Abstract
Background
In a high conflict region, war injuries to the distal lower extremity are a major source of large composite defects involving bone and soft tissues. These defects are at the edge between using a single free flap [osteo-(+/-myo) cutaneous] vs double free flap reconstruction (bone and soft tissue). In this paper, we present our experience and outcomes in treating patients with leg war injury reconstructed using a single free fibula flap.
Methods
Fifteen patients with distal leg composite defects secondary to war injuries were treated between January 2015 and March 2016. All patients were reconstructed using single barrel free fibula osteo-(+/-myo)cutaneous flap where single or double skin paddles were used according to the soft tissue defect requiring coverage.
Results
There were no cases of total or partial flap loss. Complications were limited to three cases including traumatic fibula fracture, venous congestion with negative findings, and residual soft tissue defect requiring coverage. There were no cases of wound dehiscence or infection. Mean follow-up time was 418.8 days. Mean bone healing time was nine months after which patients were allowed full weight bearing.
Conclusion
A single barrel free fibula osteo-(+/-myo)cutaneous flap is a valid and reliable tool for reconstruction composite lower extremity defects post-war injury. Adequate planning of fibula flap soft tissue components (skin, muscle) rearrangement is essential for success in such challenging reconstructions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The multiplicity of current wars is a constantly replenishing source of debilitating injuries. Advances in the arsenal of weapons and the widespread use of improvised explosive devices cause colossal volumes of severe and complex injuries. In parallel, developments in body armour and helmets are providing adequate protection to the head, neck, thorax, and abdomen, leaving the extremities relatively exposed and more liable to injury [1]. Lower extremity injuries are of particular concern as they are more common than those of the upper extremity [2, 3]. Medical and surgical advances have improved the survival outcome, decreasing the number of war casualties. Consequently, war surgeons are left with more complex and technically challenging wounds to treat, necessitating the employment of superior reconstructive procedures. Even though amputation remains an option, limb salvage is becoming more common with medical advances as in internal fixation, with success rates reported as high as 80% even when vascular injuries are present [4,5,6,7].
The nature of war-related extremity injuries is unique; there is substantial soft tissue and bone loss. The immense majority of injuries encountered in war are often high in energy and velocity. Whether it is blasts, explosives, bullets, or rifles, there frequently is concomitant burns, crush, and shockwave trauma; the injuries are frequently multilevel. The wounds are often contaminated with dirt and shrapnels, raising the risk of infection. The injury is often more extensive than expected on initial examination. In the setting of microsurgical tissue transfer, the shockwave can cause diffuse intimal damage, making the microsurgical anastomosis more surgically demanding and consequently increasing the rate of both arterial and venous thrombosis. Because of the logistics of transfer to a tertiary care centre, patients often present in the subacute phase, deeming the reconstruction a more arduous one.
This incurs prolonged hospitalization and significantly increased treatment costs [3]. Frequently, open fractures are encountered with stripped periosteum, exposed tendons, nerves, and devitalized tissue [8]. Heavy bacterial contamination is also a concern [9]. The zone of injury is usually extensive, limiting local reconstructive options. With the rapid advancement of microsurgical practice, its role in ballistic trauma has become crucial. This reconstructive option has the advantage of delivering healthy tissue far from the zone of injury [10]. Microsurgery is a technically demanding surgery, requiring special equipment and trained personnel. Although few reports exist of performing free flap transfers in low-resource settings in rural medical care centers, it can rarely be performed in the acute setting where resources are restricted, and conditions are austere [11]. Technical limitations also exist, principally in the recipient vessels, where microdamage to the endothelium may prohibit a successful anastomosis.
Segmental bone loss in lower extremity war-related injury is a particular entity. Specifically in lower extremity injuries, bone loss is a risk factor for failure of limb salvage and associated with an increased risk of early complications [12, 13]. Data regarding its prevalence and management is lacking [14]. In the non-war setting, it is generally accepted that defects less than 6–8 cm in size are be treated with bone grafts and those larger than 6–8 cm require vascularized bone transfers; this is extrapolated to war-related injuries [15]. The free fibula flap was first described by Taylor in 1975 [16]. It is a robust flap for bony reconstruction. In the literature, there is a definite scarcity in the description of this flap for lower extremity reconstruction in war-related injuries.
Our experience at the American University of Beirut Medical Center is unique due to being a tertiary care and referral centre in the Middle East region. This has facilitated exposure to cases from the recent Islamic State of Iraq and Syria (ISIS)-related war. The purpose of this study is to highlight the importance and practicality of the free fibula flap in reconstruction for lower extremity composite defects. Both military and civilian injuries are included.
Patients and methods
After obtaining Institutional Review Board approval that conforms to the Helsinki Declaration, a retrospective review was conducted on all patients who underwent lower extremity reconstruction with a vascularized free fibula flap from January 2015 to March 2016 at the American University of Beirut Medical Center. Our aim was to identify patients whose injuries were associated with the ISIS-related war. Both civilian and military injuries were included. Inclusion criteria were patients injured in the ISIS war who have underwent free fibula reconstruction of the leg at any chronological presentation. Patients were excluded from the analysis if they had undergone pedicled fibula flap reconstruction.
Patients satisfying the inclusion criteria were identified, and relevant data was extracted from the electronic medical records. Patient data collected included demographics, age, sex, body mass index, medical comorbidities, smoking status, length of follow-up, timing of reconstruction, timing of presentation, length of the bone defect, method of bony fixation, and outcome. Outcome included complications, confirmation of bone union, and time to full weight bearing. Complications were divided into early and late complications. Early complications were defined to occur in the peri-operative period; this encompassed flap complications such as problems with the arterial or venous anastomosis, venous congestion, partial or total flap loss, and bleeding. Long-term complications categories included infection, bone non-union, and fractures.
Patients were followed up with plain radiographs at -one to two month intervals. The time of bony union was defined with complete and stable integration of the fibula at both ends. After confirmation of the bone union, full weight bearing was allowed. Full weight bearing was defined as walking without assistive devices.
Results
From January 2015 to March 2016, a total of fifteen patients underwent a vascularized free fibula flap for reconstruction of a lower extremity composite defect (Table 1). The mean age was 33 years (range from 12 to 62 years). The study population consisted of three females and 12 males, with an average body mass index of 24 (range from 20 to 34). Only two patients suffered from medical comorbidities. Mean follow-up time was 418.8 days (range 176–768 days). All patients were in stable condition at presentation.
The composite defect included either skin, muscle, or bone. All the defects were in the distal half of the leg. Wounds were either open or closed depending on the injury and on what previous interventions were done; chronic patients had closed wounds with only bony loss, while patients in the acute and subacute presentation had soft tissue loss requiring either flap or skin graft coverage in addition to the bone transfer. Eleven patients had open wounds with soft tissue loss at the time of reconstruction. Seven patients had undergone previous surgery for debridement of their wounds, and five patients had underwent surgery for split thickness skin grafting for their soft tissue defect. In total, 13 of 15 patients had underwent surgical procedures for their wounds before presentation. Reconstructed areas included only the tibia. Bone defects’ mean was 12.73 cm (range from 9 to 17 cm), all necessitating vascularized bone transfer.
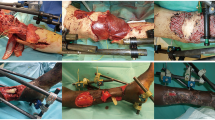
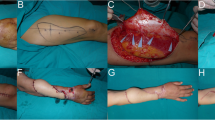
The harvested fibula flaps were either osteocutaneous (n = 7) or osteomyocutaneous (n = 8). Two patients were treated in the acute phase (≤ 2 weeks from injury) (Fig. 1), eight patients were treated in the subacute phase (between 2 and 6 weeks from injury) (Fig. 2), and five in the chronic phase (≥ 6 weeks from injury) (Fig. 3, Table 2).
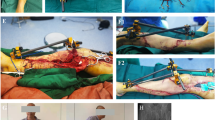
a Subacute presentation with actively infected wound. b Wound after coverage with osteomyocutaneous fibula flap and a residual soft tissue defect distally with propeller flap of the fibula skin paddle based on the distal perforator (P, proximal; D, distal). c Wound after rotating propeller flap. d, e Seventeen months post-operatively with full weight bearing on a fully healed hypertrophied fibular bone
All patients underwent between 0 and 3 surgical debridements before definite surgery. The time from presentation to our institution to definite surgery ranged from zero to 14 days. Nine patients had blast injuries as mechanism of injury while six patients had gunshot wounds.
The arterial anastomoses were done using 9.0 or 10.0 nylon sutures. The venous anastomoses were done using coupler in seven patients, and the rest were done with 9.0 or 10.0 nylon sutures. When possible, two vena comitantes were anastomosed (n = 12) (Table 3). All flaps were used as single-barrel flaps. Fixation of the fibula to the tibial stumps was done via either an intramedullary nail (n = 9) or plate and screws (n = 6) (Table 2).
Complications and functional outcomes
Only three out of the 15 patients suffered from complications (26.67%) (Table 4). The first patient required urgent exploration of the pedicle on the first post-operative day due to venous congestion of the flap. There was no evidence of venous thrombus at the anastomosis site. The congestion resolved and the patient did not require any further interventions.
The second patient required reoperation on the first post-operative day for coverage of residual bone defect using a fasciocutaneous propeller flap from the skin paddle of the flap (Fig. 2) (see video, supplemental digital content 1). We opted not to perform the propeller flap directly at the time of the primary surgery because the arterial anastomosis was difficult and had to be redone multiple times, making the viability of the flap uncertain. After viability was confirmed the next day, the propeller flap was performed. That same patient developed ankle ankylosis in the plantar flexion position, which required placement of an external fixator to correct this deformity. Consequently, the patient developed bone non-union and required one more re-operation for bone grafting. The patient was in full weight bearing ambulation at 17 months post-operatively.
The third patient fractured his fibula after falling off a tree one year after his surgery, four months after full weight bearing was allowed. The fracture was fixed by an external fixator.
None of the patients had partial or total flap loss. There were no cases of hardware exposure, wound dehiscence, infections, haematomas, or seromas. There were no takeback procedures with arterial or venous anastomosis revision. All the patients achieved full weight bearing. The mean time to full weight bearing was nine months.
Due to the small number of both patients and complications, no multivariate analysis could be carried out to link complications to any factors. The complications were divided amongst all factors such as mechanism of injury and time of presentation with no risk factors being inferred.
Discussion
The fibula and iliac crest are the most frequently used options for microsurgical bone reconstruction. The structural characteristics of the fibula make it a better option in long bone reconstruction. It is a straight cortical bone with a reliable vascular pedicle and a relative ease of dissection [15, 16]. Another principal advantage of the fibula is the ability to include skin and muscle [13, 17]. Consequently, all the segmental bone losses in our series were treated with a free fibula flap. Hence, larger bone defects with significant soft tissue loss including muscle, subcutaneous tissue, and skin require the utilization of a free fibula flap for treatment of the composite defect [13].
This procedure is not free of complications, and donor site morbidity is a serious factor to consider, especially in the combat-related injuries where the other remaining extremity is precious. Patients are at risk of deep peroneal nerve injury, adhesions at the flexor halluces longus, ankle instability, and delay in ambulation [18]. Though long-term morbidity is low, serious considerations and pre-operative planning are warranted to help limit injury to the remaining limb.
In this paper, we report a series of 15 cases of lower extremity reconstruction with a vascularized free fibula flap injured in the recent ISIS-related war. Excellent functional outcome is exemplified by a 100% flap survival and 93.33% bone union. War-related free fibula transfers to the lower extremity in the literature showed a non-union rate of 90.90%, with resorption of the bone in the rest of the cases [19]. This proves that the reliability and sturdiness of the vascularized fibular flap is not only limited to tumour resections but also has comparable result in blast injuries. Full weight bearing was achieved in our study by an average of nine months. This result is comparable or even better than the literature results where bony union after tumor resection with reconstruction using a free fibula vascularized flap occurred after 9.3 months and 1.7 years in two different studies [20, 21].
The mechanism of combat-related extremity injuries is unlike that of civilian trauma. Warfare injuries are usually heterogeneous in nature, combining blunt, penetrating, crush, and burn injuries. All these factors contribute to the challenges in dealing with these types of injuries. Irrespective of the injury, early stabilization and resuscitation are fundamental. Optimal management includes proper airway control, vascular access, and rapid recognition and treatment of haemorrhage to prevent mortality from exsanguinations [22].
All wounds should be urgently explored. These wounds are grossly contaminated, with presence of foreign bodies and devitalized tissue. The role of aggressive debridement cannot be stressed enough [23]. Serial debridements could be performed until the wound is clinically clean with signs of well-vascularized tissues [24]. On average, our patients underwent one aggressive surgical debridement prior to the definitive reconstruction.
In an ideal setting, these extremity injuries should be reconstructed in the acute setting, within the first 72 hours. Godina et al. recommended early wound coverage in civilian extremity trauma. They reported a mere 1% flap failure rate when the reconstruction was done early, as compared to 20% when the reconstruction was delayed. This was attributed to the lack of fibrosis around the vessels acutely. Other complications and infections were also lower when reconstruction was done early on [25]. It is advisable to take both deep soft tissue and bone cultures when performing internal fixation surgery at non-union sites [26].
Early reconstruction cannot be generalized to combat-related injuries. First, it is logistically arduous to perform such complicated microsurgical procedures in areas of conflict where resources are scarce. Reconstruction is often delayed until transport to a tertiary care centre is possible. In combat injuries, most reconstructions are performed in the subacute period. Kumar et al. report a reasonable 98% flap success rate with reasonably low rates of post-operative infections (8%) when reconstruction was done in the subacute period [27, 28]. In our series, although the bulk of the reconstructions were done in the subacute phase where the flap failure rates are high, we still managed a 100% flap survival.
Second, in ballistic injury, the zone of injury is difficult to define on presentation. Soft tissue injury is usually far more extensive than initially appreciated, and sufficient time must be given for it to delineate [15]. Whether blasts, explosives, or bullets, there frequently is a shockwave trauma. This shockwave causes extensive shearing and avulsion. It can cause diffuse intimal damage, making the microsurgical anastomosis more surgically demanding and consequently increasing the rate of arterial and venous thrombosis.
The pillar of reconstruction in combat injuries remains autologous tissue, whether free or pedicled. Microvascular free tissue transfer is generally a well-accepted reconstructive option. The major advantage that it offers when compared to pedicled flaps is that it provides healthy undamaged tissue, far from the zone of injury [29]. Each flap can also be tailored to the need of the recipient site, whether fasciocutaneous, musculocutaenous, or osteocutaneous. In this series, the choice of oseomyocutaneous flap over oseocutaneous flap was made based on the soft tissue defect. Including the hemi soleus or the flexor hallucis longus provided the advantage of obliterating the dead space. The muscle also provided a surface for application of skin grafts, allowing definite wound closure in the setting of skin loss.
There is a vast array of flaps that the reconstructive surgeon may choose from. Sabino et al. reported 395 flaps, both free and pedicled, for war-related extremity reconstruction. No difference in overall flap complication was reported. Difference was in the flap success rates with significantly higher failure rates reported in the muscle group when compared to the fasciocutaneous group [30].
There exist a significant number of reports and statistics about soft tissue coverage in combat-related extremity injuries. Management of large segmental bone defects remains a problematic and underreported issue, both in civilian and military settings. The most frequently encountered defects are those of the tibia; less commonly the femur, humerus, radius, and ulna are involved [31]. Bone loss stances a clinical challenge on reconstructive surgeons, especially when accompanied by concomitant vascular and complex soft tissue injuries. Most of these injuries are Gustillo type III B and C, predisposing patients to complications of infection, non-union, and amputations [32]. These extensive composite defects rarely can be reconstructed with local pedicled flaps. Vascularized bone and composite soft tissue transfers is necessary, especially when the bone defect is more than 6 cm [33]. The use of bone grafts is not a plausible option for defects more than 6 cm; bone grafts will inevitably be prone to resorption and necrosis decreasing their survival mass [34].
The Ilizarov bone lengthening method is a valid alternative option for bone defects. Its use could be very encouraging; however, it has a very long and cumbersome course. It also entails adequately trained personnel and experience to help decrease the overall complication rates reported at 30% [35]. The use of the Ilizarov method is further limited when there is significant soft tissue defect, as is the case with combat-related injuries. Microvascular bone transfer offers the advantage of a single-staged reconstruction of both bone and soft tissue defects, which would decrease the healing time, infection rates, and provide early structural stability [36].
The uniqueness of this paper stems from the fact that all injuries are war injuries from a single geographical region encountered in the same war, a unique cohort. However, these wounds vary from one injury to the other by the mechanism of injury, presentation to treatment, patient characteristics, and wound characteristics. The challenges in these combat wounds are a result of the multiplicity of war injuries, often in the same injury. Patients present at different stages of treatment with acute, subacute, and chronic wounds. The test for the surgeon’s abilities is in categorizing these wounds and catering the treatment to each while taking into consideration the singular patient factors and wound characteristics.
Limitations of the study
Our study has few limitations which may be an obstacle in the generalizability of the results. There is a deficit in the literature of free fibula transfers for the reconstruction of lower limb war injuries. Hence, our results could not be compared on all collected measures to other series in the literature. Additionally, the number of cases is limited due to the limited access by the injured patients in the war to the medical facility both logistically and financially. No association could be made between the complications and patient or wound characteristics due to the small sample number.
Conclusion
Combat-related extremity injuries are complex and technically demanding to treat. Adequate planning is essential for success in such challenging reconstructions. The single-barrel vascularized free fibula flap whether osteocutaneous or osteomyocutaenous is a valid and reliable tool for reconstruction composite lower extremity defects after blast injury. Its distinctive characteristics, high rate of bone union, acceptable healing time, and full weight bearing ambulation make it a sturdy option of reconstruction.
References
Holcomb JB, Stansbury LG, Champion HR, Wade C, Bellamy RF (2006) Understanding combat casualty care statistics. J Trauma 60(2):397–401. https://doi.org/10.1097/01.ta.0000203581.75241.f1
Dougherty AL, Mohrle CR, Galarneau MR, Woodruff SI, Dye JL, Quinn KH (2009) Battlefield extremity injuries in Operation Iraqi Freedom. Injury 40(7):772–777. https://doi.org/10.1016/j.injury.2009.02.014
Duramaz A, Bilgili MG, Bayram B, Ziroğlu N, Bayrak A, Avkan MC (2017) Orthopedic trauma surgery and hospital cost analysis in refugees; the effect of the Syrian civil War. Int Orthop 41(5):877–884. https://doi.org/10.1007/s00264-016-3378-x
Bosse MJ, Mackenzie EJ, Kellam JF et al (2002) An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. N Engl J Med 347(24):1924–1931. https://doi.org/10.1056/nejmoa012604
Casey K, Sabino J, Jessie E, Martin BD, Valerio I (2015) Flap coverage outcomes following vascular injury and repair. Plast Reconstr Surg 135(1):301–308. https://doi.org/10.1097/prs.0000000000000769
Hernigou P, Pariat J (2017) History of internal fixation (part 1): early developments with wires and plates before World War II. Int Orthop 41(6):1273–1283. https://doi.org/10.1007/s00264-016-3347-4
Hernigou P, Pariat J (2017) History of internal fixation with plates (part 2): new developments after World War II; compressing plates and locked plates. Int Orthop 41(7):1489–1500. https://doi.org/10.1007/s00264-016-3379-9
Owens BD, Kragh JF Jr, Macaitis J, Svoboda SJ, Wenke JC (2007) Characterization of extremity wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Orthop Trauma 21(4):254–257. https://doi.org/10.1097/BOT.0b013e31802f78fb
Murray CK, Hsu JR, Solomkin JS, Keeling JJ, Andersen RC, Ficke JR, Calhoun JH (2008) Prevention and management of infections associated with combat-related extremity injuries. J Trauma 64(Supplement). https://doi.org/10.1097/ta.0b013e318163cd14
Theodorakopoulou E, Mason KA, Pafitanis G, Ghanem AM, Myers S, Iwuagwu FC (2016) Free-tissue transfer for the reconstruction of war-related extremity injuries: a systematic review of current practice. Mil Med 181(1):27–34. https://doi.org/10.7205/milmed-d-15-00059
Tajsic NB, Husum H (2008) Reconstructive surgery including free flap transfers can be performed in low-resource settings: experiences from a wartime scenario. J Trauma 65(6):1463–1467. https://doi.org/10.1097/ta.0b013e318173f803
Grosset A, Pfister G, de l'Escalopier N, Plang S, Russo AP, Murison JC, Mathieu L, Rigal S. (2019) Risk factors and failures in the management of limb injuries in combat casualties. Int Orthop. https://doi.org/10.1007/s00264-019-04329-7
Fakhri RM, Herard P, Liswi MI, Boulart AL, Al Ani AMK (2019) Decision-making algorithm for sequential treatment of diaphyseal bone gaps in war-wounded patients in the Middle East. Int Orthop. https://doi.org/10.1007/s00264-019-04317-x
Pollak AN, Ficke JR, Injuries EW (2008) Extremity war injuries: challenges in definitive reconstruction. J Am Acad Orthop Surg 16(11):628–634. https://doi.org/10.5435/00124635-200811000-00003
Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ (1987) Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg 80(1):13–14. https://doi.org/10.1097/00006534-198707000-00002
Taylor GI, Miller GD, Ham FJ (1975) The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg 55(5):533–544. https://doi.org/10.1097/00006534-197505000-00002
Tu Y, Yen C, Yeh W, Wang I, Wang K, Ueng SW (2001) Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years follow-up. Acta Orthop 72(4):359–364. https://doi.org/10.1080/000164701753542014
Shpitzer T, Neligan P, Boyd B, Gullane P, Gur E, Freeman J (1997) Leg morbidity and function following fibular free flap harvest. Ann Plast Surg 38(5):460–464. https://doi.org/10.1097/00000637-199705000-00005
Maghari A, Forootan KS, Emami SA, Melow C (1992) Microvascular reconstruction of soft tissue and bone in war wounds. Scand J Plast Reconstr Surg Hand Surg 26(1):91–96. https://doi.org/10.3109/02844319209035189
Hariri A, Mascard E, Atlan F et al (2010) Free vascularised fibular graft for reconstruction of defects of the lower limb after resection of tumour. J Bone Joint Surg Br Vol 92-B(11):1574–1579. https://doi.org/10.1302/0301-620x.92b11.23832
Pollock R, Stalley P, Lee K, Pennington D (2005) Free vascularized fibula grafts in limb-salvage surgery. J Reconstr Microsurg 21(02):79–84. https://doi.org/10.1055/s-2005-864839
Fox CJ, Kreishman P (2010) High-energy trauma and damage control in the lower limb. Semin Plast Surg 24(1):5–10. https://doi.org/10.21236/ada554076
Mathieu L, Bertani A, Gaillard C, Chaudier P, Ollat D, Bauer B, Rigal S (2014) Combat-related upper extremity injuries: surgical management specificities on the theatres of operations. Chirurgie de la Main 33(3):174–182. https://doi.org/10.1016/j.main.2014.02.003
Kumar AR, Harshbarger R, Martin B (2010) Plastic surgery challenges in war wounded. Adv Wound Care 1:65–70. https://doi.org/10.1089/awc.2009.0087
Godina M (2006) Early microsurgical reconstruction of complex trauma of the extremities. Orthop Trauma Direct 4(5):29–35. https://doi.org/10.1055/s-2006-944324
Hérard P, Boillot F, Fakhri RM (2017) Bone cultures from war-wounded civilians in the Middle East: a surgical prospective. Int Orthop 41(7):1291–1294. https://doi.org/10.1007/s00264-016-3382-1
Kumar AR, Grewal NS, Chung TL, Bradley JP (2009) Lessons from the modern battlefield: successful upper extremity injury reconstruction in the subacute period. J Trauma 67(4):752–757. https://doi.org/10.1097/ta.0b013e3181808115
Kumar AR, Grewal NS, Chung TL, Bradley JP (2009) Lessons from Operation Iraqi Freedom: Successful Subacute Reconstruction of Complex Lower Extremity Battle Injuries. Plast Reconstr Surg 123(1):218–229. https://doi.org/10.1097/prs.0b013e3181904da9
Holmgaard R, Duffy J, Warburg FE, Jensen L, Bonde C (n.d.) Danish experience with free flaps in war wounds. Dan Med J 63(1):A5180
Sabino J, Polfer E, Tintle S et al (2015) A decade of conflict: flap coverage options and outcomes in traumatic war-related extremity reconstruction. Plast Reconstr Surg 135(3):895–902. https://doi.org/10.1097/prs.0000000000001025
Fleming ME, Watson TJ, Gaines RJ, O’Toole RV (2012) Evolution of orthopaedic reconstructive care. J Am Acad Orthop Surg 20. https://doi.org/10.5435/jaaos-20-08-s74
Gustilo RB, Mendoza RM, Williams DN (1984) Problems in the management of type III (severe) open fractures. J Trauma 24(8):742–746. https://doi.org/10.1097/00005373-198408000-00009
Lin C, Wei F, Chen H, Chuang DC (1999) Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg 104(4):984–992. https://doi.org/10.1097/00006534-199909020-00013
Cutting CB, Mccarthy JG (1983) Comparison of residual osseous mass between vascularized and nonvascularized onlay bone transfers. Plast Reconstr Surg 72(5):672–675. https://doi.org/10.1097/00006534-198311000-00016
García-Cimbrelo E, Olsen B, Ruiz-Yagüe M, Fernandez-Baíllo N, Munuera-Martínez L (1992) Ilizarov technique. Results and difficulties. Clin Orthop Relat Res 283:116–123
Yazar S, Lin C, Wei F (2004) One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg:1457–1466. https://doi.org/10.1097/01.prs.0000138811.88807.65
Author information
Authors and Affiliations
Contributions
All authors significantly contributed to the drafting and revision of the manuscript and approve its final form.
Corresponding author
Ethics declarations
Ethical approval
The research was approved by the Institutional Review Board of the American University of Beirut Medical Center.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
This paper adheres to the STROBE guidelines.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Video 1
Raising the propeller flap from the fibula flap skin paddle based on the distal perforator then checking the pulse with doppler. (MP4 68720 kb)
Rights and permissions
About this article
Cite this article
Karami, R.A., Ghieh, F.M., Chalhoub, R.S. et al. Reconstruction of composite leg defects post-war injury. International Orthopaedics (SICOT) 43, 2681–2690 (2019). https://doi.org/10.1007/s00264-019-04423-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-019-04423-w