Abstract
Introduction
Locked anterior shoulder dislocation (LASD) is an uncommon condition associated with bone, articular cartilage, and soft tissue damage. In selected cases, shoulder arthroplasty (SA) may be the best treatment. The purpose of this study was to assess outcomes of SA for LASD.
Materials and methods
Between 1976 and 2013, 19 SAs [three hemiarthroplasties (HA), seven total shoulder arthroplasties (TSA), and nine reverse shoulder arthroplasties (RSA), mean age 62 years] were performed for LASD. Shoulders were followed for at least two years (range, 2-30 years, mean 7.1). Clinical and radiographic outcomes were studied.
Results/discussion
Three SAs required re-operation, two TSAs for early redislocation and one HA for late, painful glenoid arthrosis. Four additional shoulders (two TSA, two HA) were unstable at most recent follow-up. Pain improved from 4.7 to 2.2 (p < 0.0001) out of 5, elevation from 51 to 94 degrees (p = 0.004), and external rotation from 1 to 34 degrees (p = 0.01). There were two excellent, seven satisfactory, and ten unsatisfactory modified Neer ratings. Compared to TSA/HA, RSA experienced fewer re-operations (0 vs. 3, hazard ratio, 2.03*10^-9, p = 0.0844) and instability (0 vs. 6, p = 0.0108). Similar post-operative pain (p = 0.2192), range of motion (p = 0.2432-0.5299), strength (p = 0.2099), satisfaction (p = 0.6563), outcomes scores (p = 0.0683-0.0933), and complication rate (p = 0.3698) were seen with RSA vs. TSA/HA.
Conclusions
RSA for the treatment of chronic LASD provides greater pain relief, and improvement in range of motion (ROM) compared to TSA/HA. Anatomic SA is associated with a high rate of instability not seen with RSA. Therefore, anatomic SA (TSA/HA) is likely not indicated in these difficult circumstances.
Level of Evidence: IV
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Locked anterior shoulder dislocation (LASD) is relatively rare. Over time, anterior soft-tissue structures are stretched and the posterior capsule and cuff may develop contractures. Additionally, the cartilage damage and bony erosion of both the humeral head and glenoid commonly occurs. Open reduction and soft tissue stabilization or coracoid transfer procedures may be considered for patients with a LASD when damage to bone stock and articular surfaces is minimal. However, in cases with severe bone loss and/or advanced arthritis, shoulder arthroplasty (SA) can be necessary [1, 2].
Several studies have reported outcomes of surgical management of LASD, with fewer examining the outcomes of SA. Many previous studies have combined anterior and posterior shoulder dislocations, soft tissue procedures and shoulder arthroplasty, making it difficult to examine disease and treatment specific outcomes [3–10]. Furthermore, no studies have compared outcomes of anatomic versus reverse components. The purpose of this study was to assess outcomes of SA for patients with LASD and to compare reverse total shoulder arthroplasty (RSA) versus total shoulder arthroplasty (TSA) and hemiarthroplasty (HA). Our hypothesis was that RSA would be associated with better outcomes due to increased shoulder stability secondary to its increased constraint.
Materials and methods
After approval from our Institutional Review Board, a single institution, registry-based retrospective review was conducted for SAs performed for LASD [11]. This registry records numerous demographic and functional variables and follows all patients after total joint arthroplasties at time points of one year, two years, five years, and then every five years thereafter [12]. Patients are asked to return for in person follow-up. Those who do not return are asked to complete a validated outcomes questionnaire which also assesses re-operation at outside institutions [13, 14].
Patient demographics, selection, and operative features
Between 1976 and 2013, 268 SAs were performed with a concomitant diagnosis of dislocation. Of these, 247 SAs were excluded because their dislocation was not locked and anterior. This left 21 consecutive SAs performed for LASD. Of these, 19 SAs had at least two year follow up and were evaluated (three HAs, seven TSAs, and nine RSAs). The median pre-operative time between the index anterior dislocation and arthroplasty was 32 weeks (range 2 weeks-32 years). In the literature, there have been widely-varying definitions of LASD [2, 8, 15]. A cutoff of two weeks was chosen in our study based on the experience of senior surgeons at our institution that soft-tissue procedures are unsuccessful after this time due to permanent peri-articular soft-tissues. Patient demographic information is presented in Table 1.
SA was indicated for LASD if pain was not amenable to non-operative treatment and was associated with: radiographic osteoarthritis; Hill-Sachs lesion deemed too large to obtain joint stability with a soft-tissue procedure (usually >40% of the articular surface); glenoid deficiency deemed too large to obtain joint stability with a soft-tissue procedure (usually >40% of the articular surface); or a combination of these factors.
Prior to the availability of RSA, the decision to perform an HA versus TSA was made based on the status of the glenoid bone stock, cartilage, rotator cuff, and stability. In cases when the surgeon determined a glenoid component would improve the patient’s pain and function without leading to instability or glenoid loosening, a TSA was performed. Sometimes this required glenoid bone grafting. However, if the surgeon determined the glenoid component placement would lead to instability or glenoid component failure, a hemiarthroplasty was utilized. Sometimes, glenoid bone grafting was required with hemiarthroplasty as well to gain stability. Due to poor results with anatomic arthroplasty, we changed our practice to utilizing RSA when this became available at our institution for which humeral head autograft was sometimes necessary to restore glenoid bone stock to obtain stability of the glenosphere.
Operative technique
The deltopectoral approach was utilized for 12 surgeries, with seven requiring additional release of the anterior deltoid. The conjoined tendon was released in one shoulder to aid in exposure. The subscapularis was found to be intact in nine, torn or attenuated in seven, and absent in three shoulders. When the subscapularis was intact, a tenotomy (seven shoulders) or lesser tuberosity osteotomy (2) was performed. The shoulder was inspected to confirm locked anterior shoulder dislocation. The posterosuperior rotator cuff was torn in nine shoulders. The humeral head was resected in an average of 44 degrees (range 20-75) of retroversion (increased retroversion was used in some early anatomic SAs to attempt to decrease dislocation). The glenoid was inspected to determine the need for grafting. Glenoid bone grafting with humeral head autograft was performed in seven shoulders (one HA, two TSA, four RSA). The rotator cuff was repaired in four shoulders (one HA, two TSAs, one RSA). The remaining five shoulders with rotator cuff tears received RSAs without rotator cuff repair.
Humeral stems were cemented in eight and uncemented in 11 shoulders. Soft tissue balancing was performed for anatomic prostheses including lysis of adhesions (12) and posterior capsular release (5). A tenuous subscapularis repair due to a thin, torn, or attenuated subscapularis was obtained in five patients (three RSA and two TSA/HA); and no repair was possible in five RSA. The deltoid and conjoint tendons were reattached to their origins when incised.
The post-operative rehabilitation program varied depending on the type and year of surgery. All patients were immobilized for at least two weeks with most immobilized for one month. In general, active assisted motion was allowed earlier after RSA.
Clinical outcomes assessment
The primary outcome studied was failure, which we defined as re-operation for instability or component revision. Secondary outcome measures included pain, range of motion, strength, subjective satisfaction with surgery, modified Neer ratings, ASES scores, shoulder subjective test scores, simple shoulder test scores, complications, and radiographic outcomes [16–18]. Pain was reported on a scale of 1 to 5 with 1 as “no pain” and 5 as “severe pain.” Range of motion was recorded using a goniometer for elevation and external rotation and as the most cephalad spinal level reached by the thumb for internal rotation. Strength was reported on a scale of 1 to 5 [19]. Post-operative subjective satisfaction was categorized as “much better,” “better,” “the same” or “worse” at last follow-up as compared to pre-operatively. Modified Neer ratings included “excellent,” “satisfactory,” and “unsatisfactory” as previously described [16, 20].
Radiographic assessment
Pre-operative, early post-operative and most recent x-ray/CT were analyzed by two orthopaedic surgeons (JMS, BSS). Review of pre-operative images determined pre-operative glenoid bone loss and presence of Hills-Sachs lesions. Radiographs obtained at most recent follow-up were reviewed to determine post-operative subluxation, dislocation, glenoid component lucency, glenoid component shift, glenoid erosion for HA, humeral component shift, humeral component lucency, and inferior scapular notching in the case of RSA. Subluxation was determined by comparing the centre of the humeral component to the centre of the glenoid component as a percentage of the surface of the glenoid component. It was graded as “none” (humeral head centred on the glenoid), “mild” (<25% shift), “moderate” (shift 25-50%), and “severe” (>50% shift) [21]. Glenoid and humeral lucencies were graded as 0-5 as previously described [22, 23]. Moderate or severe post-operative subluxation, dislocation, glenoid component shift, glenoid erosion, humeral component shift were considered to be post-operative complications when calculating a complication rate whereas glenoid lucency, humeral lucency, and inferior scapular notching were not.
Statistical methods
Data was analyzed using JMP software version 10.0.0 (SAS Institute, Inc, Cary, NC, USA). Kaplan-Meier methodology was utilized to estimate implant survival. The Wilcoxon Signed Rank test was used to evaluate changes from pre-operative to post-operative times. Cox regression analysis, Fisher’s exact test, and the Wilcoxon rank sum test were utilized to compare differences between RSA and TSA/HA. An alpha level of 0.05 was used to determine statistical significance.
Results
Re-operations
Including all RSAs, TSAs, and HAs, at an average follow-up of 7.1 years (range 2.0-30), three shoulders required re-operation due to instability after TSA (2) and glenoid arthritis after HA (1). Overall, the 5- and 10-year survival (re-operation-free) rates were 89% +/- 7% and 77% +/- 13%, respectively.
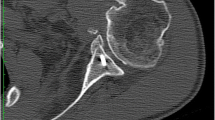
Two TSAs dislocated early and required revision. The first patient had a TSA with autogenous humeral head bone grafting to the glenoid and rotator cuff repair with the humeral component placed in 60 degrees of retroversion. On post-operative day two, the TSA dislocated and required open reduction. Unfortunately, radiographs after that revealed repeat dislocation, and the patient decided to live with the shoulder in a chronically dislocated position (Fig. 1). Five years after TSA, this patient had good pain relief, 3/5 strength in all directions, occasional dysesthesias in her ipsilateral hand, and active range of motion of 80 degrees elevation and 10 degrees external rotation.
The second patient underwent a TSA with autogenous humeral head bone grafting to the glenoid with the humeral component placed in 40 degrees of retroversion. She was placed in a plaster abduction splint post-operatively but dislocated nine days after surgery. She was successfully closed reduced and placed in a shoulder immobilizer. Unfortunately, she dislocated again four weeks after surgery, was closed reduced and placed in a Velpeau dressing. She dislocated again two months after surgery and was treated with open reduction and soft tissue balancing including rotator cuff repair and pectoralis major lengthening. At most recent follow up, 13 years after her primary surgery, her shoulder was stable with moderate pain, elevation to 50 degrees, and external rotation to -30 degrees.
One HA underwent revision to TSA six years after the index surgery for painful glenoid arthrosis. At five years after her revision surgery, she had a stable shoulder with no pain, 100 degrees of elevation, and 90 degrees of external rotation.
Clinical outcomes
Including all RSAs, TSAs, and HAs, pain scores improved from 4.7 to 2.2 (p < 0.0001) out of 5. Four (21%) patients continued to have moderate/severe pain post-operatively. Mean shoulder elevation improved from 51 (range 0-110) to 94 (range 0-180) degrees (p = 0.004). External rotation improved from 1 (range -35-70) to 34 (range -20-90) degrees (p = 0.0113). Shoulder internal rotation was relatively unchanged, from iliac crest (range abdomen-T12) pre-operatively to the SI joint (range abdomen-T12) post-operatively (p = 0.059). Shoulder strength did not improve, averaging 4.0 pre-operatively and 3.8 post-operatively (p = 0.9).
At follow-up, one patient described her subjective satisfaction as “worse,” five as “the same,” seven as “somewhat better,” and six as “much better.” Modified Neer ratings included two excellent, seven satisfactory, and ten unsatisfactory outcomes. Unsatisfactory outcomes were due to poor range of motion (3); pain and dissatisfaction (1); pain and range of motion (1); range of motion and dissatisfaction (2); and pain, range of motion, and dissatisfaction (3). ASES scores averaged 65 (range 28-100), shoulder subjective value averaged 43 (range 10-80), and simple shoulder test averaged 5.7 (range 1-12).
SAs with a strong subscapularis repair were associated with increased post-operative instability (p = 0.0345) and with lower subjective satisfaction (p = 0.0573). Notably, instability and strong subscapularis repair were associated with anatomic SA (p = 0.0108 and 0.0055, respectively). All unstable SAs were anatomic (HA/TSA) and only one RSA had a strong subscapularis repair (see discussion below). No difference was seen in outcomes of Neer rating, ASES scores, pain, range of motion, or post-operative dislocation, or complication rate when stratifying by strength of subscapularis repair (p = 0.1332-1.0), length of time dislocated (≥32 wks vs. <32 weeks, p = 0.1312-1.0), prior rotator cuff surgery (p = 0.3309-1.0), strength of subscapularis repair (p = 0.1893-1.0), >50% glenoid bone loss pre-operatively (p = 0.1758-1.0), the presence of a Hill-Sachs lesion (p = 0.4538-1.0), humeral component fixation (cemented vs. non-cemented, p = 0.0.0915-1.0), or glenoid bone grafting (p = 0.2451-1.0). There was additionally no correlation between intra-operative or post-operative humeral fracture with humeral component fixation (p = 1.0) or with RSA versus TSA/HA (p = 0.2105-1.0).
Radiographic assessment
Pre-operative radiographs were available for 15 shoulders. A Hill-Sachs lesion was seen in 14 patients. Glenoid bone loss was <25% in eight patients, 25-50% in four patients, and 50-75% in three patients (Fig. 2). Post-operative radiographs were available for 17 shoulders and radiographic outcomes are found in Table 2.
RSA vs. TSA/HA
A comparison of patients undergoing RSA and TSA/HA is shown in Table 3. The TSA/HA group had a higher proportion of strong subscapularis repairs (p = 0.0055), longer average operative time (p = 0.0031), and longer average length of follow up (p = 0.0081), and more distant surgical date (p = 0.0003).
Four of nine RSAs versus seven of 10 TSAs/HAs had a complication (p = 0.4, Table 4). Intra-operative humeral shaft fractures were treated with internal fixation followed by implantation of a humeral stem bypassing the fracture. Two dislocations were revised (see above) while the remaining unstable shoulders elected to live with an unstable SA (Fig. 2). Neer rating, ASES scores, pain, or range of motion did not differ based on instability (p = 0.1904-0.9474) or dislocation (p = 0.2059-1.0).
RSA had a five year survival rate of 100% +/- 0% compared to 80% +/- 13% in the TSA/HA group (hazard ratio 2.03*10-9, p = 0.0844).
Comparison of pre-operative variables and post-operative outcomes is detailed in Table 5. RSA showed greater post-operative elevation, external rotation, ASES scores, SST scores, and STT scores (p = 0.0683-0.5299). Clinical instability was significantly more common in the HA/TSA group (p = 0.0108) despite HA/TSA having more strong subscapularis repairs (p = 0.0055, Table 3, Figs. 1 and 3).
Discussion
LASD is a complex and rare injury that may require treatment with SA when significant damage occurs to the humeral head or glenoid. There is currently limited literature focusing on outcomes of SA for LASD. The purpose of this study was to elucidate these outcomes and to compare those of RSA with TSA/HA.
The study is limited by its retrospective nature and small numbers. We acknowledge that surgical techniques and implants have changed over time, complicating the ability to compare groups across such a long time period with significantly different length of follow-up between the TSA/HA and RSA groups. The study also lacked pre-operative outcomes scores, which would allow for better demonstration of patient improvement.
Survivorship of TSA/HA for LASD is lower than that demonstrated for other indications such as trauma, osteoarthritis, cuff-tear arthropathy, and rheumatoid arthritis, where failure rates approach 1% per year [24–31]. The rate of instability in this study (60%) is similar to Matsoukis et al, who showed 4/11 anatomic arthroplasties were complicated by post-operative instability, with two requiring re-operation [32]. Both studies show rates of instability higher than Raiss et al, who reported only one dislocation in 10 shoulders treated with HA resufacing for LASD [33]. This information is important for surgical decision-making and patient counseling.
In contrast to non-constrained SA, RSA compensates for soft tissue imbalance, leading to a more stable SA despite the fact that fewer RSAs had a strong subscapularis repair (p = 0.0055). Early failure seen with anatomic arthroplasty in this series was not seen with RSA. This finding supports Werner et al, who reported a 6% complication rate and no post-operative instability in 32 shoulders treated with bio-RSA for LASD [34]. It seems that this increased stability with RSA versus HA/TSA should lead to better survivorship and outcomes. However, no statistical association was found in the current study (Table 5). We believe that this is likely due to beta error, and a larger cohort would show the benefit of RSA. However, even if this were established, it is notable that average range of motion, outcomes scores, and complications reported in this study are suboptimal when compared to other indications. This is in agreement with a recent study by Kurowicki et al that found RSA performed for LASD compared unfavourably compared to RSA performed for more classic indications [35]. They also found that instability was only seen in two of 24 patients with LASD treated with RSA. This speaks to the severity of pathology found in shoulders with LASD and the difficulty with treating them.
Conclusion
SA provides improved pain and range of motion for LASD. TSA/HA is associated with a 60% instability rate whereas RSA eliminates post-operative instability even with less soft tissue repair. In patients requiring SA for LASD, we recommend RSA over TSA/HA due to increased stability and a potentially more functional shoulder.
References
Provencher MT, Frank RM, Leclere LE, Metzger PD, Ryu JJ, Bernhardson A, Romeo AA (2012) The Hill-Sachs lesion: diagnosis, classification, and management. JAAOS 20:242–252. doi:10.5435/jaaos-20-04-242
Sahajpal DT, Zuckerman JD (2008) Chronic glenohumeral dislocation. J Am Acad Orthop Surg 16:385–398
Cheng SL, Mackay MB, Richards RR (1997) Treatment of locked posterior fracture-dislocations of the shoulder by total shoulder arthroplasty. J Shoulder Elbow Surg 6:11–17
Flatow EL, Miller SR, Neer CS (1993) Chronic anterior dislocation of the shoulder. J Shoulder Elbow Surg 2:2–10. doi:10.1016/s1058-2746(09)80131-6
Goga IE (2003) Chronic shoulder dislocations. J Shoulder Elbow Surg 12:446–450. doi:10.1016/s1058274603000880
Pritchett JW, Clark JM (1987) Prosthetic replacement for chronic unreduced dislocations of the shoulder. Clin Orthop Relat Res 216:89–93
Rowe CR, Zarins B (1982) Chronic unreduced dislocations of the shoulder. J Bone Joint Am Am 64:494–505
Schulz TJ, Jacobs B, Patterson RL (1969) Unrecognized dislocations of the shoulder. J Trauma 9:1009–1023
Sperling JW, Pring M, Antuna SA, Cofield RH (2004) Shoulder arthroplasty for locked posterior dislocation of the shoulder. J Shoulder Elbow Surg 13:522–527. doi:10.1016/s1058274604000886
Wooten C, Klika B, Schleck CD, Harmsen WS, Sperling JW, Cofield RH (2014) Anatomic shoulder arthroplasty as treatment for locked posterior dislocation of the shoulder. J Bone Joint Surg Am 96:e19–e19. doi:10.2106/jbjs.l.01588
Berry DJ, Kessler M, Morrey BF (1997) Maintaining a hip registry for 25 years. Mayo Clinic experience. Clin Orthop Relat Res 344:61–68
McGrory BJ, Morrey BF, Rand JA, Ilstrup DM (1996) Correlation of patient questionnaire responses and physician history in grading clinical outcome following hip and knee arthroplasty. A prospective study of 201 joint arthroplasties. J Arthroplasty 11:47–57
Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH (2006) Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am 88:508–513. doi:10.2106/JBJS.E.00132
Schulz TJ, Jacobs B, Patterson RL Jr (1969) Unrecognized dislocations of the shoulder. J Trauma 9(12):1009–1023. doi:10.1097/00005373-196912000-00005
McLaughlin HL (1952) Posterior dislocation of the shoulder. J Bone Joint Surg Am 24A(3):584–590
Cofield RH (1984) Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am 66:899–906
Tashjian RZ, Deloach J, Green A, Porucznik CA, Powell AP (2010) Minimal clinically important differences in ASES and simple shoulder test scores after nonoperative treatment of rotator cuff disease. J Bone Joint Surg Am 92:296–303. doi:10.2106/jbjs.h.01296
Michener LA, McClure PW, Sennett BJ (2002) American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg 11:587-594. doi:10.1067/mse.2002.127096
Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, Donovan W, Graves D, Jha A, Jones L, Mulcahey MJ, Krassioukov A (2011) Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med 34:547–554. doi:10.1179/107902611x13186000420242
Neer CS 2nd, Watson KC, Stanton FJ (1982) Recent experience in total shoulder replacement. J Bone Joint Surg Am 64:319–337
Sperling JW, Cofield RH, Rowland CM (1998) Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am 80:464–473
Sperling JW, Cofield RH (1998) Revision total shoulder arthroplasty for the treatment of glenoid arthrosis. J Bone Joint Surg Am 80:860–867
Walch G, Badet R, Boulahia A, Khoury A (1999) Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 14:756–760
Adams JE, Sperling JW, Schleck CD, Harmsen WS, Cofield RH (2007) Outcomes of shoulder arthroplasty in Olmsted County, Minnesota: a population-based study. Clin Orthop Relat Res 455:176–182. doi:10.1097/01.blo.0000238870.99980.64
Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd (1987) Total shoulder arthroplasty. J Bone Joint Surg Am 69:865–872
Boileau P, Sinnerton RJ, Chuinard C, Walch G (2006) Arthroplasty of the shoulder. J Bone Joint Surg (Br) 88:562–575. doi:10.1302/0301-620X.88B5.16466
Boileau P, Watkinson D, Hatzidakis AM, Hovorka I (2006) Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 15:527–540. doi:10.1016/j.jse.2006.01.003
Deshmukh AV, Koris M, Zurakowski D, Thornhill TS (2005) Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg 14:471–479. doi:10.1016/j.jse.2005.02.009
Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G (2006) Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am 88:1742–1747. doi:10.2106/JBJS.E.00851
Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE (2003) Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am 85:1215–1223
Wirth MA, Tapscott RS, Southworth C, Rockwood CA Jr (2006) Treatment of glenohumeral arthritis with a hemiarthroplasty: a minimum five-year follow-up outcome study. J Bone Joint Surg Am 88:964–973. doi:10.2106/JBJS.D.03030
Matsoukis J, Tabib W, Guiffault P, Mandelbaum A, Walch G, Nemoz C, Cortes ZE, Edwards TB (2006) Primary unconstrained shoulder arthroplasty in patients with a fixed anterior glenohumeral dislocation. J Bone Joint Surg Am 88:547–552. doi:10.2106/JBJS.E.00368
Raiss P, Aldinger PR, Kasten P, Rickert M, Loew M (2009) Humeral head resurfacing for fixed anterior glenohumeral dislocation. Int Orthop 33:451–456. doi:10.1007/s00264-007-0487-6
Werner BS, Bohm D, Abdelkawi A, Gohlke F (2014) Glenoid bone grafting in reverse shoulder arthroplasty for long-standing anterior shoulder dislocation. J Shoulder Elbow Surg 23:1655–1661. doi:10.1016/j.jse.2014.02.017
Kurowicki J, Triplet JJ, Momoh E, Moor MA, Levy JC (2016) Reverse shoulder in the treatment of locked anterior shoulders: a comparison with classic reverse shoulder indications. J Shoulder Elbow Surg 25(12):1954–1960. doi:10.1016/j.jse.2016.04.019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Sources of funding
No external sources of funding were utilized for this study.
Conflict of interest
Dr. Sanchez-Sotelo reports other from Stryker (Shoulder implant design), outside the submitted work; Dr. Cofield reports other from Royalties, outside the submitted work; In addition, Dr. Cofield has a patent Smith/Nephew issued. Dr. Sperling reports other from Zimmer-Biomet, outside the submitted work; No other author has any disclosures.
Rights and permissions
About this article
Cite this article
Statz, J.M., Schoch, B.S., Sanchez-Sotelo, J. et al. Shoulder arthroplasty for locked anterior shoulder dislocation: a role for the reversed design. International Orthopaedics (SICOT) 41, 1227–1234 (2017). https://doi.org/10.1007/s00264-017-3450-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3450-1