Abstract
Purpose
Cup positioning is important for optimum hip stability, avoiding component impingement and decreasing both bearing surface wear and revision rate. Transitioning from posterior approach in a lateral position to direct anterior approach (DAA) in a supine presents unique challenges for surgeons. The aim of this study was to examine the learning curve when using standard instrumentation that was not specific to DAA.
Methods
A consecutive retrospective series of 537 total hip arthroplasty by DAA from May 2013 to December 2017. Cup positioning was analysed on radiographs and classified whether inside or outside two safe zones (inclination 30–50° and anteversion 10–30°). The demographic data (age, BMI, gender, neck shaft angle (NSA)), surgeon’s dominant side and experience were assessed as risk factors.
Results
Eighty per cent of cups (n = 426) were in the combined safe zones. Eighty-eight per cent (n = 470) were in appropriate anteversion and 87% (n = 463) abduction. Two factors that were significant were identified: Cups of left hips operated by right-handed surgeons were more anteverted (OR = 4.06) and more vertical (OR = 2.23); females had a higher anteversion of the cup (OR = 2.42). Obesity, age and NSA were not risk factors for cup malposition. There was a spike of cups too horizontal at the beginning of the experience (OR = 3.86), and no learning curve was observed in the other orientations.
Conclusion
With our DAA technique using standard instrumentation, there were no risk factors linked to the patient identified for cup malposition. DAA-specific instrumentation is not required to achieve optimum positioning of the cup. Surgeon has to be aware of an excess of abduction at the beginning of his experience and an excess of anteversion and adduction when performing THA on the opposite side of his dominant hand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally the posterior approach (PA) to THA has become the most common for total hip arthroplasty (THA) [1]. One of the main advantages of PA is the excellent exposure of the hip joint with the ability to extend proximally into the pelvis or distally along the femur as required to achieve optimum reconstruction, implant positioning and fixation. The ability to extend the exposure of DAA is technically demanding [2] and may be considered a risk for increasing the learning curve of optimum implant positioning when transitioning from PA.
An optimal acetabular position to decrease the dislocation risk was described by Lewinnek [3] as a safe zone (SZ) of inclination 40° ± 10° and anteversion of 15° ± 10°. Apart from dislocation risk, cup malpositioning is an important cause of component impingement, accelerated bearing surface wear and an increased revision rate [4]. Globally THA instability is reported from 0.3 to 10% in studies and joint registries [5, 6]. Acetabular component positioning within SZ alone will not prevent dislocation. Abdel and Reize reported that 58% of their dislocated hips had a cup in the SZ [7, 8]. Other considerations than anteversion and inclination have been found to lower dislocation risk such as matching the femoral neck version to acetabular anteversion [9], increasing the femoral head size [10], using a dual mobility cup [11] or changing the surgical approach [12, 13].
Direct anterior approach (DAA) is described in the literature as a soft tissue protecting approach [14] that decreases the risk of dislocation [15] and allows rapid recovery [16, 17] with reproducible management of leg length discrepancy (LLD) [18]. Detractors of DAA point out the separate challenges of optimal cup position and femoral exposure when transitioning from PA. Instrumentation has evolved with DAA techniques of special offset handles to aid the surgeon. We have reported that a non-DAA-specific femoral broach with a prominent lateral shoulder will increase the risk of varus femoral stem alignment when compared with a DAA-specific broach with a less prominent shoulder [19]. The influence of femoral broach shape and specific instrumentation on alignment of the femoral stem suggests that a similar situation may occur for acetabular positioning as well.
Literature suggests that cup positioning in DAA has excellent reproducibility. Kobayashi [20] report an accuracy around 79% with a tendency of increased anteversion (mean = 28°) when using a straight cup impactor, while Deacon [21] had an accuracy of 96% with less anteversion (mean = 18°) when using a DAA-specific offset cup impactor. Deacon also reported that despite using an offset impactor, there was an increase in cup inclination of obese patients.
This study aimed to assess if patient factors or the use of a straight nonspecific cup impactor could increase the risk of cup malposition when transitioning technique from PA in lateral position to DAA in supine position.
Materials and methods
Patients
Retrospectively from May 2013 to December 2017 at our hospital, 537 THA were performed on 476 patients by seven right-handed surgeons. The surgeons included one senior surgeon (n = 360) with more than ten years of experience in hip surgery practiced by posterior approach and six trainee surgeons (n = 177) who were initially trained for five years to practice hip surgery by posterior approach. The senior surgeon had learned and performed the DAA technique for a year before supervising the trainees. The inclusions included the very beginning of their experience. The inclusion criteria for our study were all patients with primary THA by DAA. The DAA is standard at our department (537 DAA (88%) vs 69 PA (12%)) unless obesity (BMI ≥ 40 kg/m2), abnormal hip anatomy requiring complex THA (e.g. congenital hip dysplasia), elderly patients (over 85 years old) with osteoporosis or when there was previous hip surgery (e.g. femoral or pelvic osteotomy). Four patients did not have pre-operative radiographs available for analysis and were excluded, and one patient died before his post-operative radiography after a pulmonary embolism. At the last follow-up, 532 THA (471 patients) were included and assessed. There were no patients lost to follow-up. Demographic data are summarized in Table 1.
Surgical technique, instrumentation and implants
The standardized approach of Hueter Gaine was used for all patients. The DAA was performed in supine position with a standard operating table as described by Lustig [22]. The acetabular cup was placed manually according to the anatomical landmarks: The transverse acetabular ligament was used to control acetabular depth, height and version, and the inclination was assessed by orientating the cup flush with the roof. The anterior and posterior horns helped for the anteversion. Fluoroscopic control was systematically utilized. Standard straight cup reamer and impactor (Fig. 1) were used for all cases. All shells were cementless (Cargos (Lepine®), Quattro (Lepine®)). Either a dual mobility or conventional liner was used. Dual mobility cup was used in patients older than 65 years old and if there was a high risk of dislocation (e.g. epilepsy, Parkinson’s disease) [23].
Radiographic evaluation
All measurements were recorded by an independent observer on the standing antero-posterior X-rays at two months after surgery. Radiographic analysis included inclination and anteversion of the cup. Anteversion was determined by the method described by Widmer [24]. Cup abduction was the angle between the cup axis and parallel between the inter-teardrop line. Cup position was analysed and classified whether inside or outside the safe zone (SZ) concerning the inclination (30–50°), the anteversion (10–30°) or both position (combined SZ).
Statistics
The continuous variables were averaged and reported with standard deviation and extremes. The multinomial logistic regression model to investigate the relationship between bad positioning of the cup and patient risk factors included patient age, gender, BMI, neck shaft angle (NSA), surgeon dominant side and experience (the 10 first DAA for each surgeon where compared with the rest the series). A p value < 0.05 was considered statistically significant in each analysis. The statistical analyses were performed using XLstat (2015.1 version, Addinsoft, France).
Results
Implant positioning
Implant positioning is reported in Table 2 and Fig. 2. Eighty per cent (n = 426) of the cups are in the combined SZ. The majority of cups that were malpositioned were either too anteverted (8.5%; n = 45) or vertical (10%; n = 54).
Risk factors (Table 3)
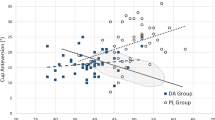
Left hips operated upon by right-handed surgeons had a risk of being too anteverted (OR = 4.06; p < 0.0001) and too much vertical (OR = 2.23; p = 0.008) (Fig. 3). Being a woman was significantly correlated to a higher anteversion (OR = 2.42; p = 0.017). Obesity, age and varus were not correlated to any risk. There was a spike of malposition in abduction at the experience beginning (OR = 3.86, p = 0.02), and no learning curve was seen in the global SZ or in other orientations (Fig. 4).
a Perfect anteversion (15°) and abduction (45°) in a patient with no risk factors (man, right size). b Excess of anteversion (35°) and adduction (51°) in a patient with both risk factors (woman, left size). THAs operated by the same right handed surgeon with the same implants (Quattro acetabular cup, Targos femoral stem, Groupe Lepine®, France)
Discussion
The important finding of this study was the use of a straight cup reamer and impactor by DAA achieved optimal acetabular positioning in the combined SZ of 80% cases. Of all cases, 88% were within optimal anteversion and 87% optimal inclination.
Kobayashi et al. [20] using a straight cup impactor in DAA concluded that the surgeon has to take care of preventing excess anteversion, and in his series, the mean anteversion was 27.6°. In our study, the anteversion was lower (mean = 22°) but still is greater than Deacon [21] using an offset cup impactor (mean = 18.3°). The inclination is not impacted by the shape of the impactor (Table 4).
Two risks factors were identified: Left hips operated by right-handed surgeons were more anteverted and more vertical, while females had more anteverted cups. The surgeons’ dominant side effect correlates with Crawford et al. [26] and Song et al. [27]. Two papers that assess right-sided surgeons only, Crawford had similar less outliers for right side hips in both the DAA and direct lateral approaches. Song also had greater accuracy for right-sided hips when comparing a posterolateral approach in 46% of the left hips in the global SZ against 62% on the right side.
Obesity, age and coxa vara were not a risk factor for malposition. There was only a learning curve concerning the excess of abduction, and no learning curve was found concerning the global positioning or the other orientations. Difficult exposure at the beginning of the learning curve induced a poor visualization of anatomical landmarks used to place the cup (transverse acetabular ligament and bony roof), fearing the excess of adduction responsible for a higher dislocation risk [28], and the surgeons tended to exaggerate the abduction. Also, the surgeons were accustomed to PA which is at risk of an excess of adduction due to a conflict with the skin or the femur, especially when using a mini-posterior approach [29]. After an initial adjustment period, based on the analysis of post-operative radiographs and the absence of dislocations, the surgeons corrected their gesture and put their cups more and more vertical (Fig. 4). The learning curve is known to be challenging in DAA with an increase of complications at the beginning of the experience [30,31,32], but none of those studies focused on a detailed modelling of the evolution of cup position. And the radiological control during the surgery avoids the important mistakes of cup positioning. Obesity is controverted, and Deacon and Callanan had a significant increase of malposition in that population [21, 33]. Hallert seemed to have more outliers without any significance [34], and Todkar had no difference [35]. Attention had been paid in our department to exclude difficult patients (obese, aged, severe coxa vara) of the anterior approach, particularly at the beginning of the learning curve. These patients with high risk of complications by anterior approach were operated by posterior approach. Probably that is why all those factors were not recognized as risk factors in this study. Appropriate indications are crucial to avoid major complications by anterior approach.
Atkinson [36] studied in 2010 the gender differences of native hip morphology. Females had a higher anteversion of the native acetabulum than males (23° vs 18°). Therefore, the difference between genders in term of anteversion can be predicted if anatomical landmarks are used to place the cup. Our aim was to reproduce the anatomy of the patient and thus the native acetabular anteversion.
The optimal anteversion described by Lewinnek was 10–30°, and we have increased this by 5 in order to avoid ilio-psoas impingement [37]. The optimal position described by Lewinnek has been questioned over time, and several studies report a lack of correlation between it and dislocation rate [7, 38,39,40]. Murphy even defined a totally new SZ based on CT scans and found a completely different SZ (anteversion 31° ± 8°, inclination 43° ±1 2°) with a special attention that must be paid to the anteversion [41]. Kamara [42] used the same “target zone” as we have during DAA guided by fluoroscopy with an accuracy of 84%, while the posterior approach without intraoperative fluoroscopy had an accuracy of 66% or guided by a robot of 97%. Other studies report increased accuracy with robot and navigation compared with without [43, 44].
Supine position in DAA changes the three-dimensional orientation of the acetabulum relative to the surgeon and needs to be taken into consideration when transitioning from the posterior approach. For us, there are two major advantages of the supine position; it creates less alteration of the pelvic orientation than the lateral decubitus position [45] and allows intraoperative fluoroscopy which for us is the reason why there was no learning curve for cup positioning.
One limitation of this study is that the assessment of implant position was on radiograph and not on CT scan. CT is more accurate, particularly for cup anteversion. However, a CT scan is not recommended for routine THA follow-up. This would have exposed study patients to increased radiation and would not reflect common practice. To compensate those drawbacks, single X-ray image based 2D/3D reconstruction technique has been proved to be a great cheaper alternative [46] and should be considered for the following studies. As a result, the quality of the radiographs could be a second limitation; however, our department X-ray technicians are specialized in the lower limb, and their images were assessed to minimize error. Another limitation is that this study is retrospective. However, the primary aims of this study were to assess implant position and their risk factors, which are not influenced by retrospective analysis.
Conclusion
With our DAA technique, specific offset instrumentation was not required to achieve optimum positioning of the cup in DAA. There were no significant patient risk factors for cup malposition. However, a surgeon has to be aware of an excess of abduction at the beginning of his experience and an excess of anteversion and adduction when performing THA on the opposite side of his dominant hand.
References
Waddell J, Johnson K, Hein W et al (2010) Orthopaedic practice in total hip arthroplasty and total knee arthroplasty: results from the Global Orthopaedic Registry (GLORY). Am J Orthop 39:5–13
Thaler M, Dammerer D, Krismer M et al (2019) Extension of the direct anterior approach for the treatment of periprosthetic femoral fractures. J Arthroplast 34:2449–2453. https://doi.org/10.1016/j.arth.2019.05.015
Lewinnek G, Lewis J, Tarr R et al (1978) Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am 60:217–220
Moskal JT, Capps SG (2010) Improving the accuracy of acetabular component orientation: avoiding malposition. J Am Acad Orthop Surg 18:286–296. https://doi.org/10.5435/00124635-201005000-00005
Brooks PJ (2013) Dislocation following total hip replacement: causes and cures. Bone Joint J 95-B:67–69. https://doi.org/10.1302/0301-620X.95B11.32645
Melvin JS, Karthikeyan T, Cope R, Fehring TK (2014) Early failures in total hip arthroplasty -- a changing paradigm. J Arthroplast 29:1285–1288. https://doi.org/10.1016/j.arth.2013.12.024
Abdel MP, von Roth P, Jennings MT et al (2016) What safe zone? The vast majority of dislocated THAs are within the Lewinnek safe zone for acetabular component position. Clin Orthop Relat Res 474:386–391. https://doi.org/10.1007/s11999-015-4432-5
Reize P, Geiger EV, Suckel A et al (2008) Influence of surgical experience on accuracy of acetabular cup positioning in total hip arthroplasty. Am J Orthop 37:360–363
Elkins JM, Callaghan JJ, Brown TD (2015) The 2014 Frank Stinchfield Award: the ‘landing zone’ for wear and stability in total hip arthroplasty is smaller than we thought: a computational analysis. Clin Orthop Relat Res 473:441–452. https://doi.org/10.1007/s11999-014-3818-0
Howie DW, Holubowycz OT, Middleton R, Large Articulation Study Group (2012) Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am 94:1095–1102. https://doi.org/10.2106/JBJS.K.00570
Gaillard R, Kenney R, Delalande J-L et al (2019) Ten- to 16-year results of a modern cementless dual-mobility acetabular implant in primary total hip arthroplasty. J Arthroplast 34:2704–2710. https://doi.org/10.1016/j.arth.2019.06.051
Miller LE, Gondusky JS, Kamath AF et al (2018) Influence of surgical approach on complication risk in primary total hip arthroplasty. Acta Orthop 89:289–294. https://doi.org/10.1080/17453674.2018.1438694
Browne JA, Pagnano MW (2012) Surgical technique: a simple soft-tissue-only repair of the capsule and external rotators in posterior-approach THA. Clin Orthop Relat Res 470:511–515. https://doi.org/10.1007/s11999-011-2113-6
Bergin PF, Doppelt JD, Kephart CJ et al (2011) Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am 93:1392–1398. https://doi.org/10.2106/JBJS.J.00557
Tsukada S, Wakui M (2015) Lower dislocation rate following total hip arthroplasty via direct anterior approach than via posterior approach: five-year-average follow-up results. Open Orthop J 9:157–162. https://doi.org/10.2174/1874325001509010157
Trevisan C, Compagnoni R, Klumpp R (2017) Comparison of clinical results and patient’s satisfaction between direct anterior approach and Hardinge approach in primary total hip arthroplasty in a community hospital. Musculoskelet Surg 101:261–267. https://doi.org/10.1007/s12306-017-0478-8
Amlie E, Havelin LI, Furnes O et al (2014) Worse patient-reported outcome after lateral approach than after anterior and posterolateral approach in primary hip arthroplasty. A cross-sectional questionnaire study of 1,476 patients 1-3 years after surgery. Acta Orthop 85:463–469. https://doi.org/10.3109/17453674.2014.934183
Lecoanet P, Vargas M, Pallaro J et al (2018) Leg length discrepancy after total hip arthroplasty: can leg length be satisfactorily controlled via anterior approach without a traction table? Evaluation in 56 patients with EOS 3D. Orthop Traumatol Surg Res 104:1143–1148. https://doi.org/10.1016/j.otsr.2018.06.020
Batailler C, Fary C, Servien E, Lustig S (2018) Influence of femoral broach shape on stem alignment using anterior approach for total hip arthroplasty: a radiologic comparative study of 3 different stems. PLoS One 13:e0204591. https://doi.org/10.1371/journal.pone.0204591
Kobayashi H, Homma Y, Baba T et al (2016) Surgeons changing the approach for total hip arthroplasty from posterior to direct anterior with fluoroscopy should consider potential excessive cup anteversion and flexion implantation of the stem in their early experience. Int Orthop 40:1813–1819. https://doi.org/10.1007/s00264-015-3059-1
Deacon M, de Beer J, Ryan P (2016) Radiological analysis of component positioning in total hip arthroplasty using the anterior approach. SA Orthop J 15:38–45. https://doi.org/10.17159/2309-8309/2016/v15n3a5
Lustig S (2015) Anterior approach (without specific table) and dual mobility acetabular component. Maitrise Orthop 243:1–5
Batailler C, Fary C, Verdier R et al (2017) The evolution of outcomes and indications for the dual-mobility cup: a systematic review. Int Orthop 41:645–659. https://doi.org/10.1007/s00264-016-3377-y
Widmer K-H (2004) A simplified method to determine acetabular cup anteversion from plain radiographs. J Arthroplast 19:387–390. https://doi.org/10.1016/j.arth.2003.10.016
Matta JM, Shahrdar C, Ferguson T (2005) Single-incision anterior approach for total hip arthroplasty on an table. Clin Orthop Relat Res 441:115–124. https://doi.org/10.1097/01.blo.0000194309.70518.cb
Crawford DA, Adams JB, Hobbs GR et al (2019) Surgical approach and hip laterality affect accuracy of acetabular component placement in primary total hip arthroplasty. Surg Technol Int 35:377–385
Song X, Ni M, Li H et al (2018) Is the cup orientation different in bilateral total hip arthroplasty with right-handed surgeons using posterolateral approach? J Orthop Surg Res 13:123. https://doi.org/10.1186/s13018-018-0789-y
Fessy MH, Putman S, Viste A et al (2017) What are the risk factors for dislocation in primary total hip arthroplasty? A multicenter case-control study of 128 unstable and 438 stable hips. Orthop Traumatol Surg Res 103:663–668. https://doi.org/10.1016/j.otsr.2017.05.014
Procyk S (2007) Initial results with a mini-posterior approach for total hip arthroplasty. Int Orthop 31:17–20. https://doi.org/10.1007/s00264-007-0435-5
de Steiger RN, Lorimer M, Solomon M (2015) What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res 473:3860–3866. https://doi.org/10.1007/s11999-015-4565-6
Hartford JM, Bellino MJ (2017) The learning curve for the direct anterior approach for total hip arthroplasty: a single surgeon’s first 500 cases. Hip Int 27:483–488. https://doi.org/10.5301/hipint.5000488
Kong X, Grau L, Ong A et al (2019) Adopting the direct anterior approach: experience and learning curve in a Chinese patient population. J Orthop Surg Res 14:218. https://doi.org/10.1186/s13018-019-1272-0
Callanan MC, Jarrett B, Bragdon CR et al (2011) The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res 469:319–329. https://doi.org/10.1007/s11999-010-1487-1
Hallert O, Li Y, Brismar H, Lindgren U (2012) The direct anterior approach: initial experience of a minimally invasive technique for total hip arthroplasty. J Orthop Surg Res 7:17. https://doi.org/10.1186/1749-799X-7-17
Todkar M (2008) Obesity does not necessarily affect the accuracy of acetabular cup implantation in total hip replacement. Acta Orthop Belg 74:206–209
Atkinson HD, Johal KS, Willis-Owen C et al (2010) Differences in hip morphology between the sexes in patients undergoing hip resurfacing. J Orthop Surg Res 5:76. https://doi.org/10.1186/1749-799X-5-76
Batailler C, Bonin N, M Wettstein null, et al (2017) Outcomes of cup revision for ilio-psoas impingement after total hip arthroplasty: retrospective study of 46 patients. Orthop Traumatol Surg Res 103:1147–1153. https://doi.org/10.1016/j.otsr.2017.07.021
Tezuka T, Heckmann ND, Bodner RJ, Dorr LD (2019) Functional safe zone is superior to the Lewinnek safe zone for total hip arthroplasty: why the Lewinnek safe zone is not always predictive of stability. J Arthroplast 34:3–8. https://doi.org/10.1016/j.arth.2018.10.034
Reina N, Putman S, Desmarchelier R et al (2017) Can a target zone safer than Lewinnek’s safe zone be defined to prevent instability of total hip arthroplasties? Case-control study of 56 dislocated THA and 93 matched controls. Orthop Traumatol Surg Res 103:657–661. https://doi.org/10.1016/j.otsr.2017.05.015
Dorr LD, Callaghan JJ (2019) Death of the Lewinnek “safe zone”. J Arthroplast 34:1–2. https://doi.org/10.1016/j.arth.2018.10.035
Murphy WS, Yun HH, Hayden B et al (2018) The safe zone range for cup anteversion is narrower than for inclination in THA. Clin Orthop Relat Res 476:325–335. https://doi.org/10.1007/s11999.0000000000000051
Kamara E, Robinson J, Bas MA et al (2017) Adoption of robotic vs fluoroscopic guidance in total hip arthroplasty: is acetabular positioning improved in the learning curve? J Arthroplast 32:125–130. https://doi.org/10.1016/j.arth.2016.06.039
Honl M, Dierk O, Gauck C et al (2003) Comparison of robotic-assisted and manual implantation of a primary total hip replacement: a prospective study. J Bone Joint Surg Am 85:1470–1478
Snijders T, van Gaalen SM, de Gast A (2017) Precision and accuracy of imageless navigation versus freehand implantation of total hip arthroplasty: a systematic review and meta-analysis. Int J Med Robot 13. https://doi.org/10.1002/rcs.1843
DiGioia A, Hafez M, Jaramaz B et al (2006) Functional pelvic orientation measured from lateral standing and sitting radiographs. Clin Orthop Relat Res 453:272–276. https://doi.org/10.1097/01.blo.0000238862.92356.45
Craiovan B, Renkawitz T, Weber M et al (2014) Is the acetabular cup orientation after total hip arthroplasty on a two dimension or three dimension model accurate? Int Orthop 38:2009–2015. https://doi.org/10.1007/s00264-014-2336-8
Author information
Authors and Affiliations
Contributions
Constant Foissey: study design, data collection, statistical analysis, literature review and manuscript writing
Cécile Batailler: study design, manuscript editing
Cam Fary: literature review, manuscript editing
Francesco Luceri: data collection, literature review
Elvire Servien: study design, manuscript editing.
Sébastien Lustig: study design, supervision, literature review and manuscript editing.
All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Conflict of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. CoFo, CB and CaFa declare that they have no conflict of interest. SL: consultant for Stryker; institutional research support from Corin and Amplitude. ES: institutional research support from Corin.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Advisory Committee on Research Information Processing in the Field of Health (CCTIRS) approved this study on June 4, 2015 under number 15-430. For this type of study, formal consent is not required.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Level of evidence: IV retrospective, consecutive case series
Electronic supplementary material
ESM 1
(XLSX 1162 kb)
Rights and permissions
About this article
Cite this article
Foissey, C., Batailler, C., Fary, C. et al. Transitioning the total hip arthroplasty technique from posterior approach in lateral position to direct anterior approach in supine position—risk factors for acetabular malpositioning and the learning curve. International Orthopaedics (SICOT) 44, 1669–1676 (2020). https://doi.org/10.1007/s00264-020-04583-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-020-04583-0