Abstract
Background
Little is known about the clinical outcome of children and adolescent patients with primary malignant sacral tumours.
Method
We retrospectively reviewed 40 patients with malignant sacral tumours aged ≤ 18 years, receiving surgery based on the previous proposed surgical resection classification at our centre from 2003 to 2018. The following data were collected in the present study: age, gender, radiological images, detailed information of the surgical procedure, pulmonary and other metastasis at presentation, complications, local recurrence, metastasis, and death during the follow-up were recorded from the clinical and follow-up files.
Results
The mean follow-up was 30.7 months (range, 5.2–146.7 months). The incidence of local recurrence was 27.5% (11/40). Seven cases had surgical site infection and there were 12 cases of wound dehiscence. One had a deep venous thrombosis and one had femoral artery thrombosis. Three had fixation breakage and then received a revision. Tenty-two patients (22/40, 55%) were free of disease. A total of 13 deaths (13/40, 32.5%) were observed and the mean overall survival period was 17.1 months (range, 6.3–34.2 months), and a pulmonary metastasis occurred in 18 patients (45%, 18/45) at the 12.0 ± 10.3 months (range, 2.2–35.3 months) after initial surgery. The overall survival rates at one, two and five years were respectively 88.3%, 62.5%, and 51.9%. In the stratification analysis of young patients with primary malignant tumours at the sacrum after surgery, it revealed the influence of pathological grade, location, and age on the oncological outcomes. Kaplan-Meier estimated a survivorship curve of patients with high and low-grade malignant tumours and showed statistical differences in the overall survival and distant relapse-free survival rates between two groups. Afterwards, the result demonstrated that paediatric patients aged ≤ 14 years had worse prognosis than those aged > 14 years.
Conclusions
It is satisfactory for the outcome of surgical treatment of children and adolescent patients with primary malignant sacral tumours based on proposed surgical classification. Furthermore, paediatric patients aged ≤ 14 years have the tendency of poor prognosis compared to adolescent aged > 14 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary malignant sacral tumours in paediatric and adolescent are difficult to manage because of their special anatomy and low incidence [1,2,3,4,5,6]. The anatomical complexities around the sacrum partly make radical operation difficult and lead to more complications. The en bloc resection, the most effective surgical intervention for the malignant sacral tumour, may result in serious complications such as huge blood loss and adjacent organ and lumbosacral trunk injury. When the tumour invades the surrounding organs or lumbosacral trunk, inadequacy of the surgical margins of excision is unavoidable. Due to the abovementioned risk factors, the prognosis of sacral malignant tumours is worse than that of extremities, especially for the young patients with sacral tumours. Alexandre et al. reported eight young patients aged from four to 18 years with sacral sarcoma received the marginal resection and six patients were alive without evidence of recurrence with the follow-up ranged from three to 204 months. They illustrated the conclusion that sacrectomy achieved local control and long-term survival, and adequate quality of life was possible in the paediatric population with high-grade sarcoma despite the high incidence of neurologic deficits and complications [7].
Little is known about the clinical prognosis of paediatric and adolescent patients with primary malignant sacral tumours, who received surgical resection, due to the low incidence in young patients and low occurrence at the sacrum region. In this study, we sought to answer the following questions regarding the surgical treatment of primary malignant tumour at the sacrum in young patients according to our previous proposed classification [8]: (1) Can the sacrectomy acquire the local control for children and adolescent patients? (2) How was the oncological prognosis of paediatric and adolescent patients after the sacrectomy? (3) Is there any difference in oncological outcome between paediatric aged ≤ 14 years and adolescent aged > 14 years?
Patients and methods
We retrospectively reviewed 40 patients with malignant sacral tumours aged ≤ 18 years, which had the intact clinical and follow-up information and who received surgical resection at our bone tumour centre from 2003 to 2018. There were 18 males and 22 females, with a mean age of 13.2 ± 4.3 years (median 13.5 years; range, 2–18 years). The clinical characteristics are shown in Table 1. The mean follow-up duration was 30.7 months (median, 17.7 months; range, 5.2–146.7 months). The inclusion criteria for the present study were as follows: (1) paediatric and adolescent population aged ≤ 18 years, (2) diagnosis as the malignant sacral tumour by the confirmation of post-operative histology, and (3) with the intact clinical and follow-up data including basic characteristics, radiological data, oncological/non-oncological complications at the last follow-up. The exclusion criteria were as follows: (1) patients aged > 18 years, (2) diagnosis as sacral benign and metastatic tumours, (3) incomplete medical records, and (4) inadequate and loss of follow-up. All patients received surgical resection and patients with Ewing sarcoma, and osteosarcoma received the neoadjuvant chemotherapy.
All patients which were included in the present study were gave written informed consent for their data in this study. All data was obtained from the clinical and radiograph records. This study was approved by the Institutional Review Board/Ethics Committee of the authors’ institution. For the initial clinical evaluation and diagnosis, all patients received plain radiographs, CT, MRI, and bone scanning. After imaging, we performed the needle biopsy to clarify the diagnosis. The routine follow-up including clinical examination and radiographs were performed every three months for the first six months, every six months for the first three years, and then annually. The chest CT scanning was performed every six months for the first three years, and then annually.
Surgical detailed information
In the previous study, we have reported that the sacrum was divided into three regions 1, 2, and 3 by the S1–S2 and S2–S3 junctions. Based on this region classification, en bloc resections were classified into five types: Type I involved regions 1; or 1 and 2; or regions 1, 2, and 3; Type II involved regions 2 and 3; and Type III involved only region 3. Type IV included sagittal hemisacrectomy and resection of a portion of the adjacent ileum. Type V included en block resection of the tumor at the sacrum and fifth lumbar vertebra. According to our surgical classification system for en bloc resection of sacral tumours, we have 17 Type I, four Type II, seven Type III, six Type IV, and six other resections which indicated en block resection was not achieved due to several reasons during operation. The surgical procedure was performed according to our previous study [8]. Post-operatively, the surgeon classified the surgical resection according to the following description: en bloc resection with wide margin or marginal and contaminated margin (if the surgeon was aiming to perform en bloc, but resulted in an intralesional resection or resection with unsatisfactory margin). If the margin was contaminated during the operation, we could use the titanium clip to help in identifying the contaminated place and advised patients to receive the radiotherapy, especially for the labeled site. If the margin was satisfactory, we would not allow them to receive the radiotherapy.
Assessment of outcome
The following data was collected in the present study: age, gender, radiological images, detailed information of surgical procedure, pulmonary, and other metastasis at presentation and follow-up information. Complications, local recurrence, metastasis, and death were recorded from the clinical and follow-up files. Furthermore, the oncological complications composed of recurrence and pulmonary and multiple metastasis. Meanwhile, the non-oncological complications included wound complication, fixation failure, and other complications. We classified patients as having no evidence of disease (NED), live with disease (LWD), and dead with disease (DWD). Oncological and non-oncological re-operations were recorded.
Statistical analysis
Actuarial overall survival, local relapse-free survival, and distant relapse-free survival were analyzed using the Kaplan-Meier survival analysis using SPSS software. Categorical variables were expressed as number of occurrences and percentage of the total patients in a category. The curves were compared using the log-rank test, where the starting point was surgery and the end point was the occurrence of complications. The SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Surgical treatment
The clinical characteristics of patients are shown in Table 1. The mean follow-up duration was 30.7 months (median, 17.7 months; range, 5.2–146.7 months). By using our sacral region classification, we found four cases at region 1; eleven at region 1 and 2; 13 at regions 1, 2, and 3; four at regions 2 and 3; and eight at region 3. Three patients had the pulmonary metastasis at the diagnosis, respectively, one epithelioid sarcoma, one malignant peripheral nerve sheath tumor, and one osteosarcoma.
All patients received the surgical treatment. Pre-operative embolization was performed in 9 (22.5%, 9/40) patients, and the surgical detailed information is shown in Table 2. Most patients underwent a posterior surgical approach (80%, 32/40), followed by seven anterior-posterior and one posterior-anterior. Furthermore, internal fixation was utilized in the majority of patients (31 cases, 77.5%). Thirty-four (85%, 34/40) patients received an en bloc resection with marginal or wide margins according to our previous proposed surgical classification, whereas six (15%, 6/40) patients received resection with contaminated margin. The typical cases of Type I, II, III and IV resection are shown in Figs. 1, 2, 3, and 4, and no Type V resection existed in our cohort.
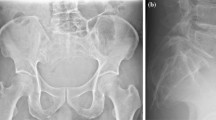
A female osteosarcoma patient receiving Type I resection, aged 13 years older, presented with low back pain for 1 month. a, b X-ray and CT scanning revealing the osteogenetic lesion at the sacrum. c, d T2-weighted MRI axial and sagittal images showing a infiltrative lesion involving S1–3. E. Post-operative X-ray. F. X-ray at 6 months after resection
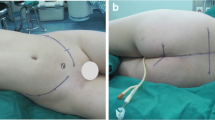
A female osteosarcoma patient receiving Type II resection, at 10 years of age, with 2 months history of low back pain. a–c Pre-operative X-ray and MRI showing osteogenesis lesion at S2–5. D. Post-operative X-ray. e X-ray at 1 year post-operation. f X-ray at 2 years post-operation and receiving the fixation removal due to patient requirement. g, h Post-operative bone scanning at 1 and 2 years
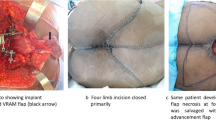
A female patient of yolk sac tumor receiving Type III resection, aged 3 years older, presented with low back pain for several months. a X-ray revealing the huge soft mass at the sacrum, indicated by the black arrow. b CT scanning showing the tumor extending into the presacral space. c, d T2-weighted MRI axial and sagittal images revealing a large soft tissue extension of the mass. e Photo of tumour specimen. f Post-operative X-ray. g Post-operative MRI scanning at 1 year
A female Ewing sarcoma patient receiving Type IV resection, at 6 years of age, with month history of leg thigh and low back pain. a Pre-operative X-ray. b CT scanning showing the eccentric bony destruction at S1–2. c Axial MRI revealing the infiltrative lesion at S1–2. d Post-operative X-ray after sagittal resection
Non-oncological complications
Nineteen out of 40 patients had wound complications, including the surgical site infection (SSI) and wound dehiscence. Among them, seven cases had SSI and 12 wound dehiscence. For seven patients with SSI, the detailed information of bacteria culture is listed in Table 3, and all cases with wound complication received the debridement. Escherichia coli was the most species and the positive rate was 71.4% (5/7). In 11 patients, one surgical debridement was sufficient; repeated debridements were necessary in eight patients: two operations in seven patients and three operations in one patient. All infections healed after debridement (Table 3).
One had the deep venous thrombosis and one had the femoral artery thrombosis. One had the rectal injury during the operation. Furthermore, three had the fixation breakage and then received the revision. The typical patient with fixation failure is shown in Fig. 5. MRI demonstrated a large infiltrative lesion at S1–5. The pathology was Ewing sarcoma. He received the total sacrectomy (Type I resection), and the fixation breakage occurred at 27 months after initial surgery. Then, the revision surgery combined with the transplantation of fibula autograft restored the spinopelvic stabilization (Fig. 5).
A male Ewing sarcoma patient receiving Type I resection, at 13 yrs. of age, who had the fixation failure at 2 years after initial surgery. a Pre-operative X-ray. b, c Axial and sagittal MRI revealing the tumour extending into the presacral space and spinal canal. d Photo of intra-operative manifestation after resection. e Photo after reconstruction. f Photo of tumour specimen. g X-ray of tumour specimen. h Post-operative X-ray. i Fixation failure at 2 years after initial surgery. j Revision surgery with the fibula autograft and renewed fixation
Analysis of overall survival, local relapse-free survival, and distant relapse-free survival
Twenty-two patients (55%, 22/40) were free of disease. A total of 13 deaths (32.5%, 13/40) were observed and the mean OS (overall survival) duration was 17.1 months (range, 6.3–34.2 months) (Table 3). Kaplan-Meier estimated survivorship curves of OS at one, two and five years were respectively 88.3%, 62.5%, and 51.9% (95% CI: 59.8–110.7%). Meanwhile, the incidence of local recurrence for young patients with primary malignant sacral tumours in the present cohort was 27.5% (11/40). Six out of 11 patients had the local recurrence that required surgery treatment, and the others refused the resection of the recurrent lesion. Time to recurrence after initial operation was 20.5 ± 17.4 months (median, 17.0 months; range, 2.3–58.6 months). Kaplan-Meier estimated survivorship curve of local recurrence-free survival rates of one and two years in young patients with sacral primary tumours were respectively 89.1% and 76.6% (95% CI: 36.5–101.3%). Furthermore, the pulmonary metastasis occurred in 18 patients (45%, 18/45) at the 12.0 ± 10.3 months (median, 6.8 months; range, 2.2–35.3 months) after the initial surgery and five cases of 18 lived with the pulmonary metastasis. Kaplan-Meier estimated survivorship curve of distant relapse-free survival rates of one and two years in young patients with sacral primary tumors was respectively 75.5% and 67.1% (95% CI: 57.7–112.7%) (Fig. 6).
a Kaplan-Meier estimated survivorship curve of the overall survival at 1, 2, and 3 years was respectively 88.3%, 62.5%, and 51.9%. b Kaplan-Meier estimated survivorship curve of local recurrence-free survival rates of 1 and 2 years in young patients with sacral primary tumours were respectively 89.1% and 76.6%. c Kaplan-Meier estimated survivorship curve of distant relapse-free survival rates of 1 and 2 years in young patients with sacral primary tumours was respectively 75.5% and 67.1%
Then, we performed the stratification analysis of young patients with primary malignant tumours at the sacrum after surgery and revealed the influence of pathological grade, location, and age on the oncological outcomes. Kaplan-Meier estimated survivorship curves of patients with high- and low-grade malignant tumours showed statistical differences in the overall survival and distant relapse-free survival rates between two groups (Fig. 7). Although Kaplan-Meier estimated survivorship curves of patients aged ≤ 14 years and > 14 years demonstrated no statistical difference in the overall survival, local recurrence-free and distant relapse-free survival rates between two groups, patients whose age was ≤ 14 years had the tendency of worse prognosis than > 14 years patients (Fig. 8). Afterwards, Kaplan-Meier estimated survivorship curves of patients with tumours above S3 and S3/below S3 revealed no significant difference in the oncological outcomes between two groups in our cohort (Fig. 9).
Kaplan-Meier estimated survivorship curve of patients with aged ≤ 14 years and > 14 years, showing no statistical differences in the overall survival, local recurrence-free and distant relapse-free survival rates between two groups. However, patients whose age was ≤ 14 years having the tendency of worse prognosis than > 14 years patients
Discussion
The present study is aiming to describe the prognosis of young patients with primary malignant sacral tumour aged less than 18 years old who received the resection based on the proposed surgical classification and compare the difference of oncological outcome between the pediatric aged ≤ 14 years and adolescent > 14 years with primary malignant sacral tumour. This series which were treated at our tumour centre provided valuable data contributing to our understanding of the clinical outcome of surgical treatment for primary malignant sacral tumours according to our resection classification in the young population.
Ewing sarcoma was one of the most common of histological types at the sacrum for young patients in the present cohort, which was concordant with other studies in the literature [7]. Our study revealed that second occurrence of primary malignant tumour at the sacrum was osteosarcoma, which was followed by malignant peripheral nerve sheath tumour and yolk sac tumour. Primary malignant tumours at the sacrum in young patients are difficult to manage because of their complex anatomy and low incidence. It has been reported that the role of surgery is crucial in the management of childhood and adolescent patients with sarcoma, especially for tumours at the sacral region. It has also been reported that the prognosis of sacral osteosarcoma was poor than other locations of spine [9]. Wang et al. reported the clinical data of 26 patients treated surgically for sacral osteosarcomas, and results showed the rates of distal metastasis and local recurrence were respectively 50% and 38.5%. It also revealed the one year and five year survival rates were 92.3% and 38.7%, respectively. It demonstrated adequate margins were very crucial for radical resection of sacral tumour, and it also significantly improved the recurrence rate and event-free survival rate for patients [6].
Considering the difficulty and complexity of tumour resection and reconstruction of spinopelvic stabilization, Kiiski et al. reported that in the most complex cases, surgery can be safely staged and final reconstruction can be carried out within one week without increasing peri-operative complications [10]. In our tumour centre, most sacral surgery were finished at one stage with the help of tumour-supplied artery embolization and intra-operative abdominal aorta balloon.
For surgery of young patients with primary malignant sacral tumours, preservation of sacral nerve is very important for them. However, adequate surgical margins should not be compromised to preserve function when they are necessary to affect tumour control. Although the anatomical complexities around the sacrum partly made radical operation difficult and lead to more complications, the en bloc resection according to our previous report of the surgical resection classification is the most effective surgical intervention for the malignant sacral tumour [8]. Total or partial sacrectomy and hemisacrectomy cause spinopelvic instability and discontinuity, and there are multiple recommendations for restoration of early stabilization and mobilization [8, 11, 12]. The iliac screw fixation combined with posterior lumbar segmental fixation is one of the most common procedures. Failure of spinopelvic fixation will eventually occur in long-time survival due to abundant loss of iliolumbar ligamentous stability; therefore, it has been reported that vascularized or non-vascularized bone reconstruction is recommended in addition to spinopelvic fixation. However, it has been reported that utilization of vascularized bone graft in the spinopelvic restoration is not necessary [10]. In the present study, fixation failure occurred in three cases and all of them received the revision fixation combined with fibula autograft (typical case shown in Fig. 5). At the last follow-up, one died with disease and the other two showed no evidence of disease.
The tumour at the sacrum has the proximity to important and sensitive neurovascular structures. When the tumour invades the surrounding organs or lumbosacral trunk, inadequacy of the surgical margins is unavoidable, whereas the en bloc resection which is the most effective surgical intervention for the malignant sacral tumour may result in serious complications such as huge blood loss and adjacent organ and lumbosacral trunk injury and post-operative complications. Look back past 20 years, the surgical technology of treating the sacral tumour has made great progress and achieved remarkable success through the unremitting effort of the orthopaedic oncologists [12]. The intra-operative massive blood loss for resection of sacral tumours is a great challenge for the orthopaedic oncologists. The effective control of the intra-operative blood loss provides a significant insurance for the thorough and safe en bloc resection of the sacrum. Pre-operative tumour-supplied artery embolization and technology of aortic balloon occlusion during the surgery are applied into the resection of sacral tumour, and these technologies improve the therapy effects. Sacral tumour-supplied arteries include iliolumbar artery and lateral and median sacral arteries [13, 14]. Thus, more attention should be paid to these arteries before surgery. In our cohort, 9 patients received the pre-operative artery embolization and 24 received aortic balloon occlusion during the operation.
It has been reported that the use of neoadjuvant chemotherapy has improved the prognosis, whereas the surgical treatment of malignant sacral tumor is still associated with poor outcomes. This is often caused by difficulty of achieving satisfactory surgical margin due to limited accessibility of satisfactory surgical margin and proximity to important and sensitive neurovascular structures. In the literature, it has been reported that the role of surgery is crucial in the management of childhood osteosarcoma, especially for tumours at the sacrococcygeal region [6, 15, 16]. Resection of tumor at the high level sacrum is associated with more complications, which include infection, hemorrhage, wound complications, mechanical failure of spinopelvic reconstruction, vascular and visceral injuries, and long-term neurological deficits including bowel, bladder, or sexual dysfunction.
Wound complications including surgical site infection (SSI) and wound dehiscence are common after sacral resection for malignancies, ranging from 25 to 53.5% in the literature [10, 17, 18], and the rate was 47.5% (19/40) in the present study of paediatric and adolescent patients. SSI often occurs after surgical procedure for sacral tumours. Due to malignant tumours being systemic diseases and chemotherapy in some patients, the immune system becomes compromised and the patient is thus predisposed to SSI [17, 18]. The rate of SSI in our study was 17.5% (7 in 40 cases). The risk factors of SSI at the sacrum include previous radiation, rectum rupture, age < 40 years, history of diabetes mellitus, maximum tumour diameter > 10 cm, complex reconstruction, and instrumentation utilization [17]. Meanwhile, SSI can occur at different time post-operatively from one day to many years, but several studies reported it commonly occurred between the 15th and 30th days after surgery [18]. Considering the fact that Escherichia coli is the most frequent species causing SSI for sacral tumour surgery, a broad-spectrum antibiotic that is effective against gram-negative should be initially considered. Afterwards, the bacteria culture is needed and adjustment of antibiotic depends on the results of bacteria cultures. Debridement is recommended if obvious symptom of SSI occurs. The incision needs to be widely opened, which can allow to totally drain and any foul-looking tissue or debris should be removed during the debridement. How we control SSI is a crucial problem for patients with sacral tumour receiving the operation. Ricciardi et al. reported that recommended strategies in the guidelines such as prophylactic antibiotics and pre-operative skin preparation of patients were considered critically important by the surveyed surgeons. Additional strategies such as ultraclean air/laminar flow, antibiotic cement, wound irrigation, and pre-operative blood glucose control were also considered highly important by surveyed surgeons [19].
Several studies showed many factors may affect the clinical outcome of patients with malignant sacral tumours. The operative resection of sacral tumour with satisfactory wide margin can offer the best long-term prognosis, and the inadequate surgical margin increases the risk of local recurrence and indicates a dismal prognosis [20,21,22,23]. It has been reported that age may be an important risk factor for the osteosarcoma prognosis [24,25,26]. Morsy et al. reported that the rate of osteosarcoma metastasis for children aged ten to 14 was higher than that aged 14–18 years old (44.4% vs 33.3%), which was concordant with our result [27]. In the stratification analysis in our study, it showed that the paediatric patients with the primary malignant sacral tumour, who had the higher pulmonary metastasis and lower survival rate, revealed the tendency of poor prognosis compared to adolescent aged > 14 years.
Alexandre et al. reported sacrectomy had the potential to be curative for children with high-grade sarcoma, with disease-free survival rate of 75%. He explained the satisfactory oncological prognosis according to the fact that chemotherapy was critical in the treatment of Ewing sarcoma and osteosarcoma, and then sacrectomy without effective chemotherapy most likely would be insufficient to achieve disease-free status [7]. In the present study, the overall survival at one, two and five years were respectively 88.3%, 62.5%, and 51.9% and this result reflected all primary malignant tumours at the sacrum. However, for the high-grade malignant tumours at the sacrum, the overall survival at one, two and five years of high-grade malignant tumours were 84.6%, 51%, and 37.2%, which was significantly lower than that of all patients. Thus, it demonstrated that patients with high-grade malignant tumours, including osteosarcoma, Ewing sarcoma, and malignant peripheral nerve sheath tumour, had poor prognosis compared to patients with low grade.
The present study had several limitations. Firstly, this was a retrospective study which may lose several cases or detailed information during the follow-up and we had no pre-established protocol for management of these patients. Secondly, our series was very heterogeneous in terms of histology and we need to respectively study the outcome of young patients with primary malignant sacral tumours at the sacrum with different pathologies in the future. Thirdly, the relatively low number of patients in this study cannot be analyzed more accurately. However, the rarity of these primary tumours at the sacrum may increase the value of our report and our cohort is the largest in the literature.
In conclusion, it is satisfactory for the outcome of surgical treatment for children and adolescent patients with primary malignant sacral tumours based on proposed surgical resection classification. Furthermore, pediatric patients aged ≤ 14 years have the tendency of poor prognosis compared to adolescent aged > 14 years.
References
Billmire DF (2006) Malignant germ cell tumors in childhood. Semin Pediatr Surg 15:30–36
De Corti F, Sarnacki S, Patte C et al (2012) Prognosis of malignant sacrococcygeal germ cell tumours according to their natural history and surgical management. Surg Oncol 21:e31–ee7
Kurugoglu S, Adaletli I, Mihmanli I, Kanberoglu K (2008) Lumbosacral osseous tumors in children. Eur J Radiol 65:257–269
Loh JK, Lin CK, Hwang YF, Hwang SL, Kwan AL, Howng SL (2005) Primary spinal tumors in children. J Clin Neurosci 12:246–248
Tsitouras V, Wang S, Dirks P et al (2016) Management and outcome of chordomas in the pediatric population: the hospital for sick children experience and review of the literature. J Clin Neurosci 34:169–176
Wang Y, Guo W, Shen D et al (2017) Surgical treatment of primary osteosarcoma of the sacrum. Spine 42:1207–1213
Arkader A, Yang CH, Tolo VT (2012) High long-term local control with sacrectomy for primary high-grade bone sarcoma in children. Clin Orthop Relat Res 470:1491–1497
Li D, Guo W, Tang X, Ji T, Zhang Y (2011) Surgical classification of different types of en bloc resection for primary malignant sacral tumors. Eur Spine J 20:2275–2281
Ozaki T, Flege S, Liljenqvist U et al (2002) Osteosarcoma of the spine: experience of the cooperative osteosarcoma study group. Cancer 94:1069–1077
Kiiski J, Kuokkanen HO, Kaariainen M, Kaartinen IS, Pakarinen TK, Laitinen MK (2018) Clinical results and quality of life after reconstruction following sacrectomy for primary bone malignancy. J Plast Reconstr Aesthet Surg 71:1730–1739
Li D, Guo W, Tang X et al (2014) Preservation of the contralateral sacral nerves during hemisacrectomy for sacral malignancies. Eur Spine J 23:1933–1939
Wei R, Guo W, Ji T, Zhang Y, Liang H (2017) One-step reconstruction with a 3D-printed, custom-made prosthesis after total en bloc sacrectomy: a technical note. Eur Spine J 26:1902–1909
Ozkan E, Gupta S (2011) Embolization of spinal tumors: vascular anatomy, indications, and technique. Tech Vasc Interv Radiol 14(3):129–140
Yang HL, Chen KW, Wang GL et al (2010) Pre-operative transarterial embolization for treatment of primary sacral tumors. J Clin Neurosci 17:1280–1285
Kerr DL, Dial BL, Lazarides AL et al (2019) Epidemiologic and survival trends in adult primary bone tumors of the spine. Spine J 19:1941–1949
Groves ML, Zadnik PL, Kaloostian P et al (2015) Epidemiologic, functional, and oncologic outcome analysis of spinal sarcomas treated surgically at a single institution over 10 years. Spine J 15:110–114
Li D, Guo W, Qu H et al (2013) Experience with wound complications after surgery for sacral tumors. Eur Spine J 22:2069–2076
Ruggieri P, Angelini A, Pala E, Mercuri M (2012) Infections in surgery of primary tumors of the sacrum. Spine 37:420–428
Ricciardi BF, Bostrom MP, Lidgren L, Ranstam J, Merollini KM, A WD (2014) Prevention of surgical site infection in total joint arthroplasty: an international tertiary care center survey. HSS J 10:45–51
Yamamoto Y, Kanzaki R, Kanou T et al (2019) Long-term outcomes and prognostic factors of pulmonary metastasectomy for osteosarcoma and soft tissue sarcoma. Int J Clin Oncol 24:863–870
Weeden S, Grimer RJ, Cannon SR, Taminiau AH, Uscinska BM, Intergroup EO (2001) The effect of local recurrence on survival in resected osteosarcoma. Eur J Cancer 37:39–46
Smeland S, Bielack SS, Whelan J et al (2019) Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer 109:36–50
Min D, Lin F, Shen Z et al (2013) Analysis of prognostic factors in 333 Chinese patients with high-grade osteosarcoma treated by multidisciplinary combined therapy. Asia Pac J Clin Oncol 9:71–79
Cho HW, Lee JW, Ma Y, Yoo KH, Sung KW, Koo HH (2018) Treatment outcomes in children and adolescents with relapsed or progressed solid tumors: a 20-year, single-center study. J Korean Med Sci 33:e260
Wang Z, Wu B, Zhou Y et al (2019) Predictors of the survival of primary and secondary older osteosarcoma patients. J Cancer 10:4614–4622
Duchman KR, Gao Y, Miller BJ (2015) Prognostic factors for survival in patients with high-grade osteosarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol 39:593–599
Morsy AM, Abdelgawad MI, Ahmed BM et al (2019) Pediatric osteosarcoma of extremities-a 15-year experience from a tertiary care cancer center in upper Egypt. J Pediatr Hematol Oncol 41:e371–ee83
Acknowledgments
Not applicable.
Funding
This work was supported by the Natural Science Foundation of China (NO:81702657) and Beijing Municipal Science & Technology Commission (No:Z181100001918025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Rights and permissions
About this article
Cite this article
Wang, J., Li, D., Yang, R. et al. Outcome of surgical treatment of children and adolescents with primary malignant sacral tumours. International Orthopaedics (SICOT) 44, 1841–1851 (2020). https://doi.org/10.1007/s00264-020-04641-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-020-04641-7