Abstract
The immunocompetence handicap hypothesis (ICHH) suggests that dominance signals are costly because their development is controlled by testosterone, which is immunosuppressive. Signal control therefore links an increased disease risk with a high quality signal. The chest bib of the house sparrow, Passer domesticus, is a signal known to be related to dominance and under control of testosterone levels. We experimentally manipulated testosterone in male sparrows during the breeding season and again independently during the post-breeding period to test whether variation in levels of testosterone could cause variation in levels of immunocompetence. There was no effect of testosterone manipulation on the cell-mediated response of birds to phytohaemagglutinin injection, nor did testosterone levels appear to affect either white blood cell ratios or red blood cell counts. In contrast, both breeding season and post-breeding season testosterone levels had significant effects upon the humoral response of the birds to sheep red blood cell injections. However, whilst testosterone during the breeding season appeared to act immunosuppressively, the role of post-breeding levels is less clear. In concordance with a previous study, there was an indication that corticosterone is involved in mediating the immunosuppressive effects of testosterone. The strength of the secondary humoral response and the cell-mediated response were negatively related suggesting the possibility of a trade-off between the different arms of the immune system. These results provide some support for the ICHH as a mechanism promoting the evolution of costly badges of status, although the results question whether the immunosuppressive cost can be mediated by testosterone at the time of badge development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current models concerning the evolution of signals predict that such signals must be costly to develop or to maintain in order to reflect individual quality honestly (Andersson 1994), but the costs associated with the production of many signals remain unclear. It has been suggested that signals indicating dominance, or 'badges of status,' remain honest indicators in part through frequent testing of individual quality, preventing the occurrence of cheating within the population (Rohwer 1975; Rohwer and Rohwer 1978; Møller 1987a; Johnstone and Norris 1993). However both theoretical (Johnstone and Norris 1993) and empirical work (Gonzalez et al. 2001) suggest that frequent testing alone is not sufficient to maintain the honesty of the signalling system, suggesting the need for other costs associated with advertisement. Folstad and Karter (1992) suggested that testosterone (T), known to be closely associated with the development of sexual characteristics and behaviour (Wingfield et al. 1990), may have detrimental effects on the immune system, such that only high quality individuals can withstand the immunosuppressive costs associated with high levels of testosterone. This immunocompetence handicap hypothesis (ICHH) has attracted much attention, though tests to date have produced mixed support (Veiga 1993; Ros et al. 1997; Birkhead et al. 1998; Gonzalez et al. 1999a, Hasselquist et al. 1999; Evans et al. 2000). A number of possible mechanisms has been suggested to link immunocompetence costs with signal quality (Westneat and Birkhead 1998). To differentiate between these mechanisms, well-designed experimental tests are needed, which manipulate endocrine control within naturally occurring levels.
Male house sparrows, Passer domesticus, possess a 'badge of status' in the form of black feathers under the bill forming a chest bib, the size of which correlates positively with male dominance (Møller 1987a, 1987b, 1988; Liker and Barta 2001; McGraw et al. 2003). The role of male bib size in female choice is unclear, as studies have variously reported that females prefer males with larger bibs (Møller 1987a, 1989b, 1990), that females prefer smaller-bibbed males (Griffith et al. 1999), or that females show no preference for bib size (Veiga 1993; Kimball 1996; Cordero et al. 1999). Gonzalez et al. (1999a) concluded that bib size is unlikely to be a condition-dependent trait, as dietary restriction experiments showed no effect on bib development in the house sparrow. They suggested as an alternative that the cost of the bib may be mediated through immunosuppressive effects associated with maintaining the signal.
Testosterone has recently been shown to affect the size of the bib in male house sparrows (Evans et al. 2000; Buchanan et al. 2001). In addition, T was also found to be connected with suppressed immune function, an effect that was associated with increased levels of corticosterone (Evans et al. 2000) and higher ectoparasite loads (Poiani et al. 2000). Correlative studies have also suggested that there may be an immune cost associated with bib moult (Møller et al. 1996; Nava et al. 2001). The possible timing for this effect is unclear, as T levels are highest at the start of the breeding season (Hegner and Wingfield 1986), and are much lower at the end of the summer, when the feathers of the new bib are being produced (Evans et al. 2000). Gonzalez et al. (1999b) found that whereas male house sparrows with large bibs had reduced cell-mediated immune response in the breeding season, they had an increased response in the autumn relative to smaller-badged males. It is unclear, however, whether T was responsible for these seasonal changes.
The sparrow, therefore, is an excellent model species with which to test the ICHH; testosterone levels are known to control the development of a sexually selected plumage signal and the absolute levels of T and the timing of the control of bib size are known (Evans et al. 2000; Buchanan et al. 2001). Earlier results from the present study demonstrated that subtle differences in the autumn levels of testosterone prior to moult are important for controlling bib development rather than the much higher breeding season levels of T which occur several months before moult (Buchanan et al. 2001). For the ICHH to mediate the cost of bib development it is important to establish whether such subtle differences could also affect immunocompetence. The first aim of this study was, therefore, to test whether individual variation in seasonal T levels produces meaningful differences in levels of immunosuppression, while still controlling individual variation in signal development. We sought to do this by manipulating testosterone levels both at the start of the breeding season and then again independently prior to the autumn moult, within the naturally occurring range for these seasonal levels.
Evans et al. (2000) found that T manipulations during the breeding season caused immunosuppression. However this work suggested that testosterone might not be directly responsible for the immunosuppression, but that the effect was mediated through covarying levels of corticosterone. A second aim was therefore to assess the relationship between T and corticosterone.
As the importance of diet quality in controlling overall immune function in the house sparrow is not clear (Gonzalez et al. 1999a), a third aim was to test experimentally whether variation in diet quality affected the degree of immune response, independently from any hormonal control.
A final aim of the study was to assess the effects of T and corticosterone on the trade-off between the cell-mediated and humoral responses, as differential allocation between these two aspects of immune function could occur and measurement of both is necessary to assess overall immunocompetence (Weiner and Bandieri 1974; Norris and Evans 2000).
In order to separate the effects of breeding and post-breeding levels of T on immune function it was necessary to manipulate the levels of T during the breeding and post-breeding periods separately. We therefore adopted a cross-over design to produce manipulated levels of T during the post-breeding period that were independent of breeding season levels. This was done to determine whether subtle variation in post-breeding levels, that has previously been shown to control variation in bib moult (Buchanan et al. 2001), was sufficient to cause meaningful variation in immune function.
Methods
Buchanan et al. (2001) reported the test of the role of testosterone in controlling bib size development and basal metabolic rate in these birds. The results reported here are the immune-response data gathered in the same experiment. For this reason the details of the experimental design and husbandry are identical to those described in Buchanan et al. (2001). Male sparrows were caught in the wild and randomly assigned to one of four experimental treatments in early March of 1998 (n=32) and 1999 (n=64). Birds in the 'high T,' 'low T' and 'castrate' treatments were all castrated, whereas birds in the 'intact' treatment were sham operated and produced natural levels of T throughout. All operations were conducted under anaesthetic. Each bird received a slow release subcutaneous T implant, designed to mimic natural T levels during the breeding season (Evans et al. 2000). Implants were medical grade silastic tubing (Dow Corning, Mich., USA, cat. no. 602-235, internal diameter 1.47 mm, external diameter 1.96 mm) filled with powdered testosterone (Sigma, Poole, UK, cat. no. T1500) and sealed with silastic adhesive. The breeding season implants were 8 mm long and 2 mm long for the high and low T treatments, respectively. Both the 'castrated' and 'intact' males received empty implants. The birds were also allocated to one of two dietary treatments, with the 'high quality diet' treatment receiving ad libitum seed mixture (Haiths' Wild Bird Seed, Cleethorpes, UK) and mealworms daily, whereas the 'low quality diet' treatment also received ad libitum seed mixture, but received mealworms only once per week. The mealworms were always consumed within 24 h confirming that they were regarded as a high quality resource. Birds were housed in groups of four (one from each treatment) per cage and remained in these social groups and under the same dietary treatment throughout the experiment. The cages were adjacent in an outdoor aviary and each cage was 2×2×1 m (height × length × breadth).
To test the relative effect on immune function of T during the autumn post-breeding period in comparison to T during the breeding period, males from the high, low and castrate treatments were randomly re-assigned to these treatments in June. The re-assignment was conducted such that birds in any breeding season experimental treatment could be re-allocated to any of the post-breeding season treatments. The breeding-period implants were removed during the re-assignment and replaced with implants producing T at levels that mimic natural post-breeding levels of T (Buchanan et al. 2001). The post-breeding season implants were 2 mm and 1 mm long for the high and low T treatments, respectively. Birds in the castrate treatment again received an empty implant. The intact birds (n=24) were given new empty implants, to control for the stress of the implant change, but remained in the intact treatment group throughout the experiment. There were therefore nine T manipulation groups: high to high (n=4), high to low (n=6), high to castrate (n=6), low to high (n=6), low to low (n=7), low to castrate (n=5), castrate to high (n=9), castrate to low (n=6) and castrate to castrate (n=7). All the birds which survived to the end of the experiment were included in the analysis. The birds suffered similar mortality levels as they would in the wild (Summers-Smith 1988) and mortality was similar in all treatment groups.
Blood samples were taken from all birds twice during the breeding season implantation period and twice during the post-breeding period to assess circulating levels of testosterone. Breeding season levels of T were calculated as the mean values from blood samples taken in March and May, whereas post-breeding values were calculated as the mean values from blood samples taken in August and October. Blood of intact birds was sampled at the same time. In addition, all birds were sampled once during the breeding season and once during the post-breeding season for estimation of corticosterone production. We used a capture-handling-restraint procedure, which has been used across a range of species to determine the short-term adrenocortical response of birds to a standardised 'stress' (Wingfield 1994). The procedure involves capturing a bird from its cage and taking a blood sample (100 μl) within 1 min in order to determine basal corticosterone concentrations. The bird is then kept in a small cloth bag and further blood samples taken at 10 and 30 min. This method of sampling is a standardised way of measuring both basal (1 min) and elevated (30 min) corticosterone production.
Immune function
All the measures of immune function were taken in September of each year, just prior to the onset of moult and during the period when the birds carried the post-breeding implants.
Cell mediated response
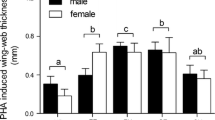
In each year the cell-mediated response of individuals was tested before the humoral response (see below) using an injection of phytohaemagglutinin (PHA) into the wing web. PHA is a plant lectin which promotes a hypersensitivity reaction and has been extensively used to investigate immune function (Lochmiller et al. 1993). The thickness of both the right and the left wing web was measured before injection using digital callipers. At this time, 50 µl of a suspension of PHA (Sigma L-8754) (24 mg of PHA in 4.8 ml of 1×PBS) was injected into the right wing web. 50 µl of 1×PBS was injected into the left wing web to control for the effect of the injection on wing web thickness. The response was measured at 6 h and 24 h post-injection and was found to be greater 24 h post-injection. After this time the swelling subsided. The 24-h response was used in the analysis. Three repeated measurements were taken with the callipers on each occasion and the measures demonstrated high repeatability (F 53,108=17.78, 81.6%, Lessels and Boag 1987).
Blood cell counts
The ratio of heterophils:lymphocytes (H:L) has previously been used as an index of avian stress (Maxwell 1993). To measure this ratio we obtained white blood cell counts from birds before and after the PHA test. Immediately before administration of the PHA a blood smear was made from a drop of blood obtained from the leg vein. This was repeated 24 h after administration of the PHA injections. The smears were air dried, fixed in methanol for 2 min, stained with a combination of May Grunwald and Geimsa stains (4 min 50% May Grunwald; 8 min 7.5% Geimsa; 6 min 50% May Grunwald), and rinsed in pH 6.8 buffer between stains and after staining, in order to differentiate the blood cell types. The cells were examined at ×100 under oil immersion and 200 white blood cells were identified and counted. Lymphocytes, heterophils, and monocytes were counted individually. Basophils and eosinophils occurred rarely and were counted as one group (Maxwell 1993).
In the autumn, 3–4 weeks after the PHA administration, the red blood cell count was assessed by drawing 100 µl of blood and diluting a portion of it with 0.15 M NaCl. This blood sample was also used to test for the presence of heterologous antibodies to sheep red blood cells (see below). An improved Neubauer haemocytometer was used to measure the number of red blood cells/mm3 of blood.
Humoral response
The birds' humoral responses were tested using intraperitoneal injections of sheep red blood cells (SRBC) (Deerenberg et al. 1997; Peters 2000) just prior to the onset of moult, during the time that the birds carried the post-breeding season implants. This test was conducted 3–4 weeks after the end of the PHA challenge in both years. A blood sample (100 µl) was drawn before the start of the immune challenge to test for the presence of heterologous antibodies, which would cross-react with the SRBC antigens. SRBCs in Alsever's solution, (TCS Microbiol) were washed three times in 1×PBS and re-suspended in 1×PBS to form a 2% solution. From this, 150 µl of the 2% solution was injected intraperitoneally. Eight days later 100 µl of blood was drawn to test for the presence of antibodies during the primary response. Fifteen days after the first injection, an identical second injection was given and 4 days after this a second blood sample was taken to test for the presence of antibodies during the secondary response. The timetable for sampling the response was based on previous work, which showed that the maximum antibody production during the primary response of sparrows to SRBC injections occurred 8 days after injection, whereas the maximum secondary response occurred 4 days after the second injection (Evans et al., unpublished data). The blood samples were spun in a benchtop centrifuge at 14,000 rpm for 15 min and the plasma removed, heat-treated at 56°C in a water bath for 30 min and stored at −20°C for further analysis. The blood samples were always tested within 3 weeks of being drawn. A haemagglutination test was used to test for the presence of antibodies against SRBC. The plasma was serially diluted across a V-form microtitre plate (Greiner). A sample of 2% SRBC solution was then added to each of the wells of the tray and the tray incubated at room temperature for 1 h. Haemagglutination is apparent when the antibodies in the plasma are present in sufficient quantity to form a film of blood cells. The most dilute titre of plasma capable of maintaining this reaction was noted.
Hormone assays
Blood samples (100 μl) were collected in heparinised capillary tubes after puncture of the brachial vein with a 25-gauge needle and centrifuged. The plasma was stored at −20°C for later hormone assay. Total androgen concentrations were measured in plasma samples by direct radioimmunoassay using anti-testosterone antiserum (code 8680-6004, Biogenesis, UK) and [125I]-testosterone label (code 07-189126, ICN, UK) (Parkinson and Follett 1995). The antiserum detects T, but also cross-reacts with other androgens present in the blood. However this cross-reactivity is low and therefore this assay represents a reliable surrogate measure of absolute T levels. The assay was run with 50% binding at 11 pg/tube and a detection limit of 0.08 ng/ml for 10 µl aliquots of plasma. After confirming that house sparrow plasma diluted parallel with the standard curve, experimental samples were measured in either 10 µl or 5 µl duplicate volumes. The interassay coefficient of variation was 15.5%.
After extraction of 20 µl aliquots of plasma in diethyl ether, corticosterone concentrations were measured by radioimmunoassay (Wingfield et al. 1992) using anti-corticosterone antiserum (code B21-42, Endocrine Sciences, Tarzana, Calif.) and [1,2,6,7-3H]-corticosterone label (Amersham, UK). The extraction efficiency was 80–90%. The assay was run with 50% binding at 150 pg/tube, and the detection limit (for 7.3 µl aliquots of extracted plasma) was 1.4 ng/ml. The interassay coefficient of variation was 9.2%.
Statistical analysis
For each immune-response variable, separate analyses were performed on the experimental and intact treatment groups. The intact group could not be combined with the experimental treatments for statistical analysis, as the T levels within the intact group during the breeding and post-breeding periods were obviously not independent. The analyses were conducted using MINITAB version 10.5 (Minitab, State College, Pa., USA). For each immune response variable a GLM ANOVA was constructed to test the effects of the experimental manipulations on immune reaction. The measure of immunocompetence was entered as the dependent variable with the experimental manipulations hypothesised to affect immune function entered into each model as categorical independent variables: breeding season treatment, post-breeding experimental treatment and dietary treatment. Mass, breeding season and post-breeding season 'basal' (bleed at 1 min after capture) and 'peak' (bleed at 30 min after capture) corticosterone production were also entered into the model as continuous independent variables. Interactions between the breeding and post-breeding treatments and between T and basal and peak corticosterone were added into the models. Each ANOVA model was reduced to its simplest form by eliminating any independent variables or interactions that failed to explain significant variation in the dependent variable. Elimination was conducted in a stepwise manner with the factors explaining least variation in the model being removed sequentially until all remaining factors explained significant variation (Zar 1984). Non-significant factors or interactions were constrained into the models only to control for experimental effects or when biologically meaningful. The model residuals were checked for normality and homoscedasticity at each step and the appropriate transformations made. Confidence intervals for the effect sizes from comparing individual experimental treatments were calculated using post-hoc Tukey tests. This was done to estimate if the sample sizes used were sufficient to demonstrate any experimental effect.
Results
The testosterone levels of the experimental birds (Fig. 1a) fell within the natural range of testosterone levels in wild sparrows (Hegner and Wingfield 1986). Within the intact group, T levels during the breeding season and post-breeding period were positively correlated (r 2=19.3, F 1,17=5.77, P=0.027). Analysis of the corticosterone samples confirmed that levels increased throughout the capture-restraint handling procedure during both the breeding and post-breeding sampling periods (Fig. 1b). Within the experimental birds, the correlation between breeding season testosterone levels and breeding season basal corticosterone was positive, but non-significant (n=60, r=0.23, P=0.077), whereas there was a significant positive correlation between post-breeding season testosterone levels and post-breeding season basal corticosterone (n=60, r=0.279, P=0.031). Within the intact group there was a significant negative correlation between breeding season testosterone levels and breeding season basal corticosterone (n=24, r=−0.412, P=0.045), whilst there was no correlation between post-breeding season testosterone levels and post-breeding season basal corticosterone (n=18, r=0.165, P=0.513). There were no correlations between either measure of testosterone and any of the measures of corticosterone taken either 10 or 30 min after capture.
a Mean testosterone levels +SE (ng/ml) for the breeding and post-breeding season experimental treatments and intact house sparrows, Passer domesticus. Values in each case are the mean of two blood sampling events for each bird within each treatment period. b Mean corticosterone levels for all birds sampled during the breeding season and post-breeding periods at 1 min after capture (= basal), 10 min and 30 min after capture
Cell-mediated response
Response to the PHA injection was higher 24 h after injection (x̄=1.41 mm±0.09 SE, n=78) than at 6 h (x̄=0.866 mm±0.08 SE, n=78). The GLM model with 24-h PHA response as the dependent variable demonstrated that neither breeding season treatment, nor post-breeding season treatment had a significant effect on the 24-h PHA response, although there was a significant effect of dietary treatment (with the lower response in the high quality diet group), (Table 1, Fig. 2). Within the intact group, none of the variables included in the analysis explained significant variation in PHA response (Table 1).
White blood cell counts
The ratio of H:L is known to be an indicator of physiological stress (Maxwell 1993) and so an ANOVA model was constructed to test the effect of the experimental manipulations on the H:L. The initial H:L (before the PHA injection) was the dependent variable with the same categorical and continuous independent variables as described above. The final ANOVA model showed that there were no significant effects of breeding season T treatment, post-breeding treatment or dietary treatment on initial H:L (Table 2). Within the intact group there was a significant negative effect of post-breeding season peak corticosterone titre (Table 2).
A similar approach was used to test the effects of the experimental manipulations on the change in the H:L before and after reaction to the PHA. Males showed a significant increase in their H:L during the 24-h reaction to the PHA injection (t=5.57, P<0.001) (Fig. 3). Change in H:L was entered into the ANOVA model as the dependent variable, with breeding season and post-breeding season experimental T treatment and dietary treatment as independent variables and initial H:L, breeding season and post-breeding season basal and peak corticosterone levels as covariates. The analysis showed that there was no significant effect of breeding season (F 2,49=0.75, P=0.476) or post-breeding season (F 2,49=0.19, P=0.825) T manipulation or dietary treatment (F 1,49=1.02, P=0.316) on the increase in H:L; however there was a significant negative effect of initial H:L (F 1,49=5.94, P=0.018). A positive effect of breeding season basal corticosterone was non-significant (F 1,49=4.01, P=0.051).
Red blood cell counts
The final model explaining variation in red blood cell counts showed that there were no significant effects of the breeding season or the post-breeding season T treatments or of dietary treatment (Table 3). However, there was a significant negative effect of breeding season basal corticosterone titre (Table 3). Within the intact group the effects of breeding season and post-breeding season testosterone titre and dietary treatment were all non-significant (Table 3).
Humoral response
The final model explaining individual variation in secondary SRBC response contained significant effects of both breeding season and post-breeding season experimental treatments (Table 4, Fig. 4). The fitted means confirmed that within the breeding season treatments the lowest response was seen in the high T treatment. Within the post-breeding treatments the difference between the treatments was less clear with the lowest response seen in the low T treatment and the high T and castrate treatments mounting marginally larger responses. The effect of dietary treatment was non-significant. There was a significant interaction between post-breeding experimental treatment and post-breeding basal corticosterone. An explanation for this interaction is that within the high and low testosterone treatments there was a negative relationship between corticosterone and immune response, which was not seen in the castrate treatment. A non-significant interaction between breeding season experimental treatment and post-breeding experimental treatment was constrained into the model as both treatments appeared to affect the response produced (Fig. 4).
The intact group was analysed separately with secondary response to the injection of SRBC as the dependent variable and with dietary treatment as an independent factor and breeding season and post-breeding season testosterone, basal and peak corticosterone titres as covariates. The final model showed a significant negative effect of post-breeding season T level, as well as a negative effect of post-breeding basal corticosterone titre (Table 4). There was also a significant interaction between post-breeding testosterone titre and post-breeding basal corticosterone. Although both hormones independently had a negative relationship with the secondary humoral response, the interaction implies that increasing corticosterone makes the relationship between testosterone and immune response less negative. There was also a significant effect of dietary treatment, such that individuals on a high quality diet produced a lower response (Table 4).
The relationship between the humoral and the cell-mediated response
Within the experimentally manipulated birds, differential resource allocation could be occurring between the different aspects of the immune system, according to the aspects of immune defence that are of greatest importance at the time (Weiner and Bandieri 1974). For this reason the relationship between the reaction to the PHA injection 24 hours after exposure and the secondary reaction to the SRBC injection, was analysed by constructing a GLM ANOVA. Secondary response to the SRBC injection was entered as the dependent variable and the PHA response and primary response to the SRBC injection were entered as continuous independent variables. Year, breeding season T treatment, post-breeding season T treatment and dietary treatment were entered as categorical independent variables. The significant predictors of the secondary SRBC response were found to be primary SRBC response and 24-h PHA reaction (Table 5). The negative relationship between the secondary humoral response and the cell-mediated response is shown in Fig. 5. This trade-off was not apparent within the intact group.
Discussion
The ICHH assumes that T promotes the development of sexually selected signals while suppressing the immune system. A number of studies has confirmed a causal role of T in increasing bib size in house sparrows (Evans et al. 2000; Gonzalez et al. 2001). In addition we have recently documented that it is T immediately prior to the onset of moult that is crucial in controlling the increase in bib size (Buchanan et al. 2001). This result is supported by the recent finding that seasonal increases in bib size are affected by the number of aggressive interactions prior to the onset of moult, probably through the effects of testosterone (McGraw et al. 2003). Therefore, the first requirement of the ICHH is fulfilled in the case of dominance signalling through chest bib production in the house sparrow.
The first aim of the work reported here was to test whether the subtle differences in post-breeding T levels previously found to influence bib size (Buchanan et al. 2001) were also sufficient to produce significant differences in immunocompetence. In short, we found little evidence that the variation in T levels during the post-breeding period could produce meaningful variation in levels of immunosuppression. There was no evidence that the cell-mediated response was affected by either breeding season or post-breeding levels of testosterone. Furthermore, neither the ratio of heterophils to lymphocytes nor overall red blood cell counts, both indicators of body condition, were affected by either breeding season or post-breeding levels of testosterone. In contrast, the secondary humoral response appeared to be affected by both breeding season and post-breeding season levels of testosterone, but only the breeding season levels showed a consistent immunosuppressive effect. The analysis confirmed that the effect of the breeding season T levels (independent of post-breeding T) was negative. However, when comparing the post-breeding treatments the lowest response was found in the low T treatment. Therefore, although post-breeding T affected the secondary humoral response, the effect could not be described as simple immunosuppression.
Although the breeding season manipulations demonstrated immunosuppression of the humoral response associated with increased testosterone levels, other immune parameters were not adversely affected by increased testosterone. Our manipulations did not produce large differences between the treatments in the cell-mediated response to PHA in white blood cell ratios or in red blood cell counts. When examining the non-significant effect of breeding season testosterone treatment on PHA response, the CIs indicate that the largest difference between the treatments occurred between the high and low T birds (CI lower −0.966, upper 0.638). The effect size generated was small relative to the difference between the treatments; therefore our sample sizes were insufficient to demonstrate any significant differences. This pattern was also true for the H:L and red blood cell counts.
There are several reasons why the breeding season treatments might show a clear immunosuppressive effect, whilst the post-breeding treatments showed no consistent effect on immunocompetence. The most parsimonious explanation of these results is that larger differences are required between individual male T levels to detect consistent differences between birds in their level of immunocompetence using these methods. However, the treatments also differ in their timing, with breeding season levels of T present some 3 months prior to the immune assays. During the experiment, the birds remained in the same social groups. If the dominance hierarchies within the cages established at the start of the experiment remained stable throughout this could have influenced the outcome of the effect of post-breeding T levels on immunocompetence, as breeding season and post-breeding season T would not in this case be truly disentangled.
The fact that breeding and post-breeding T levels were correlated in the intact birds suggests that in the wild, birds with high breeding season testosterone levels would also have high post-breeding season levels of T. This indicates that whilst no direct effect of post-breeding testosterone on immunocompetence was detected, it is entirely plausible that high levels during the post-breeding season are associated with an immunosuppressive cost, mediated at another time in the year. This supports the original idea from the ICHH that individual males have an optimum level of T that they can produce to maximise fitness, without suffering detrimental immunosuppressive costs.
The second aim of the study was to determine whether corticosterone might be directly involved in mediating the immunosuppressive effects associated with increased T. There was some evidence for this, as basal corticosterone production interacted with post-breeding testosterone levels to affect the humoral response, within both the experimental and the intact birds. Within the experimental birds, the action of corticosterone appears to be immunosuppressive, but only in the presence of T, as the castrate treatment did not demonstrate this effect. Our data therefore lend some support to the hypothesis that it is corticosterone, not T, which directly exerts the cost associated with the development of sexual traits (Evans et al. 2000) or that corticosterone and T work in tandem to facilitate immunosuppression. The direct immunosuppressive effect of each hormone could only be tested by experimental manipulations of T and corticosterone independently. Basal corticosterone appeared to covary with T levels, as has been shown in other studies (Barnard et al 1996; Evans et al. 2000). Interestingly within the intact group there was a negative relationship between corticosterone and T. This implies that males in good condition have high T and low corticosterone, but that experimental individual elevations of T will result in elevated levels of corticosterone. Corticosterone is thought to be immunosuppressive when chronically elevated (Møller 1995; Buchanan 2000) and therefore the effects seen here are likely to be linked to long-term elevation in corticosterone associated with elevated circulating T titres. There is a growing body of experimental work demonstrating the covariation of corticosterone with testosterone and the negative consequences for immunocompetence (Ketterson et al. 1991; Duffy et al. 2000; Casto et al. 2001), and recent reviews support this conclusion (Braude et al. 1999; Hillgarth and Wingfield 1997). In addition, T can also cause an increase in levels of corticosteroid-binding globulin (CBG) (Schoech et al. 1999, Deviche et al. 2001). As both T and corticosterone can bind to CBG, (although corticosterone has much greater affinity), it seems possible that T and corticosterone interact on a number of levels with consequences for immunosuppression.
Dietary treatment was found to affect the PHA response in the experimental birds and also the size of the humoral response in the intact birds. In both cases the effect was not in the expected direction, as birds on a poor quality diet mounted a larger response to the immune challenge than those on the high quality diet. This pattern is similar to some of the results found by Gonzalez et al. (1999a), who found that house sparrows kept on a protein-rich diet showed significantly elevated cell-mediated responses, but significantly suppressed humoral responses, in comparison with individuals on a protein-poor diet. This suggests that the immune response may not be directly constrained by resources (Gonzalez et al. 1999a) or access to resources (Gonzalez et al. 1999b), or that individuals are capable of re-allocating the available resources between physiological processes. One interpretation is that birds housed in poorer conditions are protecting themselves from the detrimental consequences of infection by investing proportionately more in their immune response than birds fed on a higher quality diet. If the likelihood of injury or disease varies with nutritional conditions it could be optimal to invest more in immune protection when in poor nutritional conditions. An alternative interpretation is that our 'high quality' diet is in fact not as desirable as we had intended. A recent study on captive breeding in house sparrows suggests that mealworms are a poor quality food resource for nestlings, possibly because of toxins in the cuticle of the insect larvae (Moreno-Rueda and Soler 2002). In this case our 'high quality' diet birds may have been suffering some toxic effects that could explain the direction of the change in immune investment.
The final aim of the study was to test for differential allocation between the humoral and cell-mediated immune responses. The negative relationship between the secondary humoral response and cell-mediated response to PHA 24 h after injection suggests that there may indeed be some resource-based constraint on mounting an immune response. Alternatively, the value of the two types of response may vary between individuals, such that individuals at higher risk of tissue injury, for example, might invest proportionately more in a cell-mediated response and less in the humoral response (Zuk and Johnsen 1998). That different aspects of immune function should trade-off against each other has been suggested previously (e.g. Weiner and Bandieri 1974), highlighting the problem of measuring overall immuncompetence (Westneat and Birkhead 1998). Also relevant to this trade-off are the potential costs of over-investment in immune responsiveness, in terms of causing auto-immune disorders (Råberg et al. 1998). All the birds experienced the immune tests in the same order and an alternative explanation is that birds which invested most in the earliest challenge (PHA) were unable to respond as much to the later challenge (SRBC). Further work is needed using a balanced design reversing the order of these challenges to determine the effects of one immune challenge on the other.
This study provides limited support for the immunosuppressive effects of testosterone. It is clear that at certain levels testosterone can act immunosuppressively, but it is still unclear whether this occurs directly or through the action of corticosterone. The experimental results suggest that the subtle differences between T levels of individual males during the post-breeding period, which control bib development, are not enough to cause demonstrable immunosuppression. Selection studies and survival analyses have documented a possible trade-off between immune function and the production of sexually selected signals (Nolan et al. 1998; Verhulst et al.1999). However, the direct evidence from both correlative work and manipulative experiments has been less consistent (e.g. Peters 2000; Duckworth et al. 2001; Hasselquist et al. 1999; Lindstrom et al. 2001, Westneat et al. 2003).
The results reported here suggest that seasonal changes in T levels are capable of causing variation in immunocompetence, although only in some immune traits. Our conclusion that breeding season levels of T cause immunosuppression of the humoral response supports our earlier work (Evans et al. 2000), but we found little evidence for an immunosuppressive cost associated with high post-breeding levels. Given that post-breeding levels of T control bib size during the autumn moult, we found little evidence for a direct immunosuppressive effect associated with a large badge size. However, as intact males with high T levels prior to the autumn moult also had high T levels during the previous breeding season, we suggest that it is likely that T-control of bib development has some immunosuppressive costs. When these costs are mediated or how biologically meaningful these costs are, is unclear. We would suggest, therefore, that although our study provides some support for the ICHH, it is still not clear whether the immunosuppresssive effects of T are of sufficient importance to explain the evolution of the house sparrow chest bib as an honest signal of quality.
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, N.J.
Barnard CJ, Behnke JM, Sewell J (1996) Social status and resistance to disease in house mice (Mus musculus): Status-related modulation of hormonal responses in relation to immunity costs in different social and physical environments. Ethology 102:63–84
Birkhead TR, Fletcher F, Pellatt EJ (1998) Sexual selection in the zebra finch Taeniopygia guttata: condition, sex traits and immune capacity. Behav Ecol Sociobiol 44:179–191
Braude S, Tang-Martinez Z, Taylor GT (1999) Stress, testosterone and the immunoredistribution hypothesis. Behav Ecol 10:345–350
Buchanan KL (2000) Stress and the evolution of condition-dependent signals. Trends Ecol Evol 15:156–160
Buchanan KL, Evans MR, Goldsmith AR, Bryant DM, Rowe LV (2001) Testosterone influences basal metabolic rate in male house sparrows: a new cost of dominance signalling? Proc R Soc Lond B 268:1337–1344
Casto JM, Nolan V, Ketterson ED (2001) Steroid hormones and immune function: Experimental studies in wild and captive dark-eyed juncos (Junco hyemalis). Am Nat 157:408–420
Cordero P, Wetton J, Parkin D (1999) Extra-pair paternity and male badge size in the House Sparrow. J Avian Biol 30:97–102
Deerenberg C, Apanius V, Daan S, Bos N. (1997) Reproductive effort decreases antibody responsiveness. Proc R Soc Lond B 264:1021–1029
Deviche P, Breuner C, Orchinik M (2001) Testosterone, corticosterone, and photoperiod interact to regulate plasma levels of binding globulin and free steroid hormone in Dark-eyed Juncos, Junco hyemalis. Gen Comp Endocrinol 122:67–77
Duckworth RA, Mendonca MT, Hill GE (2001) A condition dependent link between testosterone and disease resistance in the house finch. Proc R Soc Lond B 268:2467–2472
Duffy DL, Bentley GE, Drazen DL, Ball GF (2000) Effects of testosterone on cell-mediated and humoral immunity in non-breeding adult European starlings. Behav Ecol 11:654–662
Evans MR, Goldsmith AR, Norris SRA (2000) The effects of testosterone on antibody production and plumage coloration in male house sparrows (Passer domesticus). Behav Ecol Sociobiol 47:156–163
Folstad I, Karter AJ (1992) Parasites, bright males and the immunocompetence handicap. Am Nat 139:603–622
Gonzalez G, Sorci G, Møller AP, Ninni P, Haussy C, de Lope F (1999a) Immunocompetence and condition-dependent sexual advertisement in male house sparrows (Passer domesticus). J Anim Ecol 68:1225–1234
Gonzalez G, Sorci G, de Lope F (1999b) Seasonal variation in the relationship between cellular immune response and badge size in male house sparrows (Passer domesticus). Behav Ecol Sociobiol 46:117–122
Gonzalez G, Sorci G, Smith LC, de Lope F (2001) Testosterone and sexual signalling in male house sparrows (Passer domesticus). Behav Ecol Sociobiol 50:557–562
Griffith SC, Owens IPF, Burke T (1999) Female choice and annual reproductive success favour less-ornamented male house sparrows. Proc R Soc Lond B 266:765–770
Hasselquist D, Marsh JA, Sherman PW, Wingfield JC (1999) Is avian immunocompetence suppressed by testosterone? Behav Ecol Sociobiol 45:167–175
Hegner RE, Wingfield JC (1986) Behavioural and endocrine correlates of multiple brooding in the semicolonial house sparrow Passer domesticus. I. Males. Horm Behav 20:294–312
Hillgarth N, Wingfield JC (1997). Parasite-mediated sexual selection: endocrine aspects. In: Clayton DH, Moore J (eds) Host-parasite evolution: general principles and avian models. Oxford University Press, Oxford
Johnstone RA, Norris KJ (1993) Badges of status and the costs of aggression. Behav Ecol Sociobiol 32:127–134
Ketterson ED, Nolan V, Wolf JL, Zeigenfus C, Dufty AM, Ball GF, Johnsen TS (1991) Testosterone and avian life histories: the effects of experimentally elevated testosterone on corticosterone and body mass in dark-eyed juncos. Horm Behav 25:489–503
Kimball R (1996) Female choice for morphological traits in house sparrows Passer domesticus. Ethology, 102:639–648
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Liker A, Barta Z (2001) Male badge size predicts dominance against females in House Sparrows. Condor 103:151–157
Lindstrom KM, Krakower D, Lundstrom JO, Silverin B (2001) The effects of testosterone on viral infection in greenfinches (Carduelis chloris): an experimental test of the immuncompetence-handicap hypothesis. Proc R Soc Lond B 268:207–211
Lochmiller RL, Vestey MR, Boren JC (1993) Relationship between protein nutritional status and immunocompetence in northern bobwhite chicks. Auk 110:503–510
Maxwell MH (1993) Avian blood leucocyte responses to stress. World's Poult Sci 49:34–43
McGraw KJ, Dale J, MacKillop EA (2003) Social environment during molt and the expression of melain-based plumage pigmentation in male house sparrows (Passer domesticus). Behav Ecol Sociobiol 53:116–122
Møller AP (1987a) Variation in badge size in male house sparrows Passer domesticus: evidence for status signalling. Anim Behav 35:1637–1644
Møller AP (1987b) Social control of deception among status signalling house sparrows Passer domesticus. Behav Ecol Sociobiol 20:307–311
Møller AP (1988) Badge size in the house sparrow Passer domesticus: effects of intra- and intersexual selection. Behav Ecol Sociobiol 22:373–378
Møller AP (1990) Sexual behaviour is related to badge size in the house sparrow Passer domesticus. Behav Ecol Sociobiol 27:23–29
Møller AP (1995) Hormones, handicaps and bright birds. Trends Ecol Evol 10:121
Møller AP, Kimball RT, Erritzoe J (1996) Sexual ornamentation, condition and immune defence in the house sparrow Passer domesticus. Behav Ecol Sociobiol 39:317–322
Moreno-Rueda G, Soler M (2002) Breeding in captivity of House Sparrow, Passer domesticus. Ardeola 49 11–17
Nava MP, Veiga JP, Puerta M (2001) White blood cell counts in house sparrows (Passer domesticus) before and after molt and after testosterone treatment. Can J Zool 79:145–148
Nolan PM, Hill GE, Stoehr AM (1998) Sex, size and plumage redness predict house finch survival in an epidemic. Proc R Soc Lond B 265:961–965
Norris K, Evans MR (2000) Ecological immunology:life history trade-offs and immune defense in birds. Behav Ecol 11:19–26
Parkinson T, Follett B (1995) Thyroidectomy abolishes seasonal testicular cycles of Soay rams. Proc R Soc Lond B 259:1–6
Peters A (2000) Testosterone treatment is immunosuppressive in superb fairy-wrens, yet free-living males with high testosterone are more immunocompetent. Proc R Soc Lond B 267:883–889
Poiani A, Goldsmith AR, Evans MR (2000) Ectoparasites of house sparrows (Passer domesticus): an experimental test of the immunocompetence handicap hypothesis and a new model. Behav Ecol Sociobiol 47 230–242
Råberg L, Grahn M, Hasselquist D, Svensson E (1998) On the adaptive significance of stress-induced immunsuppression. Proc R Soc Lond B 265:1637–1641
Rohwer S (1975) The social significance of avian plumage variation. Evolution 29:593–610
Rohwer S, Rohwer FC (1978) Status signaling in Harris' sparrows experimental deceptions achieved. Anim Behav 26:1012–1022
Ros AFH, Groothuis TGG, Apanius V (1997) The relation among gonadal steroids immunocompetence, body mass and behaviour in young black-headed gulls (Larus ridibundus). Am Nat 150:201–219
Schoech SJ, Ketterson ED, Nolan V (1999) Exogenous testosterone and the adrenocortical response in Dark- eyed Juncos. Auk 116:64–72
Summers-Smith, J (1988) The sparrows. Poyser, Calton, UK
Veiga JP (1993) Badge size, phenotypic quality and reproductive success in the house sparrow: A study on honest advertisement. Evolution 47:1161–1170
Verhulst S, Dieleman SJ, Parmentier HK (1999) A trade-off between immunocompetence and sexual ornamentation in domestic fowl. Proc Natl Acad Sci USA 96:4478–4481
Weiner E, Bandieri A (1974) Differences in antigen handling by peritoneal macrophages from the Biozzi high and low responder lines of mice. Eur J Immunol 4:457–463
Westneat D, Birkhead T (1998) Alternative hypotheses linking the immune system and mate choice for good genes. Proc R Soc Lond B 265:1065–1073
Westneat DF, Hasselquist D, Wingfield JC (2003) Tests of association between the humoral immune response of red-winged blackbirds (Agelaius phoeniceus) and male plumage, testosterone or reproductive success. Behav Ecol Sociobiol (in press). DOI 10.1007/s0026500205779
Wingfield J (1994). Modulation of the adrenocortical response to stress in birds. In: Davey K, Peter R, Tobey Y (eds) Perspectives in comparative endocrinology . National Research Council of Canada, Ottawa, pp 520–528
Wingfield JC, Hegner RE, Dufty AM Jnr, Ball GF (1990) The 'challenge hypothesis' — theoretical implications for patterns of testosterone secretion, mating systems and breeding strategies. Am Nat 136:829–846
Wingfield J, Vleck C, Moore M (1992) Seasonal changes of the adrenocortical response to stress in birds of the Sonoran Desert. J Exp Zool 264:419–428
Zar JH (1984) Biostatistical analysis. Prentice Hall, Englewood Cliffs, N.J.
Zuk M, Johnsen TS (1998) Seasonal changes in the relationship between ornamentation and immune response in red jungle fowl. Proc R Soc Lond B 265:1631–1635
Acknowledgements
We thank Alasdair Sherman for caring for the birds, Kirsty Park and Louise Rowe for help in the field and the laboratory and local landowners for access for mistnetting. We also thank two anonymous referees for constructive comments on an earlier draft of this manuscript. K.L.B. was supported by the Natural Environmental Research Council grant GR3/11426. All experiments were conducted under Home Office Licence (PPL 60/2256) and comply with U.K. legal requirements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W.A.Searcy
Rights and permissions
About this article
Cite this article
Buchanan, K.L., Evans, M.R. & Goldsmith, A.R. Testosterone, dominance signalling and immunosuppression in the house sparrow, Passer domesticus . Behav Ecol Sociobiol 55, 50–59 (2003). https://doi.org/10.1007/s00265-003-0682-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0682-4