Abstract
Purpose
Fracture healing encompasses a succession of dynamic multifactorial metabolic events, which ultimately re-establishes the integrity of the biomechanical properties of the bone. Up to 10% of the fractures occurring annually will need additional surgical procedures because of impaired healing. The aim of this article is to review the current literature regarding the use of bone marrow aspirate concentrate (BMAC) and its effectiveness in the management of bone defects.
Methods
We have included all published clinical literature investigating the development, techniques and applications of BMAC. Language, design and risk of bias did not deter the initial inclusion of any study. Our search was exclusively limited to studies involving human subjects. A PRISMA compliant search was carried out as published in 2009. This included the online databases: PubMed, EMBASE, clinical trial.gov and the Cochrane library from 1960 to the end of May 2015. MeSH terms used included: “Bone” AND “Marrow” AND “Aspirate” AND “Concentrate” AND “Bone Defects” AND “NONUNION”. Eligible studies were independently appraised by two authors using the Critical Appraisal Skills Program checklist. For the purpose of narrative review, relevant studies were included irrespective of methodology or level of evidence.
Results
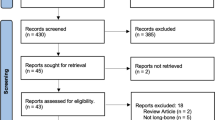
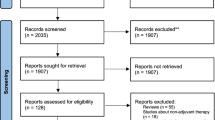
Thirty-four of the 103 (48 PubMed and 55 EMBASE) results yielded by the preliminary search were included. Exclusions included three duplicate records, six letters, 17 non-orthopaedics related studies and four records irrelevant to our search topic. The CASP appraisal confirmed a satisfactory standard of 31 studies. They all had clearly defined objectives, were well designed and conducted appropriately to meet them. The published studies reported the use of BMAC in non-union and fracture healing (15 studies), bone defects (nine studies), spine fusion (two studies), distraction osteogensis (two studies) and complications related to the use of BMAC (seven studies).
Conclusions
Stem cells found in BMAC have the potential to self-renew, undertake clonal expansion and differentiate into different musculoskeletal tissues. The commercial processing of BMAC needs to be optimized in order to achieve a consistent end product, which will provide predicable and translatable results. The future potential of cell characterization in order to determine the optimum cell for repair/regeneration of bone also needs to be explored.
Level of Evidence: Systematic Review of minimum level IV studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fracture healing encompasses a succession of dynamic multifactorial metabolic events, which ultimately re-establishes the integrity of the biomechanical properties of the bone. Einhorn et al. highlighted that up to 10% of the fractures occurring annually will need additional surgical procedures because of impaired healing [1,2,3]. Non-union is a significant orthopaedic problem, which is defined as the arrest of progression to union at a fracture site with persisting pain and mobility at the fracture site for a minimum period of six months from injury and no progression on three monthly serial x-rays [4]. Individualized surgical treatment for a fracture is warranted when the surgeon believes the fracture has little or no chance to heal. Non-unions occur in the main due to biological impairment, mechanical factors or both. The cause of a non-union can be attributed to the patient, pharmacological factors, injury and treatment related factors. Patient related factors include old age, poor compliance with rehabilitation, malnutrition, smoking, alcoholism, diabetes, peripheral neuropathy and the immunosuppression. A number of pharmacological agents have been associated with nonunion, these include steroids, cytotoxins, ciprofloxacin, NSAIDS and irradiation. Injury related factors include open fractures, significant soft tissue trauma, soft tissue interposition, infection, pathological fractures, excessive bone loss, segmental injury and comminution. Treatment related factors include excessive distraction of a fracture, inadequate stability with excessive movement and an extensive approach with vascular compromise [1,2,3, 5, 6].
The management of nonunion is a challenge to many orthopaedic surgeons and represents a significant clinical problem. The basic concept behind treatment is to provide both mechanical and biological support to the nonunion site. The aim is to restore mechanical stability with adequate strain [7] in a biologically sound milieu. The biological stimuli for the regeneration of bone involves the interaction of osteoinductive growth signals, stem cells that respond to these signals, an intact vascular bed and a scaffold that supports cellular proliferation and ingrowth [8]. The classic treatment of small defects (<3 cm) involves surgical stabilization and open autologous bone grafting with success rates of 50%–80% reported. However, this has been associated with donor-site morbidity and reduced healing potential in elderly patients [1,2,3, 5]. For larger defects (>3 cm), segmental transport represents the gold standard. However, this method is time consuming and associated with several complications, such as pin-track infections, pseudarthrosis, psychological problems, neurovascular complications and nonunion [5, 6].
There is interest in the development of alternate techniques using mechanical or biological methods, to provide the benefits of bone grafting with lower complication rates, morbidity and improved results [5]. The mechanical method includes the use of mechanical stimulation, electromagnetic fields and low-intensity ultrasound. The biological approach involves the use of osteoconductive biomaterials and osteoinductive factors to support and promote the ingrowth of newly formed bone. Osteoconductive materials include autologous bone graft, demineralized bone matrix (DBM), hydroxyapatite (HA) and tricalcium phosphate (TCP). Licenced osteoinductive factors are currently limited to bone morphogenic proteins, either naturally occurring within bone graft or from an exogenous source [2, 3, 6, 9].
Mesenchymal stem cells (MSC) are believed to have multipotent plasticity with the capability to differentiate along multiple cell lineages such as cartilage, bone, tendon, muscle and nerve [10,11,12]. Such multipotency has the potential to play a prominent therapeutic role in the repair and reconstruction of multiple tissues across a number of orthopaedic specialties [13]. Bone marrow is the most popular source of MSCs [14, 15] and historically many surgeons have utilized unprocessed bone marrow aspirate (BMA) [9, 16,17,18] in an attempt to stimulate healing. Currently, BMA is most commonly obtained from the iliac crest. There is no difference in the total number of cells obtained when harvesting from the anterior compared to the posterior pelvis, however posterior crest provides more connective tissue progenitors. Only a small percentage of mesenchymal stem cells (MSCs) can be obtained through aspiration of the marrow. Approximately 0.01% of the cells in BMA are MSCs, with the total number of viable cells obtained decreasing with age.
Bone marrow aspirate concentrate (BMAC) is an attempt to improve the recovery of the nucleated cells from marrow aspirate, while decreasing the recovery of non-nucleated cells such as RBCs. The exact mechanism of action of BMAC is currently not fully understood. Potentially the MSCs contained within BMAC will provide a direct cell source for repair of the host tissue. Alternatively or in addition to, the nucleated cells may have a paracrine effect by delivering various cytokines and growth factors into the ‘site to orchestrate and direct endogenous bone repair [19,20,21,22]. Through centrifugation the cell concentration can be increased 6–7 fold, the cellular content produces a number of growth factors, with Platelet derived growth factor (PDGF), transforming growth factor- β (TGF-β), and vascular endothelial growth factor (VEGF) likely to be the most important [23]. Fortier et al., compared the constituents in BMA and BMAC. Table 1 demonstrates that there are reduced platelets and raised white blood cells (WBC) in BMAC demonstrating that this is a very different substance to platelet rich plasma (PRP) with a likely different mechanism of action [24].
The aim of this article is to review the current literature regarding the use of bone marrow aspirate concentrate, its effectiveness and potential complications when used to manage bone defects.
Methods
Eligibility
We have included all published clinical literature investigating the development, techniques and applications of bone marrow aspirate concentrate. Language, design and risk of bias did not deter the initial inclusion of any study. Our search was exclusively limited to studies involving human subjects published in English.
Search strategy
A PRISMA compliant search was carried out as published in 2009 [25]. This included the online databases: PubMed, EMBASE, clinical trial.gov and the Cochrane library from 1960 to the end of May 2015. MeSH terms used included: “bone” AND “marrow” AND “Aspirate” AND “concentrate” AND “bone defects” AND “non-union”.
Study identification
The title and abstract from each study within the results list was reviewed independently by three authors (XX, XX and XX). Any disagreement was resolved by discussion with the senior author. Full text papers of relevant studies were subsequently obtained and reviewed against the eligibility criteria. Then, full texts of the eligible studies were further evaluated and references were checked for more suitable studies.
Critical appraisal
Two authors using the Critical Appraisal Skills Program checklist independently appraised eligible studies. For the purpose of narrative review, relevant studies were included irrespective of methodology or level of evidence.
Results
Of the results yielded by the preliminary search 103 (48 PubMed and 55 EMBASE) were included. Exclusions included three duplicate records, six letters, 17 non-orthopaedics related studies and four records irrelevant to our search topic.
On searching www.ClinicalTrials.gov, we found that five trials were registered. All trials were examining the use of stem cells in bone healing and management of defects (Table 2).
The CASP appraisal [26] confirmed a satisfactory standard of 35 studies (Fig. 1). They all had clearly defined objectives, were well designed and conducted appropriately to meet them.
The published studies reported the use of BMAC in non-union and fracture healing (15 studies), bone defects (nine studies), spine fusion (two studies), distraction osteogensis (two studies) and complications related to the use of BMAC (seven studies).
Non-union and delayed union
Four studies have looked at the efficiency of a percutaneous injection of BMA for the management of nonunion [27,28,29,30]. In these studies a total of 301 fractures were managed by BMA, 268 (89%) demonstrated union with an average healing time of 2.5 to eight months. No study documented any adverse systemic effects.
Hernigou et al. [31] evaluated the outcome of injecting 20 cm3 of BMAC obtained from the iliac crest for the management of atrophic non-union of the tibia in 60 patients. The outcomes included the volume of the callus formed and rate of clinical union. Bone union was achieved in 53/60 (88%) patients at four months following the procedure. In the seven non-united tibias, the concentration (p = 0.001) and the total number (p < 0.01) of progenitors cells injected were significantly lower than in those that united. They reported a positive association between the quantity of hard callus and the number (p = 0.04) and concentration (p = 0.01) of fibroblast colony-forming (FCF) units in the graft. Likewise, they reported the time interval needed to achieve union was negatively correlated with the FCF units’ concentration at the site of the graft (p = 0.04). They concluded that BMAC was a one-step procedure, which may prevent complications related to in vitro cultivation, for example pre-ageing, reduced viability or de-differentiate.
The use of either DBM or recombinant human bone morphogenic protein-2 (rhBMP-2) in combination with BMAC injection in the management of nonunions in long bones has been assessed [32]. This surgical procedure is called the modified Hernigou technique. Desai et al. [32] in a total of 49 patients with nonunion in tibia compared BMAC injection with DBM and/or rhBMP-2. They assessed radiologic healing of the bone gaps which were either less or more than 5 mm. They reported no significant difference in the healing rate (p = 0.81) between patients with defects less than and greater than 5 mm. This study demonstrated that the application of BMAC in combination with either DBM and/or rhBMP-2 is an effective treatment for delayed or nonunion regardless of the fracture gap size or fracture site. The use of BMAC and rhBMP-2 was linked with lower healing rates in comparison to the use of BMAC and DBM (p = 0.036). Patients who had earlier intervention, had higher union rates (p = 0.04).
Kassem [17] assessed the outcomes of percutaneous injection of BMAC in the management of fractures presenting with delayed union or non union after open reduction and internal fixation (ORIF) in 20 patients. The BMAC injection was undertaken at an average of 9.65 (4 to 24) months after ORIF. BMAC was injected percutaneously into the fracture site under fluoroscopy control. They reported an achieved clinical and radiological union rate in 95% of the cases after a mean of 2.95 months.
Hernigou et al. [33] reported the use of an injection of BMAC at the site of non-unions in 86 ankles in diabetic patients. The outcomes were compared to 86 diabetic matched non-unions treated with bone graft harvested from the iliac crest. They found that the application of BMAC resulted in healing in 82.1% of the ankles compared to only 62.3% in the control group. Major complications were observed in the control group, these included amputations (5.8%), AVN (12.7%) and infection (20%). Fewer complications were recorded in the BMAC group. They concluded that the injection of BMAC might be desirable in view of the increased risks of major complications associated with open surgery and iliac bone grafting in this population. Moreover, percutaneous BMAC application is associated with improved healing rates compared with standard iliac bone autograft treatment.
The combination of BMAC and PRP has also been reported to be effective in the management of nonunion [31, 34,35,36]. It is unclear whether BMAC or PRP or both generate this outcome since they were not evaluated independently. However, it would appear that BMAC in isolation is sufficient to promote bone healing in the view of the aforementioned studies [17, 33, 37].
Bone defects and distraction osteogensis
Petri et al. [38] assessed five patients with segmental defects ranging from 3 to 14 cm managed with BMAC on a bovine scaffold. The healing process was evaluated by positron emission tomography–computed tomography (PET-CT) at three months post surgery. PET analysis showed an increased influx of fluoride by a factor of 8.3 ± 6.4 compared with the contralateral side (p < 0.01). Bone density in the cortical area was 75 ± 16% of the contralateral side (p < 0.03). They concluded that BMAC combined with a bovine scaffold could be an alternative option to segmental bone transport in management of large bone defects. However, this statement needs to be supported by further studies to prospectively compare this procedure to autologous bone grafting and segmental transport.
Sauerbier et al. [39] and Rickert et al. [40] carried out two randomized controlled clinical trials to examine the establishment of new bone in patients with severe atrophy of the maxillary sinus. Patients obtained an augmentation of the sinus using bovine bone either with BMAC or autologous bone. Sauerbier et al. [39] studied 45 severely atrophied maxillary sinus in 26 patients in a partial cross-over design. Thirty-four sinuses in 25 patients were augmented with bovine bone mineral (BBM) and BMAC. Eleven control sinuses in 11 patients were augmented with a mixture of 70% BBM and 30% autogenous bone (AB). Biopsies were obtained after 12 to 16 weeks. The authors found comparable new bone formation in both groups three to four months after surgery, which was similar to the results reported by Rickert et al. [40].
Distraction osteogenesis for segmental bone defect reconstruction involves long duration of treatment with external fixation, which can cause considerable morbidity and high complication rates. Augmentation with percutaneously applied adjuvants to reduce consolidation time has been designated as one of the major goals for future research in this field.
Kitoh et al. [41] reported the clinical outcomes of distraction osteogenesis with BMAC and PRP in three femurs and two tibias in three patients. BMAC and autologous PRP was applied into the distracted callus. The target lengths were obtained in 100% of bones without major complications with a mean healing index of 23.0 days/cm (18.8–26.9 days/cm). Although these results were preliminary, it was concluded that the use of BMAC combined with PRP was shown to be a safe and minimally invasive therapy, which reduces the treatment period by hastening bone regeneration during distraction osteogenesis.
Lee et al. [19] demonstrated in a randomized trial superior bone healing when using BMAC during distraction osteogenesis of the tibia. Twenty patients (40 tibias) undergoing bilateral tibial lengthening were enrolled in this study. They compared patients receiving an osteotomy site injection of BMAC and PRP (treatment group) with those not receiving such an injection (control group). Twenty segments (10 patients) were included in the treatment group and 20 segments (10 patients) received no injection with a reported minimum follow-up of 24 (24–34) months. All patients undertook lengthening for familial short stature, utilizing the lengthening over an IM nail. They reported similar average distraction rates between the two groups. The mean distraction rate was 0.75 mm/day in the treatment group compared to 0.72 mm/day in the control group (p = 0.24). The mean cortical healing indexes were significantly higher in the treatment group when compared to the control group (p < 0.001), presenting quicker healing in the treatment group at each cortex. Although the callus profile and type were not different between the two groups, full weight bearing was allowed earlier in the treatment group than in the control group (index: 0.99 months/cm and 1.38 months/cm, respectively, p < 0.001).
Jager et al. reported the clinical outcomes and radiological evaluation of ten patients with bone defects treated with BMAC. Additionally, they reported the in vitro data of BMAC cultivated onto a collagen scaffold. They demonstrated that there is a rationale for a clinical application of BMAC in the treatment of osseous defects. The intra-operative harvest procedure is a safe method and does not significantly prolong the time of surgery. In addition, MSC’s isolated from the aspirate was able to adhere and proliferate onto a collagen scaffold in significant numbers after a 15 minute incubation period. These cells were then able to undergo osteogenic differentiation in vitro without any osteogenic stimuli [36]. Similar satisfactory results were reported by Hendrich et al. [42] who evaluated new bone formation after the application of BMAC in 20 patients presenting with osseous defects. They reported no adverse events in those patients.
Although Kitoh et al. [41] and Lee et al. [19] established that the combination of BMAC and PRP enriched the healing of bone defects; it is also unclear in this scenario whether BMAC or PRP or both generate this outcome since they were not evaluated independently.
Other applications
The use of BMAC has been described in the treatment of simple or unicameral bone cysts (UBC). Different treatment options have been suggested but there is no consensus regarding the best procedure [43]. Di Bella et al. [44] in a level III therapeutic study compared the healing rates and failures of multiple injections of corticosteroid versus a single injection of DBM in association with BMAC in UBC with a minimum 12 months follow-up. They retrospectively reviewed 184 patients who had received either of these two methods. They observed a healing rate of 21% in the steroid group compared with a healing rate of 58% in the BMAC group. The rate of failure observed in the steroids group was 63% compared with 24% in the BMAC group. There were no differences observed in fracture rates between both groups.
Gan et al. [45] utilized BMAC with porous beta-tricalcium phosphate (beta-TCP) to augment spinal fusion in 41 subjects. A hard fusion was attained in more than 95% of patients studied. BMAC was combined with porous beta-TCP granules using negative pressure followed by a short-time incubation. In less than three years, 95% of the cohort of patients had satisfactory spinal fusion results. Of patients 4/41 (9.7%) had exudation or moderate swelling in their wounds, and all of them were treated successfully with conservative management.
Potential risks of BMAC therapy
Although dangers are acknowledged and anticipated, bone marrow aspiration is considered to be a safe procedure, however, adverse events have been reported [46,47,48,49]. These can be categorized into risks associated with the harvest and those associated with the administration of BMAC.
Harvest
Hernigou et al. [50] has defined the sector rule for aspiration of marrow from the iliac crest, which is based on safety zones. They divided the iliac crest into six equal sectors from anterior to posterior direction. The authors studied 480 trocar entry points undertaken by six surgeons in 120 patients. They demonstrated that the sector system consistently envisaged safe and unsafe zones for placing the trocar in the iliac crest. They observed increased risk of breaches in obese patients and this risk is decreased in more experienced surgeons. Ninety-four breaches out of 480 entry points occurred with increased risks observed in the thinner sectors in the iliac crest. Additionally, there is increased risk of injuring the external iliac artery in the four most anterior sectors (1 to 4) especially in females. On the other hand, posterior sectors were associated with increased risk of sciatic nerve and gluteal vessel injury when the trocar was inserted more than 6 cm into the posterior iliac crest. They concluded that the sector rule is a reliable system to use for BMA aspiration.
In 2001, Bain surveyed 19,259 procedures from 63 hospitals over three consecutive years in the largest study looking at potential risks of the harvesting procedure. Only 11 (0.0005%) of almost 20,000 patients experienced significant haemorrhage. Recent anticoagulation therapy appears to be the most significant risk factor associated with haemorrhage. Infection has been reported only in two subjects, both were superficial infections which were successfully treated with antibiotics [46]. Whilst dependent on the site of harvest, chronic pain can be a potential concern. The pain might not spontaneously resolve, and may require treatment using neuropathic pain medications [47, 49]. Pathological fractures are a potential concern especially in the presence of osteoporosis or osteomalacia [46,47,48,49]. Finally, a drastic adverse event of death has been reported in one patient in 2001, as a result of the formation of a retroperitoneal hematoma after an aspirate from the posterior iliac crest [46].
Administration
Infection is a potential concern at the administration site, although antibiotic prophylaxis is, standard practice and overall the general risk is low, particularly in percutaneous administration. When used intra-osseously, due to bone being permeable to liquefied material, fat embolisation is a potential risk [51]. Animal studies have shown fat globules in dogs’ lungs post mortem, however in human trials, adverse clinical outcomes in the form of respiratory complications or decreased oxygen saturation have not been reported [52]. Those subjects at a greater risk of embolization, such as those with cardiac shunts should be considered as to their suitability to receive intra-osseous BMAC. In all cases of intra osseous administration, patients should be monitored for the clinical signs of fat embolism [20].
Conclusions
In conclusion, MSCs in BMAC have the potential to self-renew, undertake clonal expansion and differentiate into different musculoskeletal tissues. These include osteoblasts, chondrocytes, fibroblasts and adipocytes. MSCs are also known to regulate the immune system and have a potential positive paracrine effect. BMAC has been mostly used to encourage bone formation and treat AVN of the femoral head, with encouraging results.
Further work is needed to determine whether one preparations of BMAC performs better than others with regard to bone formation, as each system will produce an end product that will vary in cell concentration, platelet number and haematocrit.
Along side well designed clinical trials, further basic science work is required to investigate the therapeutic action of BMAC. The commercial processing of BMAC needs to be optimized in order to achieve a consistent end product, which will provide predicable and translatable results. The future potential of cell characterization in order to determine the optimum cell for repair/regeneration of bone also needs to be explored.
References
Einhorn TA (1995) Enhancement of fracture-healing. J Bone Joint Surg Am 77(6):940–956
Einhorn TA (1996) Enhancement of fracture healing. Instr Course Lect 45:401–416
Einhorn TA (1998) The cell and molecular biology of fracture healing. Clin Orthop Relat Res 355(Suppl):S7–21
Health Quality Ontario (2005) Osteogenic protein-1 for long bone nonunion: an evidence-based analysis. Ont Health Technol Assess Ser 5(6):1–57
Emara KM, Diab RA, Emara AK (2015) Recent biological trends in management of fracture non-union. World J Orthop 6(8):623–628. doi:10.5312/wjo.v6.i8.623
Hannouche D, Petite H, Sedel L (2001) Current trends in the enhancement of fracture healing. J Bone Joint Surg Br 83(2):157–164
Perren SM (2014) Fracture healing: fracture healing understood as the result of a fascinating cascade of physical and biological interactions. Part I. An attempt to integrate observations from 30 years AO research. Acta Chir Orthop Traumatol Cechoslov 81(6):355–364
Carofino BC, Lieberman JR (2008) Gene therapy applications for fracture-healing. J Bone Joint Surg Am 90(Suppl 1):99–110. doi:10.2106/JBJS.G.01546
Stevenson S (1998) Enhancement of fracture healing with autogenous and allogeneic bone grafts. Clin Orthop Relat Res 355(Suppl):S239–S246
Baksh D, Song L, Tuan RS (2004) Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 8(3):301–316
Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA (2009) Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med 37(11):2126–2133. doi:10.1177/0363546509339582
Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH (2010) Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med 38(6):1110–1116. doi:10.1177/0363546509359067
Imam MA, Mahmoud SSS, Holton J, Abouelmaati D, Elsherbini Y, Snow M (2017) A systematic review of the concept and clinical applications of bone marrow aspirate concentrate in orthopaedics. SICOT-J 3:17
Xie A, Nie L, Shen G, Cui Z, Xu P, Ge H, Tan Q (2014) The application of autologous plateletrich plasma gel in cartilage regeneration. Mol Med Rep 10(3):1642–1648. doi:10.3892/mmr.2014.2358
Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL (1998) Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 176(1):57–66. doi:10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7
Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H (2005) The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br Vol 87(7):896–902. doi:10.1302/0301-620X.87B7.16289
Kassem MS (2013) Percutaneous autogenous bone marrow injection for delayed union or non union of fractures after internal fixation. Acta Orthop Belg 79(6):711–717
Nathan ST, Fisher BE, Roberts CS, Giannoudis PV (2011) The management of nonunion and delayed union of patella fractures: a systematic review of the literature. Int Orthop 35(6):791–795. doi:10.1007/s00264-010-1105-6
Lee DH, Ryu KJ, Kim JW, Kang KC, Choi YR (2014) Bone marrow aspirate concentrate and platelet-rich plasma enhanced bone healing in distraction osteogenesis of the tibia. Clin Orthop Relat Res 472(12):3789–3797. doi:10.1007/s11999-014-3548-3
Jager M, Jelinek EM, Wess KM, Scharfstadt A, Jacobson M, Kevy SV, Krauspe R (2009) Bone marrow concentrate: a novel strategy for bone defect treatment. Curr Stem Cell Res Ther 4(1):34–43
Muschler GF, Nitto H, Matsukura Y, Boehm C, Valdevit A, Kambic H, Davros W, Powell K, Easley K (2003) Spine fusion using cell matrix composites enriched in bone marrow-derived cells. Clin Orthop Relat Res 407:102–118
McCarrel T, Fortier L (2009) Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res 27(8):1033–1042. doi:10.1002/jor.20853
Wilkins RM, Kelly CM (2003) The effect of allomatrix injectable putty on the outcome of long bone applications. Orthopedics 26(5 Suppl):s567–s570
Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, Stokol T, Cheetham J, Nixon AJ (2010) Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am 92(10):1927–1937. doi:10.2106/JBJS.I.01284
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012. doi:10.1016/j.jclinepi.2009.06.005
Hyde M, Higgs P, Wiggins RD, Blane D (2015) A decade of research using the CASP scale: key findings and future directions. Aging Ment Health 19(7):571–575. doi:10.1080/13607863.2015.1018868
Goel A, Sangwan SS, Siwach RC, Ali AM (2005) Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury 36(1):203–206. doi:10.1016/j.injury.2004.01.009
Siwach RC, Sangwan SS, Singh R, Goel A (2001) Role of percutaneous bone marrow grafting in delayed unions, non-unions and poor regenerates. Indian J Med Sci 55(6):326–336
Matsuda Y, Sakayama K, Okumura H, Kawatani Y, Mashima N, Shibata T (1998) Percutaneous autologous bone marrow transplantation for nonunion of the femur. Nihon Geka Hokan 67(1):10–17
Garg NK, Gaur S, Sharma S (1993) Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop Scand 64(6):671–672
Hernigou P, Mathieu G, Poignard A, Manicom O, Beaujean F, Rouard H (2006) Percutaneous autologous bone-marrow grafting for nonunions. Surgical technique. J Bone Joint Surg Am 88(Suppl 1 Pt 2):322–327. doi:10.2106/JBJS.F.00203
Desai P, Hasan SM, Zambrana L, Hegde V, Saleh A, Cohn MR, Lane JM (2015) Bone mesenchymal stem cells with growth factors successfully treat nonunions and delayed unions. HSS J 11(2):104–111. doi:10.1007/s11420-015-9432-1
Hernigou P, Guissou I, Homma Y, Poignard A, Chevallier N, Rouard H, Flouzat Lachaniette CH (2015) Percutaneous injection of bone marrow mesenchymal stem cells for ankle non-unions decreases complications in patients with diabetes. Int Orthop. doi:10.1007/s00264-015-2738-2
Akcay S, Kazimoglu C (2014) Bone marrow aspirate concentrate and platelet-rich plasma enhanced bone healing in distraction osteogenesis of the tibia. Clin Orthop Relat Res 472(7):2301–2302. doi:10.1007/s11999-014-3637-3
Hernigou P, Beaujean F (2002) Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res 405:14–23
Jager M, Herten M, Fochtmann U, Fischer J, Hernigou P, Zilkens C, Hendrich C, Krauspe R (2011) Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res 29(2):173–180. doi:10.1002/jor.21230
Hernigou P, Poignard A, Beaujean F, Rouard H (2005) Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87(7):1430–1437. doi:10.2106/JBJS.D.02215
Petri M, Namazian A, Wilke F, Ettinger M, Stubig T, Brand S, Bengel F, Krettek C, Berding G, Jagodzinski M (2013) Repair of segmental long-bone defects by stem cell concentrate augmented scaffolds: a clinical and positron emission tomography--computed tomography analysis. Int Orthop 37(11):2231–2237. doi:10.1007/s00264-013-2087-y
Sauerbier S, Rickert D, Gutwald R, Nagursky H, Oshima T, Xavier SP, Christmann J, Kurz P, Menne D, Vissink A, Raghoebar G, Schmelzeisen R, Wagner W, Koch FP (2011) Bone marrow concentrate and bovine bone mineral for sinus floor augmentation: a controlled, randomized, single-blinded clinical and histological trial--per-protocol analysis. Tissue Eng A 17(17–18):2187–2197. doi:10.1089/ten.TEA.2010.0516
Rickert D, Sauerbier S, Nagursky H, Menne D, Vissink A, Raghoebar GM (2011) Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res 22(3):251–258. doi:10.1111/j.1600-0501.2010.01981.x
Kitoh H, Kitakoji T, Tsuchiya H, Mitsuyama H, Nakamura H, Katoh M, Ishiguro N (2004) Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis--a preliminary result of three cases. Bone 35(4):892–898. doi:10.1016/j.bone.2004.06.013
Hendrich C, Franz E, Waertel G, Krebs R, Jager M (2009) Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthop Rev 1(2):e32. doi:10.4081/or.2009.e32
Donaldson S, Chundamala J, Yandow S, Wright JG (2010) Treatment for unicameral bone cysts in long bones: an evidence based review. Orthop Rev 2(1):e13. doi:10.4081/or.2010.e13
Di Bella C, Dozza B, Frisoni T, Cevolani L, Donati D (2010) Injection of demineralized bone matrix with bone marrow concentrate improves healing in unicameral bone cyst. Clin Orthop Relat Res 468(11):3047–3055. doi:10.1007/s11999-010-1430-5
Gan Y, Dai K, Zhang P, Tang T, Zhu Z, Lu J (2008) The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials 29(29):3973–3982. doi:10.1016/j.biomaterials.2008.06.026
Bain BJ (2005) Bone marrow biopsy morbidity: review of 2003. J Clin Pathol 58(4):406–408. doi:10.1136/jcp.2004.022178
Burkle CM, Harrison BA, Koenig LF, Decker PA, Warner DO, Gastineau DA (2004) Morbidity and mortality of deep sedation in outpatient bone marrow biopsy. Am J Hematol 77(3):250–256. doi:10.1002/ajh.20185
Bain BJ (2004) Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol 26(5):315–318. doi:10.1111/j.1365-2257.2004.00630.x
Bain BJ (2003) Bone marrow biopsy morbidity and mortality. Br J Haematol 121(6):949–951
Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P (2014) Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop 38(11):2377–2384. doi:10.1007/s00264-014-2343-9
Husebye EE, Lyberg T, Roise O (2006) Bone marrow fat in the circulation: clinical entities and pathophysiological mechanisms. Injury 37(Suppl 4):S8–18. doi:10.1016/j.injury.2006.08.036
Orlowski JP, Julius CJ, Petras RE, Porembka DT, Gallagher JM (1989) The safety of intraosseous infusions: risks of fat and bone marrow emboli to the lungs. Ann Emerg Med 18(10):1062–1067
Acknowledgements
We acknowledge the support of library staff of Royal Orthopaedic NHS Foundation Trust, UK.
Funding
Self-funded.
Author information
Authors and Affiliations
Contributions
All authors conceptualized the study and participated in study screening, selection, data extraction and manuscript preparation. All authors provided intellectual content and approved the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
NOne.
Additional information
The paper has not been submitted to any other journal.
Rights and permissions
About this article
Cite this article
Imam, M.A., Holton, J., Ernstbrunner, L. et al. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. International Orthopaedics (SICOT) 41, 2213–2220 (2017). https://doi.org/10.1007/s00264-017-3597-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3597-9