Abstract
Purpose
Patient-specific instrumentation (PSI) was usually applied in total knee arthroplasty (TKA) to acquire a favourable alignment. We hypothesized that using PSI had a potential risk of notching in the anterior femoral cortex, because the femoral component may be placed in an overextension position due to the distal femoral sagittal anteversion. The aim of this study was to figure out the relationship between the notch and the distal femoral sagittal anteversion in PSI-assisted TKA.
Methods
One hundred thirty-one patients who were to undergo total knee arthroplasty (TKA) were randomly divided into conventional instrumentation (CI) group and PSI group. The computed tomography (CT) data of lower extremities was collected and imported to the Mimics software to reconstruct the three-dimensional (3D) bone image of the femur. The angle between distal femoral anatomic axis (DFAA) and femoral mechanical axis (FMA) on sagittal plane was defined as distal femoral sagittal anteverted angle (DFSAA) and measured. The number of notch intra-operative and post-operative was recorded. Then, we calculated the incidence of the notch and analyzed its relationship with DFSAA.
Results
The average DFSAA of 262 femurs is 2.5° ± 1.5° (range, 0.0°–5.7°). When DFSAA ≥ 3°, the incidence of notch was 7.10% in CI group and 33.30% in PSI group, respectively, which shows significant statistical difference in the two groups (P = 0.016 < 0.05). When DFSAA < 3°, the incidence of notch was 6.50% in CI group and 5.30% in PSI group, respectively, which shows no significant statistical difference in the two groups (P = 0.667 > 0.05).
Conclusion
DFSAA could be taken as an indicator to predict the notch when performing TKA assisted with PSI. Especially when the DFSAA ≥ 3°, the risk of notch could be markedly increased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) is a widely used and important surgical option in the treatment of end-stage knee osteoarthritis (KOA) or other serious knee diseases. TKA can correct deformity and relieve pain by restoring limb alignment and obtaining good soft tissue balance [1]. Although at present TKA has achieved good post-operative results, about 20% of patients are still not satisfied with their results of replacement [2]. In addition to the influence of the design features and manufacturing process of the prosthesis itself, whether the implant position is correct or not has a direct relationship with the long-term survival rate of the prosthesis [3]. Malalignment of the implant after TKA may cause many complications, such as early loosening, instability of movement, persistent pain, and wear of polyethylene liner. A perfect surgical design should be inspected and reviewed from three dimensions (3D): coronal alignment, sagittal alignment, and axial alignment, which requires considerable experiences of surgeons, the poor alignment of the implant due to the limits of technical level of surgeons is a common cause of TKA revision [4]. At present, a relatively consistent definition of good lower extremity alignment after surgery is that deviation no more than 0 ± 3° from the mechanical axis in coronal plane [5].
Numerous studies have demonstrated that computer-assisted surgery (CAS) in TKA can improve prosthesis alignment and reduce outliers [6,7,8]. However, the application of CAS is somewhat limited by its complicated registration process, which has brought about a series of problems, e.g., increased surgical time and cost [9, 10], bone fracture caused by the tracer pin [11, 12], and the longer learning curve [8].
In recent years, the rapid development of 3D printing technology provides a new solution for achieving neutral alignment in TKA: individualized 3D printing osteotomy guide–assisted TKA, or better known as patient-specific instrument (PSI)–assisted TKA [13]. This technique needs to obtain CT or magnetic resonance imaging (MRI) data of full-length lower extremities preoperatively. Based on the reconstructed 3D images, surgeons can determine the size and position of the implant, and also the patient-specific cutting blocks can be designed and printed to assist TKA [14].
Although it had been proved by many researches that PSI could acquire an equivalent alignment in coronal plane [15,16,17] to or even a more favourable rotational alignment [18] than conventional instrumentation (CI), few attention has been paid on the sagittal alignment. However, sagittal alignment, especially the sagittal rotation of the femoral component, is an important factor related to the outcomes of TKA. An overly flexion position of the femoral component can lead to extension limit of the knee as well as impingement between the posterior liner and the intercondylar box [19]. On the other hand, an overly extension position can result in notching in the anterior femoral cortex [20], and the notch was often considered an adverse event for that local stress in the vicinity of the prosthesis augmented markedly which could lead to supracondylar fracture [21]. However, anatomical morphology of the distal femur is the main factor that influences the sagittal rotation of the femoral component. Ko et al. [22] came to the conclusion that sagittal bowing of the distal femur could be a risk factor for femoral component flexion in CI and notching in CAS. Since both PSI and CAS refer to the femoral mechanical axis in sagittal plane when designing the distal osteotomy, we hypothesized that in TKA, PSI has potential risk of notching in the anterior femoral cortex similar to CAS. And we also assumed that the risk of notching in the anterior femoral cortex was associated with the distal femoral sagittal anteversion, which has not been reported yet. Hence, we aimed to compare the incidence of notch in the anterior femoral cortex between PSI and CI in TKA and to analyze the relationship between notch and distal femoral sagittal anteversion.
Patients and methods
Patients
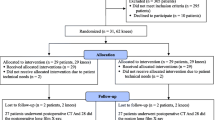
From January 2017 to December 2018, 131 patients diagnosed with knee osteoarthritis (KOA) who were undergoing primary unilateral TKA were included in this study. Patients with inflammatory arthritis, post-traumatic arthritis, or severe extra-articular deformities were excluded. There were 25 males and 106 females. The mean age of patients was 66.2 ± 8.5 years (range, 41–84 years) and the mean body mass index (BMI) was 25.2 ± 3.9 kg/m2 (range, 17.5–44.3 kg/m2). Patients were randomly divided into two groups in a ratio of 2:1with group A: 88 cases of CI group and group B: 43 cases of PSI group. All the patients received and accepted informed consent to participate in this study, which was approved by the medical ethics committee of our hospital (2018-YD084-01).
The measurement of DFSAA
Firstly, all patients included underwent full-length lower extremity radiography(AXIOM Multix M, Siemens company, Germany), then received the 64 row CT scan (Lightspeed-64, General Electric Company, USA) on both legs, with the scan thickness 0.625 mm. Position: both legs were in neutral position, and the patella was upward. The scanning range was from the pelvis to feet. Then, the CT scan data was imported into the software of Mimics 17.0 (Materialise company, Belgium) in DICOM 3.0 format to reconstruct the model of the femur. We defined the angle between the femoral mechanical axis (FMA) and the distal femoral anatomical axis (DFAA) in sagittal plane as the distal femoral sagittal anteverted angle (DFSAA), and we measured DFSAA on three dimensions by Creo 2.0 (PTC company, USA) (Fig. 1). Both group A and group B were divided into subgroup DFSAA ≥ 3°and subgroup DFSAA < 3°, respectively.
Methods to define DFSAA. A cylinder simulated femoral intramedullary (IM) guide rod was established in Mimics (diameter = 8 mm; length = 20 cm). Point (a) was the center of the femoral marrow cavity. Point (b) was the center of the femoral head. Point (c) was the entry point on the intercondylar fossa. Line (a)–(c) was the central axis of the simulated IM rod which represents the DFAA. Line (b)–(c) was FMA. The coordinate figure of these three points was recorded and imported into Creo 2.0. The cross angle of DFAA and FMA in sagittal plane was measured as DFSAA in Creo
We obtained the CT data of the patient’s legs in the outpatient department and imported them into Mimics17.0 in the format of DICOM to reconstruct the femoral and tibia models. The 3D models were converted to standard tessellation language (STL) files and loaded into Siemens NX 9.0 software (Siemens, Germany) where the alignment was measured based on anatomic landmarks. Then, we performed the simulated operation in this software including osteotomy and prosthesis matching, and completed the final prosthesis adaptation with a posterior stabilizing (PS) prosthesis (Gemini II, link, Germany) (Fig. 2). After that, the model data of osteotomy guide was imported into the software program of 3D printer (EOS P11, Germany) for printing (Fig. 3). The guide was made of medical nylon (PA2200), which was sterilized at high temperature and not easy to deform.
Surgical procedures
One hundred thirty-one patients in this group were given general anesthesia (45 cases) or intraspinal anaesthesia (86 cases). A tourniquet was applied before skin incision. Antibiotics and tranexamic acid were used 30 minutes before the operation. We adopted the anteromedial incision and medial parapatellar approach in both groups. In the CI group, an IM rod for the femur and an extramedullary guide for the tibia were applied, while in the PSI group, patients were operated by the following methods: (1) After the femoral condyle was exposed, a custom curette was used to scrape off the cartilage on the anterior surface of the femoral condyle where the osteotomy guide would be placed. (2) When the osteotomy guide was confirmed in good fit with the femoral condyle, positioning nail was implanted through the holes. Then, we replaced the PSI guides with the matched metal cutting blocks to complete the distal femur osteotomy (Fig. 4). (3) The cartilage on the tibial plateau was removed, then the PSI guides were placed in best fit before it was replaced with metal cutting blocks to complete the proximal tibia osteotomy in same way as the distal femur. (4) After removal of meniscus and osteophyte on the posterior condylar of the femur, we inserted the gap block of corresponding thickness to test the gap and soft tissue balance in extension and flexion. (5) Then, we performed external rotatory osteotomy of the distal femur with the 4-in-1 metal cutting blocks which were fixed by the positioning nail previously implanted on the distal femur. After that, we used sickle to test whether notch was formed in anterior cortex of the femur and recorded (Fig. 5A). We defined that if the lower edge of sickle was lower than the most anterior edge of anterior cortex of the femur, then anterior cortex notch was formed after osteotomy. When the sickle test showed that a notch had occurred, we moved up the positioning pin hole until no notch was formed.
(A) Using a custom curette to scrape off the cartilage on the anterior surface of the femoral condyle. (B) Confirming the good fit of the femoral osteotomy guide plate. (C) Positioning nails were implanted through the holes. (D) Using the matched metal cutting blocks to replace of the PSI guides to complete the distal femur osteotomy
The remaining procedure was then carried as the same as the conventional instrumentation. The patella in both groups was resurfaced as appropriate but without replacement. The PS cemented prosthesis was chosen as the final installation. All the operations were performed by an experienced surgeon (the corresponding author) in both groups.
Radiographic assessment of notch
The standard anteroposterior and lateral radiographs of the knee joint were performed routinely to observe the occurrence of notch (Fig. 5B) on the first post-operative day. The number of cases of femoral notch was recorded in Gujarathi’s methods, which classified the notch into 4 grades: Grade I: violation of the outer table of the anterior femoral cortex; grade II: violation of the outer and the inner table of the femoral cortex; grade III: violation up to 25% of the medullary canal (from the inner table to the center of the medullary canal); grade IV: violation up to 50% of the medullary canal (from the inner table to the center of the medullary canal) [23].
Post-operative follow-up and evaluation
All the patients were followed up by physical examination in the outpatient department for about 6 months at least. In this study, the hospital for special surgery knee scoring system (HSS) was selected to assess the function outcomes of patients at the sixth month after the operation.
Statistical analysis
We used SPSS 19.0 software (IBM Corp., NY, USA) for statistical analysis. The quantitative data were expressed as the mean ± standard deviation (SD) and range. The incidence of notch was compared by chi-square test (or Fisher’s exact test, as appropriate), and the post-operative HSS score was compared by t test. The correlation between DFSAA and height or BMI was analyzed by linear regression. A two-tailed probability level of P < 0.05 was selected as the statistical significant level.
Results
The general features of DFSAA
The average DFSAA of 262 femurs is 2.5° ± 1.5° (range, 0.0°–5.7°). DFSAA of 50% of patients was less than 2.2°, and DFSAA of the remaining 50% was 2.2°–5.7° (Fig. 6). The average DFSSA in men is 2.6° ± 1.6° (range, 0.1°–5.6°) and 2.5° ± 1.5° (range, 0.2°–5.7°) in women, respectively. There was no significant statistical difference of DFSAA between men and women (P = 0.389 > 0.05). The average DFSSA is 2.4° ± 1.5° (range, 0.1°–5.5°) in left and 2.6° ± 1.5° (range, 0.0°–5.7°) in right, respectively. There was no significant statistical difference of DFSAA between left and right (P = 0.234 > 0.05). There was no significant correlation between the left DFSAA and age (P = 0.068 > 0.05), BMI (P = 0.111 > 0.05), and between the right DFSAA and age (P = 0.091 > 0.05), BMI (P = 0.111 > 0.05).
The incidence of notch
In group A, 42 cases had DFSAA ≥ 3°and 46 cases had DFSAA < 3°. In group B, 24 cases had DFSAA ≥ 3°and 19 cases had DFSAA < 3°. The incidence of notch was 7.10% in group A and 33.30% in group B when DFSAA ≥ 3°. The incidence of notch was different in the two groups when DFSAA ≥ 3°(χ2 = 5.775, P = 0.016 < 0.05, with a power analysis of 0.81, calculated by the software of PASS 11.0 (NCSS Inc., Utah, USA). The incidence of notch was 6.5% in group A when DFSAA < 3°and 5.30% in group B when DFSAA < 3°. There was no significant difference between the two groups when DFSAA < 3° (Fisher’s accurate test P = 0.667 > 0.05) (Fig. 7).
Early clinical outcomes
All patients were followed up for six months at least, no cases of loosening and periprosthetic infection, no cases of supracondylar fracture of prosthesis and other causes of revision. The average HSS score of the patients in group A was 80.82 ± 5.30 points (range, 71–91 points) at the sixth month pre-operatively; the average HSS score of the patients in group B was 79.19 ± 6.43 points (range, 66–91 points) at the sixth month pre-operatively, with no significant difference between the two groups (P = 0.126 > 0.05).
Discussion
Notching in the anterior femoral cortex of TKA had always been treated as a predisposing factor for periprosthetic femoral fracture in early post-operative period. The incidence of notch was reported ranging widely from 1.1 to 40% [23, 24]. Although in some recent studies, whether the notch can lead to periprosthetic fracture seemed to be controversial. Most surgeons had discomfort with the notch because of the strong suspicion that it would cause the cortex stress to decrease, which was demonstrated by biomechanically evidence in the literature. Lesh et al. described that following notching a mean reduction of bending and torsional strengths was by 18% and 42%, respectively [21]. Some doctors thought the notch was irrelevant, but ignoring it was dangerous.
Usually, notching was attributed to surgical technique errors: entry point of the IM rod error, femoral component malrotation, smaller femoral prosthesis selection. However, the wide variation in the morphology of the distal femur could cause notch was more easily overlooked and ignored. And this variation had been proved to associate with ethnicity. Distal femoral sagittal anteversion (or named as distal femoral sagittal bowing) was more common in Asians with advanced osteoarthritis of the knee than that in Europeans and Americans [25, 26]. And distal femoral sagittal anteversion could lead femoral component placed in the extension position and thus causing notching in the anterior femoral cortex. We considered that these effects might be more significant in CAS and PSI because distal femoral osteotomy was based on FMA. Also, it had been reported that CAS-TKA had a higher risk of nothing than the conventional TKA, and the greater degree of flexion of the distal femur, the more likely it was to produce a femoral notch [20, 27, 28]. We also found this phenomenon to occur in PSI especially in patients with significant distal femoral sagittal anteversion showed in lateral femoral radiography preo-peratively. However, no current study had been carried out to research on the risk of notching in PSI-TKA. Therefore, we performed this research to figure out the relationship between distal sagittal femoral anteversion and the incidence of notch in PSI.
In this research, the notch incidence of the PSI group was higher than that of the CI group when DFSAA ≥ 3°, and the difference was statistically significant. There were no significant differences between the two groups when DFSAA < 3°. The result was consistent with our assumption that the risk of notching was associated with distal femoral sagittal anteversion. We analyzed the reason was that surgeons performed the distal femoral osteotomy by referring to IM rods (also we can regard it as DFAA) in CI, then the distal femoral osteotomy was conducted perpendicular to DFAA, while it was performed perpendicular to FMA in PSI. As the design principle of the 4 in 1 osteotomy guiding block required, the anterior femoral condyle osteotomy was performed perpendicular to the distal femoral osteotomy surface. When DFAA was over flexion relative to FMA in sagittal plane, DFSAA increased, then it is very likely to form a notch in the anterior cortex of the distal femur (Fig. 8).
Difference of distal femur osteotomy based on different reference lines. According to the s-DFAA (ac) and s-FMA (bc) of the femur, there were different osteotomy surfaces. Arrow 1 was the distal femur osteotomy surface which was perpendicular to s-FMA, while arrow 2 was the distal femur osteotomy surface which was perpendicular to s-DFAA, and the two surfaces were not parallel. When DFSAA was too large, femoral notch was easy to appear (arrow) in PSI-TKA
In this case, there was no obvious difference in HSS score between CI group and PSI group at sixth month after the operation. The notches observed on lateral femur radiography post-operatively were all Gujarathi types I–II. And no periprosthetic fracture occurred during the early post-operative period. This short-time clinical outcome was consistent with Gujarathi’s research, which concluded that no relationship existed between minimal anterior femoral notching and supracondylar fracture of the femur [23]. Notches of types III and IV would significantly increase the risk of supracondylar fracture of the femur. Zalzal et al. [29] showed that the notch of the anterior cortex of the femur could cause local stress concentration, especially when the notch of the femur was more than 3 mm, sharp, and adjacent to the prosthesis. In our research, not more than notches above type III were observed post-operatively, not least because surgeons operated the anterior femoral osteotomy quite carefully. When a deep notch that might happen observed by the sickle tester, the 4 in 1 cutting guide would be adjusted anteriorly.
PSI is a simple modern technique to assist TKA, and it is getting popular in recent years due to the benefits of accurate preo-perative planning, fewer intra-operative steps and reduction in surgical time [30], a lower risk of blood loss, and fat embolism [31]. Also, PSI is especially applicable in cases where the use of an IM rod would be problematic due to previous femoral trauma [32]. According to the results of this study, we recommended that when choosing PSI for TKA, attention should be paid on distal femoral sagittal anteversion pre-operatively. A CT scanning of lower extremity may be helpful in this evaluation procedure for the measurement of DFSAA. A conventional instrumentation may be more appropriate than PSI for mitigating the risk of notching in patients with an over large DFSAA. However, if PSI must be done, a design of the cutting guide with the reference of the DFAA is recommended.
There were also some limitations in this study: Firstly, our research was not a strictly randomized controlled trial (RCT). Selection bias may exist in surgery option, and double blind for patients and surgeons would be perfect. Secondly, we did not measure the depth of the notch in anterior femoral cortex. We believed that analyzing the relationship between the depth of notch and DFSAA would make the conclusion more convincing. Thirdly, sagittal femoral bowing was related to ethnicity. All the subjects in this study were KOA patients in Southwest China. Therefore, the results of this study could not directly explain whether there would be similar conclusions in other regions or other ethnic groups. There is still a need for further multicenter and diversified research.
Conclusion
Surgeons should be aware of the risks associated with notching of the anterior femoral cortex when choosing PSI in TKA. DFSAA could be taken as an indicator to predict notch when performing TKA assisted with PSI. Especially when the DFSAA ≥ 3°, the risk of notching could be markedly increased.
Data availability
Not applicable.
References
Kim SJ, Bamne A, Song YD, Kang YG, Kim TK (2015) Patients still wish for key improvements after total knee arthroplasty. Knee Surg Relat Res 27(1):24–33. https://doi.org/10.5792/ksrr.2015.27.1.24
Choi YJ, Ra HJ (2016) Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res 28(1):1–15. https://doi.org/10.5792/ksrr.2016.28.1.1
Choong PF, Dowsey MM, Stoney JD (2009) Does accurate anatomical alignment result in better function and quality of life? Comparing conventional and computer-assisted total knee arthroplasty. J Arthroplast 24(4):560–569. https://doi.org/10.1016/j.arth.2008.02.018
Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM (2002) Insall Award paper. Why are total knee arthroplasties failing today? Clinical orthopaedics and related research (404):7-13. doi:https://doi.org/10.1097/00003086-200211000-00003
Abdel MP, Ollivier M, Parratte S, Trousdale RT, Berry DJ, Pagnano MW (2018) Effect of postoperative mechanical axis alignment on survival and functional outcomes of modern total knee arthroplasties with cement: a concise follow-up at 20 years. J Bone Joint Surg Am 100(6):472–478. https://doi.org/10.2106/jbjs.16.01587
MacDessi SJ, Jang B, Harris IA, Wheatley E, Bryant C, Chen DB (2014) A comparison of alignment using patient specific guides, computer navigation and conventional instrumentation in total knee arthroplasty. Knee 21(2):406–409. https://doi.org/10.1016/j.knee.2013.11.004
Li Y, Tian H, Geng X (2018) Effect of the surgical time and coronalmechanical alignment after total knee arthroplasty using computer navigation system, traditionalor 3D printing patient-specific instruments. Zhonghua Yi Xue Za Zhi 98(27):2157–2161. https://doi.org/10.3760/cma.j.issn.0376-2491.2018.27.006
Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N (2010) Relationship between cutting errors and learning curve in computer-assisted total knee replacement. Int Orthop 34(5):655–662. https://doi.org/10.1007/s00264-009-0816-z
Cheng T, Zhang G, Zhang X (2011) Clinical and radiographic outcomes of image-based computer-assisted total knee arthroplasty: an evidence-based evaluation. Surg Innov 18(1):15–20. https://doi.org/10.1177/1553350610382012
Lee WT, Chin PL, Lo NN, Yeo SJ (2015) Short-term outcome after computer-assisted versus conventional total knee arthroplasty: a randomised controlled trial. Journal of orthopaedic surgery (Hong Kong) 23(1):71–75. https://doi.org/10.1177/230949901502300117
Jung KA, Lee SC, Ahn NK, Song MB, Nam CH, Shon OJ (2011) Delayed femoral fracture through a tracker pin site after navigated total knee arthroplasty. J Arthroplasty 26 (3):505.e509-505.e511. doi:https://doi.org/10.1016/j.arth.2010.01.006
Hoke D, Jafari SM, Orozco F, Ong A (2011) Tibial shaft stress fractures resulting from placement of navigation tracker pins. J Arthroplasty 26 (3):504.e505-508. doi:https://doi.org/10.1016/j.arth.2010.05.009
Camarda L, D’Arienzo A, Morello S, Peri G, Valentino B, D'Arienzo M (2015) Patient-specific instrumentation for total knee arthroplasty: a literature review. Musculoskelet Surg 99(1):11–18. https://doi.org/10.1007/s12306-014-0339-7
Kwon OR, Kang KT, Son J, Suh DS, Heo DB, Koh YG (2017) Patient-specific instrumentation development in TKA: 1st and 2nd generation designs in comparison with conventional instrumentation. Arch Orthop Trauma Surg 137(1):111–118. https://doi.org/10.1007/s00402-016-2618-2
Chotanaphuti T, Wangwittayakul V, Khuangsirikul S, Foojareonyos T (2014) The accuracy of component alignment in custom cutting blocks compared with conventional total knee arthroplasty instrumentation: prospective control trial. Knee 21(1):185–188. https://doi.org/10.1016/j.knee.2013.08.003
Hamid KS, Matson AP, Nwachukwu BU, Scott DJ, Mather RC 3rd, DeOrio JK (2017) Determining the cost-savings threshold and alignment accuracy of patient-specific instrumentation in total ankle replacements. Foot & ankle international 38(1):49–57. https://doi.org/10.1177/1071100716667505
Maus U, Marques CJ, Scheunemann D, Lampe F, Lazovic D, Hommel H, Vogel D, Haunschild M, Pfitzner T (2018) No improvement in reducing outliers in coronal axis alignment with patient-specific instrumentation. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 26(9):2788–2796. https://doi.org/10.1007/s00167-017-4741-1
Mannan A, Smith TO (2016) Favourable rotational alignment outcomes in PSI knee arthroplasty: a level 1 systematic review and meta-analysis. Knee 23(2):186–190. https://doi.org/10.1016/j.knee.2015.08.006
Yehyawi TM, Callaghan JJ, Pedersen DR, O’Rourke MR, Liu SS (2007) Variances in sagittal femoral shaft bowing in patients undergoing TKA. Clin Orthop Relat Res 464:99–104. https://doi.org/10.1097/BLO.0b013e318157e4a2
Lee JH, Wang SI (2015) Risk of anterior femoral notching in navigated total knee arthroplasty. Clinics in orthopedic surgery 7(2):217–224. https://doi.org/10.4055/cios.2015.7.2.217
Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr (2000) The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. J Bone Joint Surg Am 82(8):1096–1101. https://doi.org/10.2106/00004623-200008000-00005
Ko JH, Han CD, Shin KH, Nguku L, Yang IH, Lee WS, Kim KI, Park KK (2016) Femur bowing could be a risk factor for implant flexion in conventional total knee arthroplasty and notching in navigated total knee arthroplasty. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 24(8):2476–2482. https://doi.org/10.1007/s00167-015-3863-6
Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF (2009) Risk of periprosthetic fracture after anterior femoral notching. Acta Orthop 80(5):553–556. https://doi.org/10.3109/17453670903350099
Singh JA, Jensen M, Lewallen D (2013) Predictors of periprosthetic fracture after total knee replacement: an analysis of 21,723 cases. Acta Orthop 84(2):170–177. https://doi.org/10.3109/17453674.2013.788436
Mullaji AB, Marawar SV, Mittal V (2009) A comparison of coronal plane axial femoral relationships in Asian patients with varus osteoarthritic knees and healthy knees. J Arthroplast 24(6):861–867. https://doi.org/10.1016/j.arth.2008.05.025
Yau WP, Chiu KY, Tang WM, Ng TP (2007) Coronal bowing of the femur and tibia in Chinese: its incidence and effects on total knee arthroplasty planning. Journal of orthopaedic surgery (Hong Kong) 15(1):32–36. https://doi.org/10.1177/230949900701500108
Minoda Y, Kobayashi A, Iwaki H, Mitsuhiko I, Kadoya Y, Ohashi H, Takaoka K, Nakamura H (2010) The risk of notching the anterior femoral cortex with the use of navigation systems in total knee arthroplasty. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 18(6):718–722. https://doi.org/10.1007/s00167-009-0927-5
Minoda Y, Watanabe K, Iwaki H, Takahashi S, Fukui M, Nakamura H (2013) Theoretical risk of anterior femoral cortex notching in total knee arthroplasty using a navigation system. J Arthroplast 28(9):1533–1537. https://doi.org/10.1016/j.arth.2013.02.015
Zalzal P, Backstein D, Gross AE, Papini M (2006) Notching of the anterior femoral cortex during total knee arthroplasty characteristics that increase local stresses. J Arthroplast 21(5):737–743. https://doi.org/10.1016/j.arth.2005.08.020
Boonen B, Schotanus MG, Kerens B, van der Weegen W, van Drumpt RA, Kort NP (2013) Intra-operative results and radiological outcome of conventional and patient-specific surgery in total knee arthroplasty: a multicentre, randomised controlled trial. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 21(10):2206–2212. https://doi.org/10.1007/s00167-013-2620-y
León VJ, Lengua MA, Calvo V, Lisón AJ (2017) Use of patient-specific cutting blocks reduces blood loss after total knee arthroplasty. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie 27(2):273–277. https://doi.org/10.1007/s00590-016-1893-5
Chan WC, Pinder E, Loeffler M (2016) Patient-specific instrumentation versus conventional instrumentation in total knee arthroplasty. Journal of orthopaedic surgery (Hong Kong) 24(2):175–178. https://doi.org/10.1177/1602400211
Acknowledgments
We appreciated the assistance of measurement and software application from Professor Mingjun Fu in Naton Institute of Medical Technology, Beijing.
Funding
The current study was supported by Clinical Research Fund (no. 2016YLC21), the second affiliated hospital to army medical university of Chinese People’s Liberation Army, and the National Natural Science Foundation of China (grant no. 81501606).
Author information
Authors and Affiliations
Contributions
Dr. Wang conceived and designed the study. Dr. Ke, Dr. Ran, and Miss He measured the DFSAA. Dr. Wang performed the surgery, Dr. Xu, Dr. Song, and Dr. Lv were assistants. Miss Zhou and Miss He completed the follow-up work. Dr. Ke wrote the paper. Dr. Wang edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
All the patients received and accepted informed consent to participate in this study, which was approved by the medical ethics committee of our hospital (2018-YD084-01).
Conflict of interest
The authors declare that they have no conflicts of interest
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Level of evidence III, clinically case-controlled study
Rights and permissions
About this article
Cite this article
Ke, S., Ran, T., He, Y. et al. Does patient-specific instrumentation increase the risk of notching in the anterior femoral cortex in total knee arthroplasty? A comparative prospective trial. International Orthopaedics (SICOT) 44, 2603–2611 (2020). https://doi.org/10.1007/s00264-020-04779-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-020-04779-4