. Here we report that acoustic features of wahoos also reveal information about male competitive ability. High-ranking males give wahoos with higher fundamental frequencies (F0) and longer ‘hoo’ syllables. Within-subject analyses revealed that, as males fall in rank, the hoo syllables tend to shorten within a period of months. As males age and continue to fall in rank, F0 declines, hoo syllables shorten, and formant dispersion decreases. Independent of age and rank, within bouts of calling F0 declines and hoo syllables become shorter. Because wahoos are often given while males are running or leaping through trees, variation in these acoustic features may function as an indicator of a male’s stamina. The acoustic features of contest wahoos thus potentially allow listeners to assess a male’s competitive ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual selection has favored the evolution of exaggerated male traits in numerous species. Such traits may enhance a male’s competitive success, make him more attractive to females, or both. Exaggerated male traits may include distinctive vocalizations. In a number of species of birds, amphibians, and mammals, for example, males produce loud, repetitive calls that apparently function as displays of size, condition or fighting ability (e.g. Davies and Halliday 1978; Clutton-Brock and Albon 1979; Reby and McComb 2003). If the production of such calls is more costly or difficult for individuals in poor condition than for those in good condition, they provide listeners with accurate information about signallers’ relative competitive abilities (Zahavi 1975; Grafen 1990, but see also Maynard Smith 1994; Silk et al. 2000; Vehrencamp 2000).
However, not all signal features need to bear some cost to provide accurate information about the signaller. In the case of so-called index signals, physiological constraints determine the quality of the signal (Vehrencamp 2000). In several species of amphibians and birds, for instance, the fundamental frequency (F0) of male display calls is significantly correlated with body size. Since large size typically confers an advantage in male–male competition, F0 functions as a reliable indicator of fighting ability (Davies and Halliday 1978; Ryan and Brenowitz 1985; Bee et al. 1999; Bee 2002). Other acoustic features also potentially serve as indicators of size and hence competitive ability. In mammals, the filter function of the vocal tract is directly related to its length, which in turn is correlated with body size (Fitch 1997). As the vocal tract increases in length, successive resonant frequencies (formants) become more closely spaced. As a result, the spacing of formant frequencies (‘formant dispersion’) provides an accurate cue to overall body size. The roars of red deer, for instance, exhibit a close correlation between formant dispersion, age, and weight (Reby and McComb 2003). Simply due to the mechanics of sound production, therefore, the roars function as honest indicators of a male’s competitive ability (Fitch and Hauser 2003).
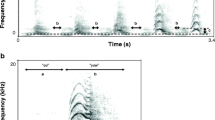
In this paper we examine the loud calls given by free-ranging male baboons (Papio cynocephalus ursinus) to test whether acoustic features vary in relation to several measures of male condition, including body size, dominance rank, age, and apparent exhaustion after a lengthy display. The loud calls—or ‘wahoos’ (Hall and DeVore 1965)—of male savannah baboons consist of a loud bark, the ‘wa’, and a second, lower amplitude ‘hoo’ (Fig. 1). Wahoos are the loudest calls in the baboons’ repertoire and appear to be adapted for long-range communication. Depending on wind conditions they may be audible from more than one kilometer.
Wahoos are given by males to predators (‘alarm’ wahoos) and during aggressive interactions with conspecifics (‘contest’ wahoos) (Saayman 1971; Cheney and Seyfarth 1977). Despite their superficial similarity, alarm and contest wahoos are significantly different from each other acoustically. Contest wahoos are given at a much higher rate, exhibit lower frequency characteristics, have longer hoo durations, and a relatively louder hoo portion than alarm wahoos (Fischer et al. 2002). In playback experiments, alarm and contest wahoos elicit different responses from listeners (Kitchen et al. 2003a).
Contest wahoos appear to function as displays of male competitive ability (Kitchen et al. 2003b). They are frequently heard at dawn, when males may participate in a chorus of calling, and are also given during aggressive interactions, as males either chase each other or herd females (Buskirk et al. 1974). Males involved in such chases often engage in conspicuous displays by running through the group or leaping from branch to branch in trees while calling. Prolonged calling and the physical activity that often accompanies calling may require considerable stamina. Following protracted wahoo bouts, males often appear to be physically exhausted.
Previous research (Kitchen et al. 2003b, 2003c) found that high-ranking males are more likely to participate in wahoo bouts, give wahoos at higher rates, and produce longer bouts than low-ranking males. Regardless of rank, however, all males are most likely to participate in wahoo bouts with opponents whose dominance ranks are similar to their own (Kitchen et al. 2003b). Supporting the view that males use wahoos to assess their opponent’s competitive ability, bouts involving males of similar rank (and, presumably, similar competitive abilities) are longer and involve higher rates of calling than bouts involving disparately-ranked males. They are also more likely to escalate into physical fighting (Kitchen et al. 2003b).
Here we focus on acoustic variation in contest wahoos and its potential relation to male competitive ability. Our analysis consists of three parts: First, we examine calls for acoustic variation in relation to dominance rank. Second, we test whether call features change as individual males drop in rank both over relatively short periods of time (on average 9 months) and over longer periods of time (3–10 years). Third, we analyze the variation within a given bout of calling, to test whether acoustic features also change over very short periods of time, presumably as a result of exhaustion. On the assumption that contest wahoos have been selected to reflect male condition, we predicted that the calls of high-ranking males would differ from those of other males not only in rate of delivery and bout length (Kitchen et al. 2003b) but also in their acoustic features.
Methods
Study site and subjects
The study site lies in the Moremi Wildlife Reserve in the Okavango Delta, Botswana, a huge inland delta fed by the Okavango River. Yearly rainfall in Angola causes the Okavango and its tributaries to rise and flood the grasslands. Only slightly elevated patches of woodland, or ‘islands’, which range in size from less than one to over hundreds of hectares, remain uncovered (Hamilton et al. 1976; Ross 1987). During the flood, baboons ford the floodplains to travel from one island to the next.
The main study group, C, has been observed since 1978. All individuals are easily identifiable and matrilineal relatedness of all natal animals is known. Subjects are fully habituated to observers on foot. During this study, C group contained 78–88 animals, including 17 different adult males. The mean number of males on any given day was 12 (range=10–13). The number of males fluctuated due to immigrations, emigrations, maturation and death. Subjects included all immigrant and natal males 8.5 years of age or older, the earliest age at which males have been observed to transfer between groups in this area (D.L. Cheney and R.M. Seyfarth, unpublished data).
Data collection
Most calls used in the analysis were tape-recorded during a 23-month study period (June 1999–May 2001). Details of behavioral observations and data collection are given elsewhere (Kitchen et al. 2003b, 2003c). Calls were recorded using digital audio tape (DAT) recorder (Sony Walkman TCD-100) and Sennheiser ME66 directional microphones. To examine whether call features change over longer periods of time, we also analyzed calls that were tape-recorded from four males as part of several earlier studies (1992–1999) on the vocal communication of this species (Cheney et al. 1995; Palombit et al. 1997; Fischer et al. 2002). During these studies, calls were recorded using the equipment described above or analogue cassette recorders (SONY WM-DC6).
Acoustic analyses
We first visually inspected and sampled calls that were not disturbed by background noise (i.e. bird song, other animals calling) at a sample frequency of 44,100 Hz using Cool Edit 2000 (Syntrillium, Phoenix, Ariz.). For the spectral analysis, we reduced the sample frequency to 11,025 Hz to obtain a better frequency resolution. Next, we used the SIGNAL sound analysis system (Engineering Design, Belmont, Mass.) to conduct a fast Fourier transform (1,024-pt FFT; time step: 5 ms; frequency range:4,410 Hz; frequency resolution: 10.8 Hz). We submitted the resulting frequency time spectra to a custom software program (LMA 8.4) that extracts call parameters from acoustic signals (Hammerschmidt 1990; Schrader and Hammerschmidt 1997).
Additionally, we conducted a linear predictive coding (LPC; Markel and Gray 1976) analysis using Speechstation2 (Sensimetrics, Somerville, Mass.). LPC analysis is an alternative to Fourier analysis for describing the spectrum of a signal segment (Owren and Linker 1995; see Owren et al. 1997 for an application of LPC analysis to baboon grunt vocalizations). We calculated 12 LPC coefficients at a sampling rate of 22,050 Hz and a frequency range of 11,025 Hz. To obtain a better characterization of the energy at higher frequencies, we used a pre-emphasis filter of 6 dB/octave. We determined the location of the first five peaks, the amplitude ratio between the 1st and 2nd peak for the mid segment of the wa syllable, which exhibited a nearly constant F0. We identified the peaks using a cursor on the screen, guided by the formant tracks as indicated by the software. We checked observer reliability on a random selection of approximately 10% of the calls. The correlation between the values determined by two independent observers varied in relation to the variable under analysis. Pearson correlation coefficients ranged between 0.886 and 0.978 for all variables except the fifth formant, where observer agreement was 0.768. The formant peaks were located at around (mean±SD) 850±50 Hz, 2,100±200 Hz, 5,200±250 Hz, 7,050±450 Hz, and 8,900±200 Hz (n= 14 adult males). From these results, we determined the formant dispersion, i.e. the average distance (Hz) between adjacent peaks for all five peaks. Because of the lower observer reliability for the 5th peak, we additionally considered the difference between first and second formant (f1f2 difference). Finally, we determined the amplitude ratio between first and second formant (f1f2 ratio). Formants were not evenly spaced in the spectrum, indicating that the vocal tract of male baboons does not fit the model of a straight tube (see Fant 1960 for examples of changes in the width of a vocal tract that can produce shifts in resonance frequencies). Therefore, the model of a straight tube should be used cautiously when vocal tract length is inferred from the location of the formants.
We ran two types of analysis, one based on the quantitative description of variables derived from the spectrogram and one based on LPC analysis. Based on a previous analysis of acoustic variation in relation to context, age, and sex (Fischer et al. 2002), we examined the following seven variables in relation to male condition: F0 (Hz), wa duration (ms), hoo duration (ms), dfa1 start (Hz) (frequency at which the distribution of frequency amplitudes reaches the 1st quartile at beginning of call), formant dispersion (Hz), f1f2 difference (Hz), and f1f2 ratio. We were unable to measure call amplitude in the field because of the varying distances between males and the observers.
We determined the inter-call interval (ICI) between successive calls to the nearest second and examined whether it was related to the acoustic features of the previous or subsequent call. We measured ICIs before (n=282) and after a call (n=276). The median ICI was 3 s (inter-quartile range: 2–7 s). None of the acoustic variables was related to either the preceding or the following ICI in either analysis (Pearson correlation coefficients <0.2).
Dominance rank
Dominance ranks were established using data collected by both focal animal and ad libitum methods (Kitchen et al. 2003b). Dyadic dominance relations were defined according to the direction of approach-retreat interactions and submissive behavior. Submissive behavior included the ‘fear grimace’ (lips pulled back exposing clenched teeth), ‘fear bark’ (a cough-like vocalization) and ‘lean-away’ (the subordinate glances at and then turns head and extends body away from approaching dominant). Dominance rank orders were linear and unidirectional, at least over the short term. Among all males, there was an average of 1.5 rank reversals per month (Kitchen et al. 2003b).
For every display bout, a relative dominance rank was assigned to each participant based on his place in the hierarchy at the time. A male’s rank was then expressed as the percent of males dominated, which controlled for changes in the total number of males residing in the group. Percent males dominated was calculated as (n−r)/(n−1), where n=the number of male subjects and r=an individual’s rank (Kitchen et al. 2003b). In the analysis of the relation between dominance rank and wahoo features, results were unaffected when these ranks were replaced with absolute ranks.
Age and size
The ages of all natal adult males were known. Although we could use residence length to estimate the relative ages of non-natal males who had lived in the group for several years, it was more difficult to estimate the ages of recent immigrants, because male baboons often transfer between groups more than once during their lives. We therefore estimated the ages of immigrant males through tooth wear, classifying males as young, middle-aged, or old (for details see Kitchen et al. 2003b). Male body size was determined through measures of shoulder height (Kitchen et al. 2003b). On average (mean±SD), the shoulder height was 711±17 mm (range: 683–736 mm). Repeated measurements of the same male yielded consistent, repeatable differences among individuals: the mean standard error for males measured on multiple days was 3.8 mm (range=1.2–8.6 mm). The average weight of the males (n=6) was 27.2 kg (range: 25–29.3 kg).
Statistical analysis
To analyze between-male differences, we calculated the mean for each acoustic variable across all calls recorded from a given male while he held a given rank. For each male, we only considered the entry that was based on the largest number of calls. However, as some of the bins were quite small, we lumped neighbouring entries. For instance, male BJ contributed 2 calls while he ranked second and 14 calls while he ranked third (during the next month). We then calculated the average rank for the male across all calls contributed. In this way, males could be ordered in a linear dominance hierarchy based on their average ranks. Ultimately, our analysis was based on 196 calls recorded from 14 males, sampled from 2–18 different bouts per male. Calls were sampled approximately evenly within and between males from beginning (46.5% of calls) and end (53.5%) of high (43.5%) and low intensity bouts (56.5%; see Kitchen et al. 2003b). Calls sampled within the same bout were separated by an average of 4.8 calls (SEM=1.3) and by an average of 20.8 s (SEM=4.0), far longer than the average inter-call interval of 3 s. We used GLM (general linear model) analysis, which allows the modelling of continuous dependent variables compared with continuous and categorical predictor variables (Dobson 2002). We used rank, height, and tooth score as predictor variables, and applied the backward stepwise selection process to identify the best model: that is, the model that included only those variables that explained the largest part of the variance.
To analyze within-male variation, we used two independent data sets. For the first analysis, we sampled calls when a male held a given rank, and again after he had fallen in rank. The average time span between the ‘high-ranking’ and the ‘low-ranking’ condition was 9 months (range: 2.5–19.5 months). During this time, males fell an average of 4.3 rank positions (range: 2–7). This data set contained a total of 256 calls recorded from eight males, with 22–36 calls per male sampled from 3–13 different bouts per male. We sampled calls approximately evenly within and between males from bouts of both high (36.3% of calls) and low calling intensity (63.7%) and from the beginning (42.5%) and end (57.5%) of bouts. Calls sampled within the same bout were separated by an average of 1.7 calls (SEM=0.3) and by an average of 12.6 s (SEM=1.7). For each male, we used the mean value for each acoustic variable, creating a matched-pair dataset in which each male could be tested using each acoustic measure in his high- and low-ranking condition. Acoustic variation was tested with a repeated measures ANOVA based on a permutation approach (Mundry 1999). In a second analysis, we examined 143 calls recorded from four males over 3–10 years. As with the analysis based on rank, we calculated, for each male, mean values for each acoustic measure in each year. We then used a generalized mixed model (GLMM) analysis with individual as a random factor to identify acoustic variation in relation to relative age (as estimated by tooth wear and the duration of a male’s residence in the group). GLMM analyses are an extension to GLM, which allow the incorporation of ‘subjects’ as random effects to control for replicated observations (Pinheiro and Bates 2000).
To examine acoustic changes within a bout of calling, we analyzed three calls each from the beginning and end of call bouts that lasted at least 2 min. Altogether, we considered ten bouts in this analysis, recorded from six males (four males contributed two bouts each). We calculated the mean values for the variables under interest across the calls taken from the start and end of the bout and entered these into a GLMM analysis with position in bout as a fixed factor, subject as a random factor, and total number of calls in the bout and bout length as covariates. In a second test, we analyzed 1–3 calls sampled approximately 10 s into the bout, and 1–3 calls sampled approximately 60 s into the bout. As above, we calculated the mean values for the acoustic variables across the 10 s and 60 s positions. In this analysis, we considered 12 bouts recorded from 11 individuals. Statistical analyses were calculated using Statistica and SPSS 9.0. To control for multiple tests, we calculated adjusted P values based on Hochfeld’s improved Bonferroni method (Hochfeld 1988; Shaffer 1995). All tests are two-tailed.
Results
Rank, age, and size
As already reported (Kitchen et al. 2003b), we found no consistent relation between a male’s dominance rank and either tooth score (Pearson’s correlation coefficient r=0.329, n=14, P=0.251) or shoulder height (r=−0.313, n=14, P=0.276). Although all males judged to be old by tooth wear or long-term demographic data were either low- or middle-ranking, not all young males were high-ranking. As a result, there was no clear relation between age and dominance rank. The lack of a correlation between age, size and dominance rank also arose because of instability in male ranks. During the 2-year study, each male occupied a mean of 3.4 different ranks (range: 2–7). An average of 3.2 males occupied each of the 12 rank positions (range: 2–6).
Rank, age, and acoustic variation
When we analysed acoustic variation across males, the best model identified rank as the only variable to explain variation in F0 and hoo duration (Table 1). The same was true if absolute instead of relative ranks were used (F0 adjusted R 2=0.454, P<0.005; hoo duration: adjusted R 2=0.362, P<0.013). Males who held a higher average rank produced calls with a significantly higher F0 and significantly longer hoo syllables than lower-ranking males (Fig. 2). Furthermore, tooth score explained 75% of the observed variation in formant dispersion. Males with teeth in poor condition, who were typically old and low-ranking, produced wahoos with a lower formant dispersion than those produced by higher-ranking males (Fig. 3). Notably, male height showed only a weak effect for dfa1 start and was unrelated to all other variables. The remaining acoustic variables revealed no significant relation with any of the predictor variables.
When individual males dropped in rank over a relatively short period of time, hoo duration decreased, although not significantly (Table 2). Over longer periods of time, several acoustic variables showed changes in relation to a male’s relative age (Table 3). In particular, the difference between the first and second formant decreased significantly as males aged (Fig. 4a), while the ratio between the first and second formant decreased. Older males, who also typically occupied lower ranks, also produced calls with a lower F0 and a shorter hoo syllable than younger males (Fig. 4b). In the latter analysis, dfa1 start increased significantly, while it decreased over a shorter period of time.
Changes within a call bout
When we considered the ten call bouts with a minimum length of 120 s, two acoustic variables revealed significant changes in relation to call position within a bout (Table 4). First, F0 decreased slightly. Second, hoo duration was significantly shorter at the end of a bout than at the beginning. When we compared changes from 10 s into the bout to 60 s into the bout, none of the variables revealed significant differences, although the F0 once again decreased (P<0.1).
Discussion
Compared with the wahoos of other males, the wahoos of high-ranking male baboons had a significantly higher F0 and hoos of significantly longer duration. As individual males fell in rank, over periods of both months and years, their hoo syllables became shorter. In addition, both the distance between the first two formants and their relative amplitude changed (see below). By elongating the hoo syllable, high-ranking males exaggerated one of the acoustic feature that distinguished males’ from females’ calls, adults’ from adolescents’ calls, and contest from alarm wahoos (Fischer et al. 2002). Relative to alarm wahoos, therefore, the contest wahoos of high-ranking males displayed exaggerated contest features.
Similarly, wahoos produced at the beginning of a calling bout were significantly different acoustically from those produced at the end. During calling bouts, the F0 of a male’s wahoos declined and his hoos became shorter in duration. Changes in both measures were evident over periods as short as 2 min.
Are wahoos energetically costly, thereby providing honest evidence of a male’s competitive ability? Data on modes of delivery support this view. High-ranking males participated more often in wahoo displays, called at higher rates, and produced more wahoos per bout than did low-ranking males (Kitchen et al. 2003b). In theory, then, listeners could assess the competitive ability of a rival by attending to these measures of wahoo behavior.
Could listeners make a similar assessment from the acoustic features of wahoos alone, independent of their mode of delivery? At present, it is difficult to assess the direct costs of animal vocalizations under field conditions (but see Thomas 2002). The energetic cost of producing just one call is probably not very great. When male baboons wahoo, however, they usually produce several consecutive calls (mean=20 wahoos in 120 s), often while chasing other individuals. A male’s stamina and endurance may have a significant effect on whether a male can maintain certain acoustic features within his call, particularly in conjunction with physical activity. Data provide some support for this view, particularly as it applies to F0 and hoo duration.
The evidence that higher-ranking males produced wahoos with higher F0s seems at first counterintuitive, because high F0s are typically associated with subadults and smaller body size (Fischer et al. 2002). It is important to emphasize, however, that the higher F0s exhibited by high-ranking males (320–340 Hz) were still considerably lower than the average F0 exhibited by adolescent males (380 Hz, Fischer et al. 2002). That is, by their acoustic features, high-ranking males were still, clearly, fully adult males. One possible explanation for the rise in F0 is that it may also be affected by the motivational state of the animal (Morton 1977) or vocal effort (Lienard and Di Benedetto 1999). In fact, Hsiao et al. (1994) showed that F0 in dogs increases linearly with subglottal pressure. Thus, the higher F0 of high-ranking males may have occurred because these males called at higher amplitudes than low-ranking males (see also Reby and McComb 2003). Unfortunately, the difficulty of obtaining accurate amplitude measurements from individuals who are running or leaping through trees currently prevents us from testing this hypothesis. Whatever the proximate mechanisms underlying the relatively high F0 of high-ranking males, the significant correlation between rank and F0 suggests that listeners could potentially use this cue to assess a male’s competitive ability.
We do not yet know exactly how hoos are produced: by exhaling, inhaling, or both. It is clear from field observations, however, that wahoo displays lead to short, rapid breathing, either because of call production itself, the running displays that accompany calling, or both factors. Shortness of breath would lead to shorter hoos regardless of whether they were produced by inhalation or exhalation. However, there was no direct effect of call rate on hoo duration, even when rank was held constant (J. Fischer, D.M. Kitchen, R.M. Seyfarth, D.L. Cheney, unpublished data). Since we did observe a significant decline in hoo duration during a bout of calling, the only feasible explanation for the lack of correlation between hoo duration and inter-call interval is that exhaustion does not necessarily manifest itself in short term changes in call rate. Here again, whatever the proximate mechanisms underlying hoo production, the significant correlation between rank and hoo duration suggests that listeners could potentially use this cue as an honest indicator of competitive ability.
Finally, the acoustic features of wahoos changed with age. Over periods as short as 3 years, the F0 of a male’s wahoos declined, his hoos became shorter, and the amplitude difference between the first and second formant of his calls became smaller, reflecting a general upward shift in the overall spectrum of his calls. By all of these measures (but particularly the first two), an older male’s calls became acoustically less like those of younger, high-ranking males. Apparently, older males cannot (or will not) produce wahoos with the same acoustic features as those produced by individuals in better condition. This, in turn, suggests that listeners can assess the relative age—and hence competitive ability—of an immigrant male even if they have not yet interacted with him or observed him interacting with others.
At least some of the observed acoustic variation in wahoos is perceptible to the human observer, and possibly also to the baboons themselves. For instance, higher ranking males have a F0 around 70 Hz higher than lower ranking males, at an average F0 of 260 Hz. Also, the 100–300 ms difference in hoo duration is clearly audible. Whether or not the 100 Hz difference between old and young males over the 2,000 Hz formant dispersion is meaningful to subjects seems less likely, but ultimately must be determined empirically. Playback experiments are currently underway to test the perceptual salience of the variation in these measures.
The lack of a significant correlation between weight, height, and acoustic measurements can be explained as follows. Weight and height appear to be uncorrelated because males in their prime tend to put on a lot of muscle and fat, whereas most of them become skinny and bony when they age, with no changes in shoulder height. Weight and height appear to be uncorrelated with acoustic features because the two former measures may exhibit relatively little variation among males who have reached fully adult size. Fischer et al. (2002) found that F0 and formant dispersion decreased significantly from juveniles to adolescents and adults. However, within an age class, this significant relationship disappeared. Pfefferle and Fischer (2003) found a similar result in Hamadryas baboons. We therefore believe that the observations presented in this paper are robust and not simply related to a measurement error.
One may argue that age alone caused the changes we observed in relation to rank, simply because old males are typically middle or low ranking. However, as mentioned before, not all young males were high ranking. In the analysis in which we examined the acoustic variation in relation to rank we entered age, rank, and height as predictor variables. In this particular analysis, we examined calls from high and low ranking young males, high and low ranking ‘middle aged’ males, as well as low ranking old males. Only rank was a significant predictor variable of F0 and hoo duration. This result and the analysis of within bout variation (demonstrating that variation can occur over the short term), suggest that stamina and endurance (or the lack thereof) underlie the variation in acoustic features, instead of some morphological process related to aging.
Intriguingly, there was a significant relation between age (as measured by tooth wear) and formant dispersion: older males—that is, those with more yellowed, worn teeth—had a significantly lower formant dispersion than males with teeth in good condition. Our behavioral observations suggest that males with good teeth tend to open their mouths more widely when they call (and also when they yawn), thus shortening their vocal tracts and increasing formant dispersion, whereas males with worn teeth open their mouths less widely, decreasing formant dispersion (see also Ohala 1984). Detailed analyses of video footage will be required to quantify this observation.
The precise determinants of male rank in our population are not clear and lie beyond the scope of this paper. Dominance rank is not correlated with the ability to recruit allies, because baboons in this population do not form alliances (Bulger 1993; personal observation). Newly immigrant and young adult males are more likely than other males to achieve alpha status (Hamilton and Bulger 1990; R.M. Seyfarth, D.L. Cheney, unpublished data; see also van Noordwijk and van Schaik 2001 for similar patterns in long-tailed macaques). Immigrant and young adult males also appear to be more willing to challenge other males and to escalate fights, perhaps not only because they are in better condition but also because they have not yet fathered offspring whom they need to protect against potentially infanticidal males (Hamilton and Bulger 1990; Bulger 1993; Palombit et al. 2000). It is likely that rank is determined by a combination of factors such as age, size, fighting ability, and social skills.
Although the factors that can lead to honesty in acoustic signals are the focus of much theoretical debate, the results of our study support the view that at least in some cases, honest signals arise because certain features are costly and not all individuals can produce them (e.g. Grafen 1990; Vehrencamp 2000). Possibly, high levels of testosterone—which typically are associated with display behaviours (e.g. Van Duyse et al. 2002)—also incur a cost to the signaller (Buchanan et al. 2001; Wingfield et al. 2001). Whichever way the costs are mediated, our data suggest that listeners could potentially assess a male’s competitive ability by attending to both his mode of delivery (frequency of participation, rate and duration of calling) and the acoustic features of his calls (F0, hoo duration, formant dispersion). Playback experiments to test the salience of each of these features are currently under way.
References
Bee MA (2002) Territorial male bullfrogs (Rana catesbeiana) do not assess fighting ability based on size-related variation in acoustic signals. Behav Ecol 13:109–124
Bee MA, Perrill SA, Owen PC (1999) Size assessment in simulated territorial encounters between male green frogs (Rana clamitans). Behav Ecol Sociobiol 45:177–184
Buchanan KL, Evans MR, Goldsmith AR, Bryant DM, Rowe LV (2001) Testosterone influences basal metabolic rate in male house sparrows: a new cost of dominance signalling? Proc R Soc Lond Ser B 268:1337–1344
Bulger J (1993) Dominance rank and access to estrous females in male savanna baboons. Behaviour 124:89–122
Buskirk WH, Buskirk RE, Hamilton WJ (1974) Troop-mobilizing behavior of adult male chacma baboons. Folia Primatol 22:9–18
Cheney DL, Seyfarth RM (1977) Behaviour of adult and immature male baboons during inter-group encounters. Nature 269:404–406
Cheney DL, Seyfarth RM, Silk JB (1995) The role of grunts in reconciling opponents and facilitating interactions among adult female baboons. Anim Behav 50:249–257
Clutton-Brock TH, Albon SD (1979) Roaring of red deer and the evolution of honest advertisement. Behaviour 69:145–170
Davies NB, Halliday TR (1978) Deep croaks and fighting assessment in toads (Bufo bufo). Nature 274:683–685
Dobson AJ (2002) An introduction to the generalized linear models, 2nd edn. Chapman and Hall/CRC Press, Boca Raton, Fla.
Fant G (1960) Acoustic theory of speech production. Mouton, The Hague
Fischer J, Hammerschmidt K, Cheney DL, Seyfarth RM (2002) Acoustic features of male baboon loud calls: influences of context, age, and individuality. J Acoust Soc Am 111:1465–1474
Fitch WT (1997) Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J Acoust Soc Am 102:1213–1222
Fitch WT, Hauser MD (2003) Unpacking honesty: generating and extracting information from acoustic signals. In: Simmons AM, Popper AN, Fay, RR (eds) Animal communication. Springer, Berlin Heidelberg New York
Grafen A (1990) Sexual selection unhandicapped by the Fisher process. J Theor Biol 144:473–516
Hall KRL, DeVore I (1965) Baboon social behavior. In: DeVore, I (ed) Primate behavior: field studies of monkeys and apes. Holt, Rinehart and Winston, New York, pp 53–110
Hamilton WJ, Bulger JB (1990) Natal male baboon rank rises and successful challenges to resident alpha males. Behav Ecol Sociobiol 26:357–363
Hamilton WJ, Buskirk RE, Buskirk WH (1976) Defense of space and resources by chacma (Papio ursinus) baboon troops in an African desert and swamp. Ecology 57:1264–1272
Hammerschmidt K (1990) Individuelle Lautmuster bei Berberaffen (Macaca sylvanus): Ein Ansatz zum Verständnis ihrer vokalen Kommunikation. PhD dissertation, Freie Universität, Berlin
Hochfeld Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–802
Hsiao T, Solomon NP, Luschei ES, Titze IR, Kang L, Fu T, Hsu M (1994) Effect of subglottic pressure on fundamental frequency of the canine larynx with active muscle tensions. Ann Otol Rhinol Laryngol 103:817–821
Kitchen DM, Cheney DL, Seyfarth RM (2003a) Female baboons’ responses to male loud calls. Ethology 109:401–412
Kitchen DM, Seyfarth RM, Fischer J, Cheney DL (2003b) Loud calls as indicators of dominance in male baboons (Papio cynocephalus ursinus) Behav Ecol Sociobiol 53:374–384
Kitchen DM, Cheney DL, Seyfarth RM (2003c) Factors mediating inter-group encounters in savanna baboons (Papio cynocephalus ursinus) Behaviour (in press)
Lienard JS, Di Benedetto MG (1999) Effect of vocal effort on spectral properties of vowels. J Acoust Soc Am 106:411–422
Markel JD, Gray AH (1976) Linear prediction of speech. Springer, Berlin Heidelberg New York
Maynard Smith J (1994) Must reliable signals always be costly? Anim Behav 47:1115–1120
Morton ES (1977) On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am Nat 111:855–869
Mundry R (1999) Testing related samples with missing values: a permutation approach. Anim Behav 58:1143–1153
Noordwijk MA van, van Schaik CP (2001) Career moves: transfer and rank challenge decisions by male long-tailed macaques. Behaviour 138:359–395
Ohala JJ (1984) An ethological perspective on common cross-language utilization of F0 of voice. Phonetica 41:1–16
Owren MJ, Linker C (1995) Some analysis methods that may be useful to acoustic Primatologists. In: Zimmermann E, Newman JD, Jürgens U (eds) Current topics in primate vocal communication. Plenum, New York, pp 1–28
Owren MJ, Seyfarth RM, Cheney DL (1997) The acoustic features of vowel-like grunt calls in chacma baboons (Papio cyncephalus ursinus): implications for production processes and functions. J Acoust Soc Am 101:2951–2963
Palombit RA, Seyfarth RM, Cheney DL (1997) The adaptive value of ‘friendships’ to female baboons: experimental and observational evidence. Anim Behav 54:599–614
Palombit R, Cheney D, Seyfarth R, Rendall D, Silk J, Johnson S, Fischer J (2000) Male infanticide and defense of infants in chacma baboons. In: van Schaik C, Janson C (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 123–152
Pfefferle D, Fischer J (2003) Sounds and size—identification of variables that reflect body size in Hamadryas baboons. Folia Primatol 74:212
Pinheiro JC, Bates DM (2000) Mixed-effect models in S and S-Plus. Springer, Berlin Heidelberg New York
Reby D, McComb K (2003) Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav 65:519–530
Ross K (1987) Okavango: jewel of the Kalahari. Macmillan, New York
Ryan MJ, Brenowitz EA (1985) The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am Nat 126:87–100
Saayman GS (1971) Behaviour of adult males in a troop of free-ranging chacma baboons. Folia Primatol 15:36–57
Schrader L, Hammerschmidt K (1997) Computer-aided analysis of acoustic parameters in animal vocalizations: a multi-parametric approach. Bioacoustics 7:247–265
Shaffer JP (1995) Multiple hypothesis testing. Annu Rev Psychol 46:561–584
Silk JB, Kaldor E, Boyd R (2000) Cheap talk when interests conflict. Anim Behav 59:423–432
Thomas RJ (2002) The costs of singing in nightingales. Anim Behav 63:959–966
Van Duyse E, Pinxten R, Eens M (2002) Effects of testosterone on song, aggression, and nestling feeding behavior in male great tits, Parus major. Hormon Behav 41:178–186
Vehrencamp S (2000) Handicap, index, and conventional elements of bird song. In: Espmark Y, Amundsen T, Rosenqvist G (eds) Animal signals: signalling and signal design in animal communication. Tapir Publishers, Trondheim, Norway, pp 277–300
Wingfield JC, Lynn SE, Soma KK (2001) Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain Behav Evol 57:239–251
Zahavi A (1975) Mate selection—selection for a handicap. J Theor Biol 53:205–214
Acknowledgements
We thank the Office of the President and the Department of Wildlife and National Parks of the Republic of Botswana for permission to conduct research in the Moremi Wildlife Reserve. Jim Nicholson, Kurt Hammerschmidt, Mokupi Mokupi, and Keena Seyfarth assisted with data collection. Ryne Palombit kindly made some of his recordings available for analysis. We are grateful to Dana Pfefferle for support with the acoustic analysis, and Daniel Stahl for statistical advice. Three anonymous reviewers provided valuable comments on the manuscript. This research was supported by the Deutsche Forschungsgemeinschaft (Fi 707/2; 707/4), and by National Science Foundation grant IBN 9514001, National Institutes of Health grants MH62249, the Leakey Foundation, and the Research Foundation of the University of Pennsylvania. This research complies with the laws of the Republic of Botswana and was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Nunn
Rights and permissions
About this article
Cite this article
Fischer, J., Kitchen, D.M., Seyfarth, R.M. et al. Baboon loud calls advertise male quality: acoustic features and their relation to rank, age, and exhaustion. Behav Ecol Sociobiol 56, 140–148 (2004). https://doi.org/10.1007/s00265-003-0739-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0739-4