Abstract
The ability to resist infections can differ between individuals of different social rank. This study investigates if the clearance of an avian virus infection (Sindbis virus) was related to the social status of greenfinches (Carduelis chloris) and if infected birds would decline in social status. The results showed that virus clearance patterns were related to social status. Within groups of birds with similar plumage color (a social status signal), body mass and age, a bird’s social status could be used to predict virus clearance patterns. Dominant birds had higher virus concentrations early in the infection and lower virus titres during later stages of the infection as compared to birds that were subordinates. The infection had no significant effect on previously established social ranks, and ranks that were established during the infection did not appear to be influenced by a bird’s infection status. In conclusion, this study exemplifies a case where the social rank of an individual was a predictor of the ability to clear a viral infection. The underlying physiological mechanisms of this relationship remain to be further investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An animal’s social status position in the group can influence many fundamental aspects of its biology such as the probability of survival, being able to reproduce or resist infectious disease (Pusey and Packer 1997; Zuk and Johnsen 1999). In some animal species, including the greenfinch (Carduelis chloris), both social status and parasite resistance ability are accurately revealed by plumage characteristics (Rohwer 1975; Hamilton and Zuk 1982). In greenfinches, the brightness of a male’s plumage (i.e., more or less yellow) functions both as a badge of social status (Eley 1991), and as an indicator of a male’s parasite resistance ability. Males with a brighter plumage have been found to have fewer blood parasites (Merilä et al. 1999) and faster clearance of a Sindbis virus infection (Lindström and Lundström 2000), and are able to mount stronger humoral immune response after challenge (Saks et al. 2003). To explain how ornamental traits such as plumage characteristics in birds can be related to parasite resistance two alternative ideas have been put forward. First, the relationship between signaling traits and parasite resistance can be related through the protective properties of antioxidants, for example carotenoids that are used both during clearance of infections and in many animal signals, such as the greenfinch plumage (Olson and Owens 1998; Saks 2003). Alternatively, the interrelationship could be mediated by the immunosuppressive effects of steroid hormones such as testosterone or stress hormones (Folstad and Karter 1992; Buchanan 2000). Both these hormones are influenced by social behaviors. In some flock living animals, subordinate animals are subject to higher levels of stress hormones (Creel 2001). If dull greenfinch males are exposed to chronic social stress as a consequence of their low social status, and this has a negative impact on their immune functions, it would explain their reduced ability to resist parasites. This study was performed to investigate the interrelationship between social status and disease resistance in male greenfinches, and to test if the maintenance of a low social status was associated with reduced virus clearance ability.
Being infected by a parasite can also affect an individual’s social status. If the maintenance of a high social rank is costly, individuals might be forced to allocate resources to maintain a high rank at the expense of other costly activities such as parasite clearance. Thus animals might respond to infections by conceding rank (Freeland 1981; Zuk et al. 1998). The second objective of this study was to test if the experimental virus infection would affect an individual’s ability to maintain or obtain a high status position.
Methods
Study system
The study was performed on greenfinches infected with the Sindbis virus (Togaviridae, Alphavirus). The greenfinch is a medium sized (ca. 30 g) seed-eating passerine that lives gregariously during large parts of the year (Cramp and Perrins 1994). In the study area, the greenfinch is one of many avian hosts for the mosquito-transmitted Sindbis virus (Lundström 1999). In the summer months, the prevalence of natural Sindbis virus infections is around 10 % in greenfinches in our study area (Lundström and Lindström, unpublished data). There is currently no evidence that this virus infection has pathological effects and causes disease in avian hosts. For greenfinches, the size of the male tail patch is positively related to overall plumage brightness, and negatively related to Sindbis virus clearance rate (Lindström and Lundström 2000).
Greenfinches were caught in mist-nets in the Uppsala area (59°50′N; 17°50′E) in January, and were kept alone in indoor cages (50×50×50 cm) on a natural daylight cycle with unlimited access to water and sunflower seeds until the experiment started in March 1999, when birds were placed together in pairs in the cages. After capture, all birds were ringed, color-banded and aged into two age classes, yearlings or older. Their body mass was measured with a Pesola scale (to the nearest 0.1 g). The length of the right yellow tail patch was measured with a ruler to the nearest mm. The 32 male greenfinches in the study were divided into eight experimental groups consisting of four birds each. The groups consisted of birds that were matched according to age, tail patch length and body mass, so that within groups, birds were of the same age and as similar as possible with respect to tail patch length (average group differences max-min were 10%) and body mass (average group difference max-min were 9%). Body masses were measured on three occasions; directly after capture, the second day of the experiment, and 4 days after the infection. All birds were blood-sampled before the experiment and all were found seronegative for Sindbis virus neutralizing antibodies in a plaque reduction neutralizing test in Vero cell cultures where 80% plaque-reduction was used as the cut-off limit (Earley et al. 1967; Francy et al. 1989).
Dominance tests
Six mornings before the infection and the first four mornings after the infection, dominance tests were performed for each pair. For these tests, the food and water bowl was removed at 1600 hours the day before the observations and then reintroduced to them between 1000 and 1200 hours the following day. The third day after infection, observations were made a second time in the afternoon. The food and water bowl was then removed at 1300 hours, and pairs were observed between 1400 and 1600 hours. The order of these tests was altered each day so that the pair that was observed last on one test day was observed first on the next. When pairs were shifted, both males in a pair were transferred to a new cage, so that no male would have prior experience of the cage.
In all tests, the pair was observed from a hiding place at 2-m distance immediately after the food and water bowl was replaced in the cage. Each interaction that took place between the males was recorded and for each interaction a winner and a loser was appointed. The behaviors that were performed by the male initiating these contacts were typically either threat display or attack (Senar 1990). An individual was considered to be a winner if its opponent gave a submissive posture or withdrew after having been exposed to a threat display or an attack. The winners could thereby gain or retained access to food, water or perch. When one male had won significantly more than 50 % of the encounters as indicated by a binomial test, that male was classified as the dominant bird of the pair that day.
Since all males were ranked relative to the other three birds within each experimental group they could be assigned a within-group rank ranging from 1 to 4, with 1 being the lowest rank. The two infected birds in each group were classified either as dominants or subordinates relative to each other. Since some infected birds that were classified as subordinates relative to the other infected bird were actually ranked higher than other birds within their experimental group, the within-group ranks (1–4) gave more detailed information of their social status. In some cases the within-pair ranks changed between days, in such cases males were classified as being higher ranked than their counterparts if they had been dominant in the majority of the observation days.
Social rank
Within experimental groups of four birds, birds were randomly divided into two subgroups (1 or 2). Within these subgroups, one bird was assigned to a virus treatment (V) and the other to a control treatment (C). Thus, within groups, each bird had unique assignments to subgroup/treatment (V1, V2, C1 and C2). The week before the infection, within group ranks were determined for each individual relative to two other birds within groups during three subsequent days. This was done as a control to estimate how often birds would change their ranks within the pairs without being subject to any treatment. In all groups, the V1-assigned birds were ranked against the C1 birds and the V2 birds. Thus, V1 bird was used twice, but the pairs were unique. The observations made before the treatment showed that the dominance hierarchies that were measured tended to stabilize over time. Between day one and two, 20/32 or 62 % of the ranks were stable, and between day two and three, 28/31 or 90% were stable. Since the pairs that were kept together after receiving the treatment had spent more than 3 days together, I made the assumption that 90% of the dominance hierarchies would be stable between subsequent test days if the hierarchies were unaffected by the infection.
After these control observations, birds were kept in the V1-C1 and V2-C2 combination until the infection, when one bird in each group was infected. The study was designed as a one-tailed test, to test if dominant birds would decrease in rank when infected. To increase the number of cases where the dominant would receive the infection treatment, I used the information from the control observations and switched treatment assignments between birds if both C assigned males had been dominant relative to V assigned males in the control tests. Of the birds that were given the infection treatment, 11 of 16 had been dominants in relation to their control.
On the first 4 days after one bird had received the infection, dominance tests were performed. Since the Sindbis virus titre in the blood typically peaks around the third day after infection, any behavioral consequences of the infection was expected to be most pronounced at this time. Therefore, on day three, all infected (V) birds were ranked relative to both control treated (C) birds within the experimental group. Thus, for the first time in the experiment, birds within all groups were combined in the previously untested V1-C2 and V2-C1 combination.
Virus infection and detection
The 16 males that had been assigned to the V-treatment were infected with Sindbis virus. Each of these males received a subcutaneous injection containing 104 plaque-forming units (PFU) of Sindbis virus diluted in 100 μl water with 0.09% NaCl. The virus dose was selected to match the dose transmitted by a mosquito during a natural infection (Chamberlain and Sudia 1961). For the infection, the Eds 82/5 strain of Sindbis virus was used. This is genetically a typical northern European strain (Norder et al. 1996). The strain was taken through two passages in Vero cells before use in this study. Birds assigned to the C treatment received an injection of 100 μl water with 0.09% NaCl.
On the first 7 days following the infection, blood samples were taken from birds in both treatment groups by extracting 0.1 ml blood from the jugular vein with a 27-gauge needle and a 0.5-ml syringe each afternoon between 1600 and 1700 hours. Handling and sampling required less than 5 min for each bird. Whole blood samples were immediately diluted 1:10 in Hank’s balanced salt solution supplemented with 2% Hepes buffer and 10% fetal calf serum (Life Technologies). Samples were kept on ice for a maximum of 2 h, and then stored at −70°C until assayed for virus content in a plaque test as described in Lundström et al. (1990). The lowest detectable virus titre in the assay was 101 PFU/ml whole blood, and virus titres ranged between 101 and 104.7 PFU/ml in this experiment. Using the daily virus titres, I calculated the total viremia for each bird, defined as the sum of log10 virus titers measured days 1-7. To test for differences in virus proliferation patterns at different stages of the infection, the virus titres of each bird was divided into an early (sum of virus titers days 1–3) and late (sum of virus titers days 5-7) phase. These stages represented a proliferation phase, where the average virus titres was rapidly increasing, and a clearance phase, where viruses were being neutralized by the immune system. The fourth day after infection represented an intermediate stage and was excluded from this comparison. After the experiment, control treated birds were released and virus treated birds were terminated and dissected for other studies.
Data analyses
The data were analyzed with Statistica for Windows. Parametric tests were used where data met the required assumptions. For other data, I used non-parametric tests. In the text, r s is the correlation coefficient from Spearman rank correlations. To perform power analyses, the G-power program was used (Buchner et al. 1997).
Results
Changes in social status during the infection
Each day following the infection, observations were made to determine ranks in pairs where one male was infected. Between day 0 (when birds received the infection) and day 1, all ranks remained stable; between days 1 and 2, 81% of all ranks remained stable; and between days 2 and 3, 75%. On this day, 3/11 dominant infected birds decreased in rank, and 1/5 subordinate infected birds increased in rank. Between day 3 and 4, 87% of all pairs remained stable (n=16 in all cases). When these observed frequencies of stable ranks were compared to the expected frequency 90% (rank shifts that occurred without treatment in the control week) there was no significant increase in rank shifts during the infection. (Day 1–2: χ 2=0.24, P=0.62; Day 2–3: χ 2=0.82, P=0.36; Day 3–4: χ 2=0.00, P=1.00). I also tested if those dominance relationships that were first formed while one bird was infected would be influenced by infection status. During the peak of the virus infection (day 3), there was no association between infection status and the outcome of the dominance test (χ 2=0.0, P=1.00, n=16) in newly formed pairs. Since each bird had been ranked against two other birds in the group, the outcome of the dominance tests could be predicted based on their previous encounters in 11 out of 16 cases by assuming that the dominance hierarchies would be linear within groups. In none of these 11 cases did the outcome of the dominance test differ from the predicted outcome, despite the fact that one of the birds in each pair was infected. Thus, overall there was no evidence that infection status had influenced the outcome of the dominance tests.
Social status and infection clearance
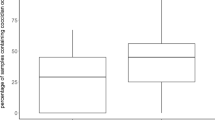
When virus titres were compared between infected dominant and subordinate birds (Fig. 1), these results showed that dominant birds had significantly higher early viremia than subordinate males (t 14=3.15, P=0.007). Among infected males, there was also a significant positive correlation between their within-group rank and early viremia (r s =0.69, P=0.03, n=16), thus birds with higher ranks had faster virus proliferation during the early stage of the infection (Fig. 2a). Total viremia was not different between dominants and subordinates (t 14=1.12, P=0.28) and not significantly associated with within-group rank (r s =0.25, P=0.34). Late viremia was significantly higher among subordinates birds as compared to dominants (U=15, P=0.05, n=8 in each group), and there was a significant negative relationship between social rank and late viremia (r s =−0.56, P=0.02, n=16) (Fig. 2b). When comparing the reductions in virus titers between days four and five, dominant birds had significantly larger reductions in virus titers (U=13.5, P=0.05, n=8 in each group). Males with large tail patches had lower total viremia. However, this result was not statistically significant (r s =−0.45, P=0.07, n=16).
Viremia comparison between dominant and subordinate greenfinches (Carduelis chloris). a Daily virus titers of subordinates and dominants for 7 days following an experimental infection with Sindbis virus; b early (day 1–3) and late (day 5–7) viremia in dominants and subordinates. Bars represent means and standard errors of virus titers measured as plaque forming units (PFU)/ml whole blood. Differences between groups are denoted above bars, * P<0.05, ** P<0.01 from t-test for early viremia, and Mann-Whitney U-test for late viremia, n=8 in each group)
Viremia in relation to within group rank (1–4, with 4 being the highest rank). a Early viremia (day 1–3), was positively correlated to rank (r s =0.69, P=0.03, n=16), In contrast, b the relation between rank and late viremia (day 5–7) was negative (r s =−0.56, P=0.02, n=16). Sample sizes within each rank were 1: n=2; 2: n=2; 3: n=7; 4: n=5
Body masses
At the time of capture, birds with low ranks had though not significantly, a high body mass compared to high ranked birds (r s =−0.33, P=0.06, n=32), thus body mass in the field tended to be inversely related to rank. Body mass was measured on two occasions during the experiment; on the second day after the experiment started, and 4 days after the infection. On both these occasions, body masses were not significantly related to rank (r s =−0.21, P=0.46, n=32, r s =−0.14, P=0.25, n=31). There was a small but significant decrease in body mass (paired t-test, t=2.55, P=0.02, n=32) from the body mass at capture (mean±SD=27.4±1.50 g) until the second day of the control measurements (27.0±1.30 g). From this day until 4 days after the virus or control injection, the average body mass of the birds (27.8±1.55) was significantly increased (paired t-test, t=5.29, P<0.001, n=31). This increase in body mass was observed for both virus-infected birds (t=−4.57, P<0.01, n=16) and controls (t=-3.05, P<0.001, n=16). There were no significant relationships between a bird’s body mass during the experiment and total viremia (r s=0.41, P=0.11, n=16), early viremia (r s=−0.33, P=0.21, n=16) or late viremia (r s=0.41, P=0.11, n=16).
Discussion
The results of this study show that virus proliferation and clearance patterns were related to an individuals’ social status. This implies that there were underlying physiological differences between high and low ranking birds that influenced their ability to resist the experimental virus infection. In contrast to the initial expectations there was no clear evidence that males of low social status had reduced virus clearance ability. Instead, social rank was related to virus infection patterns: subordinate birds with low ranks had slower virus proliferations and subsequently a slower virus clearance as compared to dominants. These differences in viremia patterns could reflect underlying differences in genetic quality, immunological investments or hormonal profiles between the behavioral strategies.
First, the patterns observed may reflect underlying genetic quality differences in disease resistance. Dominant males could possess better parasite resistance abilities as a result of their superior genetic quality, and male rank could be used as a cue by females to gain information about the males’ parasite resistance ability (Qvarnström and Forsgren 1998). In this study, there was no difference in total viremia that related to rank and it is thereby hard to conclude which virus clearance strategy of the ones observed that would be most beneficial for an individual’s fitness. We have previously found that greenfinches had a reduced take-off speed after a simulated predator attack after an experimental infection (Lindström et al. 2003) and it may be important for an individual’s survival to clear the virus infection in the shortest possible time to minimize the risk of being predated upon while infected.
The difference in virus clearance pattern could reflect underlying physiological differences between the behavioral strategies. One such difference between could lie in their nutritional status. Nutritional status can affect immune functions and resistance to disease in birds (Klasing 1998). Since dominant individuals have priority to access of food resources, they could also be in better nutritional condition. In this experiment, birds were fed the same diet and the only information relating to nutritional status available is the measurements of body mass. In another experiment, Sindbis virus-infected greenfinches increased in body mass relative to controls (Lindström et al. 2003), suggesting that this could be a response to increased energetic demands inflicted by the infection. In the present study, both virus-infected and control birds increased in body mass during the experiment and there was an initial decrease in body mass from capture until the experiment started. It has previously been shown that greenfinches decrease their body reserves in response to a more predictable food supply (Ekman and Hake 1990; Hake 1996). During the experiment the food supply became more unpredictable, due to increased competition accompanied by the experimental procedure of repeatedly removing the food supply a few hours each day. The mass changes observed in the current experiment could therefore be interpreted either as a response to the infection or to the altered predictability of the food supply. There was no significant relationship between bird body mass and virus clearance. Furthermore, body mass during the experiment was not significantly related to social rank and thus overall there was no evidence suggesting that the relationship between social rank and virus clearance was influenced by body mass.
Dominant and subordinate birds differed in virus clearance patterns and, from an immunological perspective, this could represent different strategies of virus clearance. Different components of the immune system are active at different stages during a virus infection (Biron 1999), and potentially the slower virus replication that was observed in subordinates could be the result of a more effective early immunological defense, while dominant birds had a more efficient humoral immune response. Steroid hormones such as testosterone or stress hormones such as corticosterone can regulate immune functions and the levels of these hormones can be related to social rank. Particularly during the establishment of social hierarchies in birds, testosterone levels are increased in response to the social challenge (Wingfield et al. 1990). In a previous study, the effect of elevated testosterone levels on viremia was examined (Lindström et al. 2001). In this study, the virus clearance pattern in the testosterone-implanted group was similar to the one found in subordinates in this study, with low initial virus titres and a delayed clearance. Thus the viremia patterns observed in subordinates could be attributed to immunomodulatory effects of testosterone acting stronger on subordinates. An alternative explanation is that the virus clearance patterns in both these studies were modulated by stress hormones. Since both testosterone implantation and social subordination can be associated with elevated stress hormone levels, the delayed viremia pattern in testosterone implanted birds and subordinates, could have been an effect of an increase in stress hormone levels (Evans et al. 2000; Poiani et al. 2000; Zuk and Johnsen 2000). In agreement with our previous findings (Lindström and Lundström 2000), males with bright tail patches had lower viremias, thought not significantly.
In this study, being infected did not seem to have any strong influence on a bird’s ability to either maintain or acquire a dominant position. The small sample size limits any firm conclusions on this point and it should be pointed out that the power of detecting even a large effect was low (0.41). However, there were no tendencies for ranks to be altered and even during the peak of the infection, when rank changes were most common, decreases in rank were as frequent as increases. There are examples of studies that show that disease decreases social status of infected individuals (Freeland 1981; Zuk et al. 1999), although such effects may be more common in chronic infections or infections causing severe disease. The Sindbis virus infection may be too transient to have any social repercussions. In a previous study, we did find evidence that this virus influenced the behaviors of infected birds (Lindström et al. 2003). Since social rank can be intimately coupled with an individual’s fitness it may not be a beneficial strategy to reallocate resources from rank maintenance to infection clearance. Although there was no clear effect on rank during the acute phase of this infection, it is still possible that effects of this virus infection would influence an individual’s rank on a longer time scale. In humans, infections with the Sindbis virus can in some cases lead to persistent symptoms (Niklasson et al. 1988) and it can not be excluded that this virus infection can also have long lasting effects in birds.
References
Biron CA (1999) Initial and innate responses to viral infections- pattern setting in immunity or disease. Curr Opin Microbiol 2:374–381
Buchner A, Erdfelder E, Faul F (1997) How to use G-Power. http://www.psycho.uni-duesseldorf.de/aap/projects/gpower/how_to_use_gpower.html. Cited 6 October 2003
Buchanan K (2000) Stress and the evolution of condition-dependent signals. Trends Ecol Evol 15:156–160
Chamberlain RW, Sudia WD (1961) Mechanisms of transmission of viruses by mosquitoes. Annu Rev Entomol 6:371.390
Cramp S, Perrins CM (1994) The birds of the western Palearctic, vol 8. Oxford University Press, Oxford
Creel, S (2001) Social dominance and stress. Trends Ecol Evol 16:491–497
Earley E, Peralta PH, Johnson KM (1967) A plaque neutralization method for arboviruses. Proc Soc Exp Biol Med 125:741–747
Ekman J, Hake MK (1990) Monitoring starvation risk: adjustments of body reserves in greenfinches (Carduelis chloris L.) during periods of unpredictable food supply. Behav Ecol 1:62–67
Eley C (1991) Status signalling in the western greenfinch, Carduelis chloris. PhD thesis, University of Sussex, Brighton, U.K.
Evans MR, Goldsmith AR, Norris SRA (2000) The effect of testosterone on antibodyproduction and plumage coloration in male house sparrows (Passer domesticus). Behav Ecol Sociobiol 47:156–163
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
Francy DB, Jaenson TGT, Lundström JO, Schildt E-B, Espmark Å, Henriksson B, Niklasson B (1989) Ecologic studies of mosquitoes and birds as hosts of Ockelbo virus in Sweden and isolation of Inkoo and Batai viruses from mosquitoes. Am J Trop Med Hyg 41:355–363
Freeland WJ (1981) Parasitism and behavioral dominance among male mice. Science 213:461–462
Hake M (1996) Fattening strategies in dominance-structured greenfinch (Carduelis chloris) flocks in winter. Behav Ecol Sociobiol 39:71–76
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Klasing KC (1998) Nutritional modulation of resistance to infectious diseases. Poult Sci 77:1119–1125
Lindström KM, Lundström JO (2000) Male greenfinches with brighter ornaments have higher virus infection clearance rates. Behav Ecol Sociobiol 48:44–51
Lindström KM, Krakower DS, Lundström JO, Silverin B (2001) The effect of testosterone on a viral infection in greenfinches (Carduelis chloris): an experimental test of the immunocompetence handicap hypothesis. Proc R Soc Lond B 268:1–5
Lindström KM, van der Veen IT, Lundström JO, Legault B-A (2003) Activity and predator escape performance of Greenfinches infected with Sindbis virus. Ardea 91:103–111
Lundström JO (1999) Mosquito-borne viruses in Western Europe: a review. J Vect Ecol 24:1–39
Lundström JO, Niklasson B, Francy BD (1990) Swedish Culex torrentium and Cx. pipiens (Diptera: Culicidae) as experimental vectors of Ockelbo virus. J Med Entomol 27:561–564
Merilä J, Sheldon BC, Lindström K (1999) Plumage brightness in relation to hematozoan infections in the Greenfinch, Carduelis chloris: bright males are a good bet. Ecoscience 6:1-7
Niklasson B, Espmark Å, Lundström J (1988) Occurrence of arthralgia and specific IgM antibodies three to four years after Ockelbo disease. J Infect Dis 157:832–835
Norder H, Lundström JO, Kozuch O, Magnius LO (1996) Genetic relatedness of Sindbis virus strains from Europe, Middle East and Africa. Virology 222:440-445
Olson VA, Owens IPF (1998) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13:510–514
Poiani A, Goldsmith AR, Evans MR (2000) Ectoparasites of house sparrows (Passer domesticus): an experimental test of the immunocompetence handicap hypothesis. Behav Ecol Sociobiol 47:230–242
Pusey AE, Packer C (1997) The ecology of relationships. In: Krebs JR, Davies NB (eds) Behavioural ecology an evolutionary approach, 4th edn. Blackwell, Cambridge, pp 254–284
Qvarnström A, Forsgren E (1998) Should females prefer dominant males? Trends Ecol Evol 13:498–501
Rohwer S (1975) The social significance of avian plumage variability. Evolution 29:593–610
Saks L, Ots I, Horak P (2003) Carotenoid-based plumage coloration of male greenfinches reflects health and immunocompetence. Oecologia 134:301–307
Senar JC (1990) Agonistic communication in social species: what is communicated? Behaviour 112:270–283
Wingfield JC, Hegner RE, Dufty AM, Ball GF (1990) The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846
Zuk M, Johnsen TS (2000) Social environment and immunity in the red jungle fowl. Behav Ecol 11:146–153
Zuk M, Kim T, Robinson SI, Johnsen TS (1998) Parasites influence social rank and morphology, but not mate choice, in female red junglefowl, Gallus gallus. Anim Behav 56:493–499
Acknowledgements
J. Lundström, I. Ahnesjö, M. Zuk, A. Qvarnström and J. Höglund provided constructive discussions when planning this experiment, and/or gave valuable comments on the manuscript. The experiment was approved by the ethical committee of animal research (C216/96) in Uppsala and animals were maintained in accordance with the laws regulating animal care in Sweden.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Brown
Rights and permissions
About this article
Cite this article
Lindström, K.M. Social status in relation to Sindbis virus infection clearance in greenfinches. Behav Ecol Sociobiol 55, 236–241 (2004). https://doi.org/10.1007/s00265-003-0703-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0703-3