Abstract
Purpose
Cases of fracture-fixation device infection involving Staphylococcus lugdunensis are not frequent. The clinical characteristics and the choice of treatment strategies of these infections are not obviously known to date.
Methods
We performed a review of fracture-fixation device infection involving S. lugdunensis managed by our centres.
Results
Among the 38 cases of fracture-fixation device infection involving S. lugdunensis, 53% were located in the tibia. Most of our cases (87%) were chronic infections. Purulent discharge, which occurred in 79% of cases, was the most frequent clinical symptom, followed by pain in 63%, local inflammation in 55%, and fever in 37%. Bacteremia and severe sepsis occurred in 10% and 18% of cases, respectively. Four cases (10%) were treated exclusively with antimicrobial treatment alone. Thirty-four cases (89%) were treated with a combination of surgery with antimicrobial therapy including surgical debridement, antibiotics and osteosynthesis device retention in six cases (16%), and osteosynthesis device removal in 27 cases (71%). The mean length of antibiotic treatment was 119 days. The relapse rate was high that was not related to selection of resistant strains. Polymicrobial infection had no impact on clinical outcome. A combination of surgery with antimicrobial therapy was identified as a significant prognostic factor associated with remission (p = 0.042).
Conclusions
S. lugdunensis is probably involved in more infections than has been reported. Using appropriate microbiological methods laboratories should routinely identify the species of all coagulase-negative Staphylococci isolates involved in fracture-fixation device infection to better achieve the treatment strategies of fracture-fixation device infection involving S. lugdunensis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unlike other coagulase-negative staphylococci that is usually involved in a subacute or chronic infection, Staphylococcus lugdunensis is an aggressive pathogen that shares major virulence factors with S. aureus [1]. Cases of S. lugdunensis infections are often under reported due to its lack of detection in clinical laboratories using conventional phenotypic identification including biochemical assays like the Api-Staph test (Biomerieux, Marcy l’Etoile, France) and automated phenotypic identification systems including MicroScan (Dade Behring, West Sacramento, CA, USA), Vitek 2 (BioMérieux, Marcy l’Etoile, France), and Crystal GP (Becton Dickinson, Sparks, MD, USA) [2]. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry and molecular techniques have improved the identification of S. lugdunensis strains [2].
S. lugdunensis is a virulent coagulase-negative staphylococcus that emerges in several human infections such as infectious endocarditis, vascular infections and blood stream infections [3]. S. lugdunensis has been reported as a pathogen able to form biofilms that seems to play a determinant role in orthopaedic device infection particularly in prosthetic joint infection [2, 4, 5]. Only a few reports of osteoarticular infection caused by S. lugdunensis have been published [6–16]. However, we believe that the organisms may still be under-recognized as a pathogen in osteoarticular infections. In this study, we review the clinical features and outcomes of the cases of infection associated with fracture-fixation devices caused by S. lugdunensis treated by our referral centre for the treatment of bone and joint infection and in collaborating centres in southern France.
Material and methods
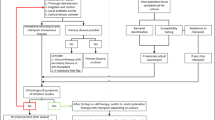
All the cases of infection associated with fracture-fixation devices caused by S. lugdunensis were managed in our centre and in collaborating centres between January 2002 and December 2014, and were diagnosed based on medical history with clinical evidence of infection using biological and/or radiological compliant data, with positive culture of S. lugdunensis identified from ≥2 surgical biopsies. The cases of osteomyelitis without orthopaedic devices and prosthetic joint infection involved S. lugdunensis were not included in this study. Infections involving fracture-fixation devices were classified according to the time of onset after implantation: early infection when infection is diagnosed within <3 weeks after implantation of the orthopaedic device, delayed infections when infection develops between three and ten weeks, and late infections occur more than ten weeks after implantation. [17, 18].
Surgical biopsies obtained from all patients, i.e., joint fluid, bone biopsies or tissue samples around fracture-fixation devices were crushed in Eppendorf (Hamburg, Germany) tubes and inoculated on 5% sheep-blood, chocolate, Mueller-Hinton, trypticase soy, and MacConkey agar plates (BioMérieux, France) and incubated at 37 °C in a 5% CO2 atmosphere and in an anaerobic atmosphere for ten days. Pure bacterial cultures, obtained by picking isolated colonies, were identified with conventional phenotypic identification such as Gram staining (Aerospray Wiescor; Elitech), catalase and oxidase activity tests, automated phenotypic identification systems included the Vitek 2 system (BioMérieux, Marcy l’Etoile, France), MALDI-TOF mass spectrometry or molecular methods, as previously described [19]. The antimicrobial susceptibilities of S. lugdunensis isolates to fosfomycin, fusidic acid, clindamycin, benzylpenicillin, rifampicin, quinolones, cotrimoxazole, doxycycline, aminoglycoside, vancomycin, and teicoplanin were determined and interpreted according to the recommendations of the French Society for Microbiology and the European Committee on Antimicrobial Susceptibility Testing (CA-SFM/EUCAST) available at http://www.sfm-microbiologie.org. Susceptibility to methicillin was screened by agar diffusion using cefoxitin disks (BioRad, Marnes-La-Coquette, France). All of our cases were treated by standard treatment protocol (Supplementary Table S1).
We evaluated treatment success as the remission defined by the absence of infection signs evaluated at least 12 months after the end of the antimicrobial treatment. We used the last follow-up time as the endpoint. Relapse was defined by the occurrence of pain and swelling of the bone or joint, wound drainage, implant site erythema, induration or oedema, joint pain, joint effusion, fever, purulent discharge from the wound, sinus tract drainage and persistent positive culture from deep samples based on surgical procedures after the end of treatment during follow-up examinations at the clinic. Time to relapse was calculated from the date of the end of curative treatment to relapse.
We performed a descriptive analysis of our population using the IBM® SPSS® Statistics software v.20.0. Then, we analyzed the clinical outcome and prognostic factors such as co-morbidities, location of fractures-fixation devices, clinical signs, C-reactive protein levels, polymicrobial infection, and infection cases by S. lugdunensis resistant to antimicrobials following medico-surgical treatment strategies including antimicrobial treatment without surgery, surgical debridement, antibiotics and osteosynthesis device retention, and antibiotic with osteosynthesis device removal. First, we analyzed factors for relapse versus non-relapse cases using univariate analysis to identify prognostic variables strongly associated with remission or relapse risk (p < 0.2), then multivariate analysis was performed to assess the predictions, after adjusting for significant variables in the univariate analysis and/or risk factors. A p-value less than 0.05 was considered statistically significant.
Results
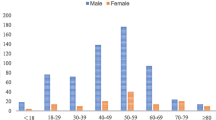
Thirty-eight cases of S. lugdunensis infection associated with fracture-fixation devices treated by our centre. The mean age was 49 years ±16 years (range 28–85 years), yielding a male/female sex ratio of 3.22. Seventy-six percent of our cases had comorbidities and risk factors including diabetes mellitus and a history of open fracture, which were identified in six cases (16%) and 15 cases (39%), respectively (Table 1). The mean of the Charlson comorbidity index [20] (a combined age-comorbidity score used to estimate relative risk of death from prognostic clinical covariates) was 0.66 ± 2.
We identified 20 cases (53%) located in the tibia. Another 18 cases were located in the ankle (eight cases), knee (four cases), femur (two cases), foot (two cases), and vertebra (two cases). The mean time delay between orthopaedic device implantation and infection onset was 311 days ±285 days (range 5–2237 days). Most of our cases (87%) were delayed and late infections occurring after three weeks of implantation. Only five cases (13%) were early infections that occurred within three weeks of implantation.
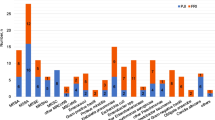
Purulent discharge, which occurred in 30 cases (79%), was the most frequent clinical symptom, followed by pain in 24 cases (63%), local inflammation in 21 cases (55%), and fever in 14 cases (37%). Bacteremia occurred in four cases (10%) and severe sepsis was recorded in seven cases (18%). Twenty-two cases (58%) presented an elevated C-reactive protein level (≥ 40 mg/mL). We did not observe any difference of clinical features between cases of early infections that occurred within three weeks of implantation and delayed and late infections occurring after three weeks of implantation. Cases of S. lugdunensis infection associated with fracture-fixation devices were polymicrobial infection in 23 cases (60%). Coagulase negative staphylococci is the most frequent pathogen identified in fracture-fixation devices infection involving S. lugdunensis (Supplementary Fig. 1). All S. lugdunensis isolates were susceptible to classical anti-staphylococcal drugs used in osteoarticular infection, with the exception of three isolates resistant to fosfomycin, one isolate that was resistant to methicillin, one isolate resistant to fusidic acid, and one isolate resistant to clindamycin. Seventy-three percent of isolates were resistant to benzylpenicillin. No isolate resistant to rifampicin, quinolones, cotrimoxazole, doxycycline, aminoglycoside, vancomycin, and teicoplanin was identified.
Four cases (10%) were treated exclusively with antimicrobial treatment alone, without surgery. Thirty-four cases (89%) were treated with a combination of surgery with antimicrobial therapy including surgical debridement, antibiotics and osteosynthesis device retention in six cases (16%), osteosynthesis device replacement in one case (3%), and osteosynthesis device removal in 27 cases (71%). The mean length of antibiotic treatment was 119 days ±72 days (range 10–328). A total of 37 patients were evaluated with an average follow-up time of 30 months including 30 cases that were followed more than 12 months after the end of antimicrobial treatment. Remission was recorded in 32 cases (84%). Relapse was observed in five cases (13%). The mean time to relapse was 243 days ±85 days (range 150–365 days) after the end of treatment.
In the univariate analysis, two significant prognostic factors associated with relapse were identified, including history of open fracture (p = 0.007) and conservative therapy with antimicrobial alone without surgery (p = 0.042). A combination of surgery with antimicrobial therapy was an independent factor associated with remission of fracture-fixation devices infection caused by S. lugdunensis (p = 0.042). However, polymicrobial infection was not associated with relapse of fracture-fixation devices infection caused by S. lugdunensis. The choice of surgery treatment strategies between osteosynthesis device removal and debridement, antimicrobials with osteosynthesis device retention appeared to have no impact on clinical outcome in our study. From the results of the univariate analysis, six variables including alcoholism, history of open fracture, polymicrobial infections, antimicrobial treatment alone without surgery, surgical treatment, and osteosynthesis device removal were included in the multivariate analysis. We did not identify other specific factors in the multivariate analysis adjusted for diabetes mellitus and polymicrobial infection (Table 2).
Discussion
S. lugdunensis infection is more invasive compared to those caused by other coagulase-negative staphylococci [2]. The relationship between S. lugdunensis and osteoarticular infections has been reported in prosthetic joint infection [6–8, 14] and in osteomyelitis without orthopedic device infection including diabetic foot osteomyelitis [16, 21]. More recently, Lourtret-Hascoët et al. reported that the cases of prosthetic joint infection caused by S. lugdunensis were usually acute infection and have similar relapse rate to those caused by S. aureus.
Cases of infections associated with fracture-fixation devices caused by S. lugdunensis currently appear to be under reported. To date, only 13 cases have been reported included three cases of knee fracture-fixation devices [22, 23], two cases of ankle fracture-fixation devices [23], two cases of tibia fracture-fixation devices [23], two cases of toe fracture-fixation devices [23], and four cases of infection associated with vertebral arthrodesis [9, 23, 24]. Nevertheless, 38 cases were identified in our centers and in collaborating centres in the area of Marseille, Nimes, and Toulouse.
Conventional phenotypic identification tools using morphological and biochemical characteristics fail to identify S. lugdunensis [2]. Some automated identification systems have solved part of the problem of S. lugdunensis strains identification [2]. The arrival of MALDI-TOF mass spectrometry in clinical microbiology laboratories presents an alternative molecular identification tool for identification of S. lugdunensis strains from other coagulase-negative staphylococci [2]. The organisms can still be under-recognized as a pathogen in fracture-fixation device infections that result from improper identification of S. lugdunensis [3, 25] or are dismissed as contaminants of normal skin flora in surgical biopsies. In addition, physicians should be aware that a high number of S. lugdunensis cases tend to be with another pathogen associated with fracture-fixation device infection. The significant role of S. lugdunensis in polymicrobial fracture-fixation device infection should be considered when the organism was isolated from ≥2 surgical biopsies.
S. lugdunensis is known as a commensal coagulase-negative staphylococci that usually colonizes the skin in inguinal areas, perineum regions, on the lower extremities, and in the axillae [26]. These localizations may play a role in the potential subcutaneous dissemination of fracture-fixation device infection in patients with a history of open fracture. Among other coagulase-negative staphylococci, S. lugdunensis has a distinct position due to its pathogenic capacity, clinical implication that can cause an acute and more severe infection with a higher relapse rate. These clinical characteristics can be explained by the fact that S. lugdunensis shares the same virulence factors with S. aureus including hemolytic activity, resistance to lysozyme, ability to adhere to host cells by adhesins, and capacity to form biofilm [2].
In comparison to other coagulase-negative staphylococci, S. lugdunensis isolates were much more susceptible to narrow-spectrum antimicrobial agents [5, 27]. Despite the low rate of antimicrobial resistant S. lugdunensis isolates, we observed a high relapse rate in our study. Lower favourable outcome was observed in patients with S. lugdunensis prosthetic joint infection compared to patients with S. epidermidis prosthetic joint infection [5]. Surprisingly, relapse was not related to polymicrobial infection in our study. In addition, we found that relapses were associated with a history of open fracture and treatment option with antimicrobial alone without surgery. All five patients with early infection (≤ 3 weeks of implantation) and 87% of patients with delayed or late infection (> 3 weeks of implantation) have favorable outcome. These results were not significant in this population.
Our study suggests that a combination of surgery (i.e., debridement, antibiotics, and osteosynthesis device retention or osteosynthesis device removal) with prolonged antimicrobial therapy (nearly 4 months) were required to control fracture-fixation device infection caused by S. lugdunensis. We did not find any difference between cases treated with surgical lavage and debridement and those cases treated with osteosynthesis device removal in our study. Further prospective and randomized trial with a short term (e.g., 6 weeks) versus a long term (e.g., 4 months) of antimicrobial therapy and with surgical lavage and debridement versus osteosynthesis device removal would be required to confirm our findings. A combination of rifampicin and fluoroquinolone were used as a first-line treatment for 71% of patients in this study. Other second-line antimicrobials such as fusidic acid, clindamycin, doxycycline, co-trimoxazole, linezolid, vancomycin, and teicoplanin were used for the treatment of polymicrobial fracture-fixation device infection involving the resistance of coagulase negative staphylococci.
This study is also limited in its retrospective character and the fact that analyzed data derived from only nine hospital centers in two regions in southern France may have biased our finding by local epidemiology. We believe that increasing the description of S. lugdunensis in infections associated with fracture-fixation devices should allow for a better understanding of treatment strategies.
Conclusion
Although S. lugdunensis infections associated with fracture-fixation devices are rare, the organism should not be dismissed as a contaminant when isolated from surgical biopsies and the diagnosis should be further investigated. This organism is probably involved in more infections than has been reported. Particularly, when a laboratory does not routinely identify the species of all of its coagulase-negative Staphylococci isolates involved in infections associated with fracture-fixation devices. The choice of appropriate microbiological methods such as MALDI-TOF mass spectrometry or molecular methods to correctly identify the organism as Staphylococcus lugdunensis should allow clinicians to reach accurate diagnosis of S. lugdunensis infections associated with fracture-fixation devices. The treatment success rate for fracture-fixation device infection caused by S. lugdunensis was the same overall whatever the type of surgery. In general, osteosynthesis device removal should be practiced in the cases of delayed or late fracture-fixation device infection caused by S. lugdunensis with unstable fracture, in relapsed cases previously treated with surgical lavage and debridement or patients suffering from sepsis.
References
Freney J, Brun Y, Bes M et al (1988) Staphylococcus lugdunensis sp. nov. and staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Evol Microbiol 38:168–172. doi:10.1099/00207713-38-2-168
Ravaioli S, Selan L, Visai L et al (2012) Staphylococcus lugdunensis, an aggressive coagulase-negative pathogen not to be underestimated. Int J Artif Organs 35:742–753. doi:10.5301/ijao.5000142
Frank KL, Del Pozo JL, Patel R (2008) From clinical microbiology to infection pathogenesis: how daring to be different works for staphylococcus lugdunensis. Clin Microbiol Rev 21:111–133. doi:10.1128/CMR.00036-07
Hussain M, Steinbacher T, Peters G et al (2015) The adhesive properties of the staphylococcus lugdunensis multifunctional autolysin AtlL and its role in biofilm formation and internalization. Int J Med Microbiol IJMM 305:129–139. doi:10.1016/j.ijmm.2014.11.010
Lourtet-Hascoët J, Bicart-See A, Félicé MP et al (2016) Staphylococcus lugdunensis, a serious pathogen in periprosthetic joint infections: comparison to Staphylococcus aureus and Staphylococcus epidermidis. Int J Infect Dis 51:56–61. doi:10.1016/j.ijid.2016.08.007
Shah NB, Osmon DR, Fadel H et al (2010) Laboratory and clinical characteristics of staphylococcus lugdunensis prosthetic joint infections. J Clin Microbiol 48:1600–1603. doi:10.1128/JCM.01769-09
Sanzéni L, Ringberg H (2003) Fistulating periprosthetic staphylococcus lugdunensis hip infection cured by intra-articular teicoplanin injections—a case report. Acta Orthop Scand 74:624–625. doi:10.1080/00016470310018072
Szabados F, Anders A, Kaase M et al (2011) Late Periprosthetic joint infection due to staphylococcus lugdunensis identified by matrix-assisted laser desorption/ionisation time of flight mass spectrometry: a case report and review of the literature. Case Rep Med 2011:608919. doi:10.1155/2011/608919
Guttmann G, Garazi S, Van Linthoudt D (2000) Spondylodiscitis due to staphylococcus lugdunensis. Clin Exp Rheumatol 18:271–272
Fleurette J, Bès M, Brun Y et al (1989) Clinical isolates of staphylococcus lugdunensis and S. Schleiferi: bacteriological characteristics and susceptibility to antimicrobial agents. Res Microbiol 140:107–118
Greig JM, Wood MJ (2003) Staphylococcus lugdunensis vertebral osteomyelitis. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 9:1139–1141
Thomas S, Hoy C, Capper R (2006) Osteomyelitis of the ear canal caused by staphylococcus lugdunensis. J Inf Secur 53:e227–e229. doi:10.1016/j.jinf.2006.02.005
Kragsbjerg P, Bomfim-Loogna J, Törnqvist E, Söderquist B (2000) Development of antimicrobial resistance in staphylococcus lugdunensis during treatment-report of a case of bacterial arthritis, vertebral osteomyelitis and infective endocarditis. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 6:496–499
Sampathkumar P, Osmon DR, Cockerill FR 3rd (2000) Prosthetic joint infection due to staphylococcus lugdunensis. Mayo Clin Proc 75:511–512. doi:10.4065/75.5.511
Huang HL, Soo Rui Ting M, Olszyna DP et al (2016) Localization of staphylococcus Lugdunensis Clavicular osteomyelitis using FDG-PET/CT. Am J Med 129:e9–e11. doi:10.1016/j.amjmed.2015.10.040
Seng P, Traore M, Lagier J-C et al (2017) Staphylococcus lugdunensis: an underreported pathogen in osteomyelitis. J Foot Ankle Surg Off Publ Am Coll Foot Ankle Surg. doi:10.1053/j.jfas.2016.10.018
Trampuz A, Zimmerli W (2006) Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 37(Suppl 2):S59–S66. doi:10.1016/j.injury.2006.04.010
Zimmerli W (2014) Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med 276:111–119. doi:10.1111/joim.12233
Seng P, Barbe M, Pinelli PO et al (2014) Staphylococcus Caprae bone and joint infections: a re-emerging infection? Clin Microbiol Infect 20:O1052–O1058. doi:10.1111/1469-0691.12743
Roffman CE, Buchanan J, Allison GT (2016) Charlson comorbidities index. J Physiother 62:171. doi:10.1016/j.jphys.2016.05.008
Kear S, Smith C, Mirmiran R, Hofinger D (2016) Staphylococcus lugdunensis: a rare pathogen for osteomyelitis of the foot. J Foot Ankle Surg Off Publ Am Coll Foot Ankle Surg 55:255–259. doi:10.1053/j.jfas.2014.06.019
Karnani R, Myers JP (2008) Bone and joint infections caused by staphylococcus lugdunensis: report of 2 cases and review of the literature. Infect Dis Clin Pract 16:94–99
Douiri N, Lefebvre N, Riegel P et al (2016) Staphylococcus lugdunensis: a virulent pathogen causing bone and joint infections. Clin Microbiol Infect. doi:10.1016/j.cmi.2016.05.031
Johnson LB, Burket JS, Kauffman CA (1999) Staphylococcus lugdunensis infection following spinal fusion. Infect Dis Clin Pract 8:206–208
Szabados F, Nowotny Y, Marlinghaus L et al (2011) Occurrence of genes of putative fibrinogen binding proteins and hemolysins, as well as of their phenotypic correlates in isolates of S. Lugdunensis of different origins. BMC Res Notes 4:113. doi:10.1186/1756-0500-4-113
van der Mee-Marquet N, Achard A, Mereghetti L et al (2003) Staphylococcus lugdunensis infections: high frequency of inguinal area carriage. J Clin Microbiol 41:1404–1409
McHardy IH, Veltman J, Hindler J et al (2017) Clinical and microbiological aspects of β-lactam resistance in staphylococcus lugdunensis. J Clin Microbiol 55:585–595. doi:10.1128/JCM.02092-16
Acknowledgements
The authors would like to thank Catherine Peruffo for their helpfulness in this study.
Author information
Authors and Affiliations
Contributions
Piseth Seng: 1st author, he was involved clinical data collection, conception, design and drafting the manuscript.
Madou Traore: 2nd author, clinical data collection, microbiological data collection, manuscript revision.
Jean-Philippe Lavigne: 3rd author, microbiological data verification, manuscript revision.
Laurence Maulin: 4th author; clinical data verification, manuscript revision.
Jean-Christophe Lagier: 5th author, clinical data verification, manuscript revision.
Jean-François Thiery: 6th author, surgical data verification, manuscript revision.Jean-François Thiery: 6th author, surgical data verification, manuscript revision.
Pierre-Yves Levy: 7th author, microbial data verification, manuscript revision.
Pierre-Marie Roger: 8th author , clinical data verification, manuscript revision.
Eric Bonnet: 9th author , clinical data verification, manuscript revision.
Albert Sotto: 10th author , clinical data verification, manuscript revision.
Andreas Stein: final author, clinical data verification, discussion section, final approval of the version to be published.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
There is no funding source.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study. This study was approved by the institutional research ethics board (Comité de Protection des Personnes Sud Méditerranée 1). Copies of the written approval are available for review by the Editor-in-Chief of this journal.
Electronic supplementary material
Supplementary Fig. 1
(PPTX 96 kb).
Table S1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Seng, P., Traore, M., Lavigne, JP. et al. Staphylococcus lugdunensis: a neglected pathogen of infections involving fracture-fixation devices. International Orthopaedics (SICOT) 41, 1085–1091 (2017). https://doi.org/10.1007/s00264-017-3476-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3476-4