Abstract
Purpose
Sciatic nerve palsy after periacetabular osteotomy (PAO) is a serious complication. The purpose of this study was to determine whether a multimodal sciatic monitoring technique allows for identification of surgical steps that place the sciatic nerve at risk.
Methods
Transcranial electrical motor evoked potentials (TcMEPs), somatosensory evoked potentials (SSEPs), and spontaneous electromyography (EMG) were monitored in a consecutive series of 34 patients (40 hips) who underwent PAO for the treatment of symptomatic hip dysplasia between January 2012 and November 2014. There were 29 females (85%) and five males (15%) with an average age of 19 years (range, 12–36 years) at the time of surgery.
Results
We detected eight temporary sciatic nerve monitoring alerts in six patients (incidence of 15%). The events included decrease in amplitude of the TcMEPs related to the position of the hip during incomplete ischium osteotomy and placement of a retractor in the sciatic notch during the posterior column osteotomy (N = 3), generalized bilateral decrease in TcMEPs during fragment manipulation and fixation in association with acute blood loss (N = 2), and a change in SSEPs during a superior pubic osteotomy and supra-acetabular osteotomy (N = 1). At the end of the procedure, TcMEPs and SSEPs were at baseline and there was no abnormal pattern on EMG in all patients. Post-operatively, at two, six, 12 weeks, and six and 12 months, no motor weakness or sensory deficits were noted.
Conclusion
Multimodal neuromonitoring allowed for identification of intra-operative steps and maneuvers that potentially place the sciatic nerve at higher risk of injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Bernese periacetabular osteotomy (PAO) is a well-established procedure for the treatment of symptomatic hip dysplasia [1]. Sciatic nerve injury is one of the most serious complications of PAO with previously reported incidence between 0 and 15% [1–8]. In a multicenter study of 1677 patients who had undergone PAO, sciatic nerve palsy occurred in 28 (1.7%) cases [9]. The mechanisms causing sciatic nerve injury are not well elucidated but due to its close proximity, the nerve may be at risk of direct trauma during the incomplete osteotomy of the ischium and the posterior wall osteotomy.
Intra-operative nerve monitoring has been applied to several orthopaedic procedures [10, 11]. The goal of intra-operative monitoring is to identify early changes in neural activity to guide for procedural actions and adjustments that ultimately may avoid definitive injury to the nerve. Different monitoring techniques have been described in the past, however, currently a multimodal technique including somatosensory evoked potentials (SSEPs) to provide information of the sensory pathway, continuous spontaneous elicited electromyography (EMG), and transcranial motor evoked potentials (TcMEPs) to monitor descending motor pathway is recommended to increase sensitivity and accuracy [12–15]. Although multimodal sciatic monitoring has been reported to be reliable and effective in alerting the surgeon of the possibility of nerve injury during complex hip surgery [14], its application to PAO has been limited [14, 16].
The purpose of this study was to report our preliminary experience with a multimodal sciatic monitoring technique using EMG, SSEPs, and TcMEPs to determine whether neurophysiologic monitoring allows for identification of surgical steps that place the sciatic nerve at risk; and to investigate whether actions taken after detection of early changes in nerve activity would avoid definitive nerve injury during Bernese PAO.
Material and methods
Patients
Multimodal intra-operative monitoring of the sciatic nerve was performed in a consecutive series of 34 patients (40 hips) who underwent Bernese PAO between January 2012 and November 2014. There were 29 females (85%) and five males (15%). The average age of the patients at the time of surgery was 19 years (range, 12–36 years) and the average body mass index (BMI, kg/m2) was 22.9 (range, 18.7-28.4). The right hip was involved in 22 patients (55%) and there was bilateral involvement in six patients (17.6%). All patients had hip or thigh pain for minimum of three months and radiographic evidence of acetabular dysplasia with a lateral center edge angle inferior to 20° on an anteroposterior pelvic plain radiograph. A retrospective review of the electronic medical records was performed to collect data on post-operative evaluation in regards to neurological examination of the involved lower extremity for all patients at two, six, 12 weeks, and six and 12 months after surgery. An Institutional Review Board approval was obtained prior to the commencement of this study.
Anesthesia protocol and surgical technique
General endotracheal anesthesia with a total intravenous anesthetic (TIVA) was utilized in all cases without the use of non-depolarizing neuromuscular blockade [17] and sevoflurane [17–19], which may influence monitoring. A lumbar epidural was placed prior to the operation to facilitate pain management after surgery; however, no local anaesthetic was administered until neuromonitoring was completed. This study includes the first 40 osteotomies performed by a single author according to a previously described technique [20]. Briefly, an anterior approach to the hip through the interval between the sartorius and tensor fascia lata is used. The iliopsoas and hip joint capsule interval is exposed to allow for the incomplete osteotomy of the ischium under fluoroscopy imaging. Then, the inner table of the ilium is exposed and a Hohmann retractor is placed in the quadrilateral surface or in the sciatic notch to allow for exposure for the subsequent osteotomies. The second step is an osteotomy of the superior pubic ramus. The third step is a supra-acetabular cut performed with an oscillating saw and followed by the fourth osteotomy through the posterior column aiming toward the first osteotomy. After the acetabular fragment is completely cut, a Shanz pin is inserted to allow for manipulation and positioning of the acetabulum. The osteotomy is then fixed with three or four 4.5 mm screws.
Sciatic nerve monitoring protocol
All patients were monitored by parallel use of TcMEPs, SSEPs, and EMG using a Cadwell/Cascade acquisition system (Cadwell Laboratories, Inc. Kennewick, WA). A three-channel electroencephalogram (EEG) was also recorded to monitor anesthesia depth. For TcMEPs monitoring, stimulating electrodes were placed overlying the motor cortex at C1’ and C2’ scalp sites. Trains of seven stimuli, 50 μsec pulse duration, and interstimulus interval of 3 ms were used with stimulation intensities ranging from 200 to 500 V. The time base for recording was set at 10 ms/division. Subdermal needle electrodes for EMG and TcMEPs recordings were positioned in bilateral tibialis anterior, medial gastrocnemius, and abductor hallucis muscles. Routine acquisition of TcMEPs was elicited after the first (incomplete ischial) osteotomy, second (pubic) osteotomy, third (supra-acetabular) osteotomy, and at the completion of the posterior column osteotomy, after manipulation and provisional fixation of the fragment and after definitive fixation of the osteotomy (Figs. 1 and 2). Other acquisitions of TcMEPs were elicited with direct notification to or from the surgeon. The criteria for alarm were a complete loss of the TcMEPs signal from the baseline recording (disappearance) or a relative or asymmetric change in the amplitude of the compound muscle action potential [21].
Somatosensory evoked potentials were elicited by stimulation of the posterior tibial nerve on both ankles. Recordings were acquired from the scalp from C2 to C4 [17]. The SSEPs were generated in both ankles ranging from 10–20 mA. The repetition rate was set to 4.13 stimulations per second and pulse width was set at 0.3 ms. The low frequency filters were set at 50 Hz and high frequency filters at 500 Hz. The recording time-base was set at 10 msec/division. Throughout the surgical procedure, new SSEPs averages were acquired and compared to baseline data. Any recordings with a 50% reduction in amplitude and/or a 10% increase in latency from baselines were considered criteria for alarm and the surgeon was promptly notified.
Spontaneous EMG was recorded from bilateral tibialis anterior, medial gastrocnemius, and abductor hallucis muscles. The time-base for recording spontaneous EMG was set to 50 ms/division. The filter settings were set 30 Hz for low frequency and 1500 Hz for high frequency. Criteria for notifying the surgeon included any continuous, repetitive EMG firing with change in waveform morphology. At the time of any persistent neurotonic discharges, TcMEPs were elicited.
Results
We detected eight events of transient sciatic nerve monitoring changes in six patients, for an incidence of 15% of the surgeries (Table 1). Three events were decrease in amplitude of the TcMEPs related to the position of the hip in flexion and adduction during incomplete ischium osteotomy (n = 1) and related to the position of the hip and retractor placement close to the sciatic notch during the posterior column osteotomy (n = 2). TcMEPs returned to baseline in all these events after the hip was repositioned to flexion to about 30° and slightly abducted and the retractors were moved distally toward the ischial spine in the quadrilateral plate. Generalized bilateral decrease in TcMEPs was found in two patients during fragment manipulation and fixation in association with blood loss and arterial hypotension (Fig. 3). In one patient there was a generalized loss of TcMEPs found during the initial step of the PAO because of a break in the anesthesia protocol. Change in SSEPs was found in a 13-year-old girl who had undergone an intertrochanteric femoral osteotomy for treatment of coxa-valga and excessive anteversion one year before PAO. During subperiosteal exposure of the quadrilateral surface and posterior column, the periosteum was thick and found to be under tension. A reverse Hohmann retractor was placed in the quadrilateral surface at the level of the ischial spine to facilitate exposure. However, during the pubic and the supra-acetabular osteotomy there was more than 50% reduction in amplitude of SSEPs. In both instances, SSEPs returned to baseline with reducing the tension on the retractor and changing the position of the leg to slight flexion in neutral adduction/abduction (Fig. 4).
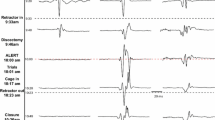
TcMEPs during left hip PAO in a 17-year-old female demonstrates a reduction of TcMEPs from baseline during the posterior column osteotomy and full recovery after adjustment of hip positioning and removal of Hohmann retractor from the sciatic notch and placement at the quadrilateral surface (black arrow). The impact of blood loss and relatively low arterial blood pressure is shown (*) with full recovery of the TcMEPs after autologous transfusion and final fixation
Neuromonitoring during PAO on a 13-year-old female shows decrease of amplitude of SSEPs during the pubic osteotomy and supra-acetabular osteotomy related to placement of Hohmann retractor and tension associated with hip position. There is complete restoration of SSEPs to baseline after appropriate hip and retractor repositioning
At the end of the procedure, TcMEPs and SSEPs were at baseline and there was no abnormal pattern on EMG in all patients. Postoperatively, at two, six, 12 weeks, and six and 12 months no motor weakness or sensory deficits on the deep and superficial peroneal and tibial nerve distribution were seen in any of these patients.
Discussion
Transitory or permanent sciatic nerve injury is a rare but major complication following PAO [9]. In this study, we investigated the role of a multimodal technique for monitoring the sciatic nerve in a consecutive series of PAOs. We found a 15% rate of abnormal nerve findings associated with hip position in flexion, adduction, and internal rotation, placement of Hohmann retractors with excessive tension and in close proximity to the sciatic notch, and with blood loss. All nerve changes resolved after a pause in the procedure followed by identification and correction of the underlying cause.
We acknowledge several limitations of this study. First, we report on a relatively small sample of 40 hips that may be too small to determine if it would be possible to reliably identify a nerve injury that has been reported to occur in only 2% of all PAO [9]. However, based on the fact that this study describes the first patients undergoing PAO by a young hip preservation surgeon, we would have expected to have some cases of nerve dysfunction based on the incidence reported in studies describing similar surgeons’ learning curve [5, 6]. Second, it is not clear from our findings whether the cases that had neuromonitoring changes would have developed clinical nerve palsy if the actions taken to correct those changes had not taken place. However, neuromonitoring allowed for valuable information about the surgical steps of PAO that place the sciatic nerve at risk of injury and for corrective actions including repositioning of the limb, adjustments of retractors away from the sciatic notch and control of blood loss and blood pressure. We do not have enough data to support routine use of neuromonitoring during PAO but we believe that it gives helpful information especially to the novice surgeon. In addition, it may be helpful in difficult cases with history of multiple previous hip surgeries. Although we showed the ability of multimodal monitoring to identify surgical steps that place the nerve under the risk of injury, further studies using larger number of patients would be necessary to test whether multimodal monitoring would allow for prevention of nerve injury during PAO. Finally, multimodal neuromonitoring has its own limitations. It requires general anesthesia and extensive communication with the anesthesiology team to avoid interference with the readings. In our study we found one case in which communication was broken and the patient received an epidural test dose with local anesthetic, which eliminates the possibility of accurate monitoring. The success of neuromonitoring depends further on the availability of an experienced neurophysiology team that is familiar with PAO surgical steps.
To the best of our knowledge only two previous studies have evaluated the neuromonitoring during PAO [14, 16]. Pring et al. [16] reported a study of 127 patients who underwent PAO with nerve monitoring using continuous EMG. Neurotonic discharges were recorded in 36 (26%) of the patients, which is a higher occurrence rate compared to the present study. Seven patients had various degrees of sciatic nerve dysfunction after surgery. Intra-operative EMG identified abnormal neurotonic discharges in 5/7 patients but in the remaining two patients no EMG abnormality was detected. The authors concluded that EMG monitoring contributed to the lower incidence of nerve injury by allowing the surgeon to take quick action when a neurotonic discharge was identified. However, EMG did not identify all nerve irritation and did not allow for prevention of nerve dysfunction in all patients. We believe that monitoring of the sciatic nerve should include SSPEs and TcMEPs in addition to EMG as major nerve trauma, including a direct laceration, may not produce neurotonic discharges on EMG [22]. Exclusive use of SSEPs should also be discouraged because it only effectively evaluates the sensory pathway and may not reflect the status of motor fibres within the nerve. Adding TcMEPs to the monitoring protocol allows for direct assessment of the motor function pathway and due to its high sensitivity, false negatives are very infrequent. In addition, TcMEPs deterioration occurs before and even without SSEPs changes, which allows for earlier recognition and action to prevent a nerve injury.
In a more recent study, Sutter et al. [14] reported on 18 patients who underwent PAO with multimodal nerve monitoring that included EMG, SSEPs, and TcMEPs. In 11 (61%) patients there were intra-operative alerts given to the surgeon following deterioration of the neurophysiological parameters. In 4/11 alerts, the surgical action associated with the alert was positioning of the leg; 3/11 alerts were given at time of mobilization of the acetabular fragment; in 4/11 cases the alert was related to an osteotomy being performed. In most cases the neurophysiological parameters returned to baseline, however, one patient with acetabular dysplasia and previous femoral osteotomy experienced neurological deficit while the hip was in flexion and internal rotation. We also noted a double occurrence of SSEPs drop in one patient with previous femoral osteotomy. Although we reported a lower occurrence of sciatic nerve event in this study compared to the findings by Sutter et al. [14], our findings showed similar intra-operative steps as a cause of nerve alert. Positioning of the leg in flexion, adduction, and internal rotation and placement of retractors too close or into the sciatic notch were the main reasons for nerve alert in our study. Further investigation is warranted to determine whether patients with previous femoral osteotomy are at higher risk of sciatic nerve dysfunction after PAO.
An experimental study showed that nerve injuries occur if a nerve is elongated by > 6% of its length [23]. Sciatic nerve injury may also have been induced by ischemia secondary to traction. Ogata demonstrated that the average stretching of more than 15.7% caused complete arrest of blood flow in the stretching nerve [24]. Nerve tissue ischemia may be potentially aggravated by blood loss that is associated with PAO. In fact, we found two nerve-monitoring changes after blood loss exceeded 2000 ml associated with arterial hypotension. In both cases the nerve function returned to baseline after blood transfusion and reestablished normal arterial blood pressure. One change in neuromonitoring baseline occurred during the incomplete ischial cut. Return to baseline occurred with repositioning of the hip. This finding is in line with a previous investigation of the effect of hip position on the location of the sciatic nerve using MRI in which the nerve was found to move away from the infracotyloid groove to an average of 20 mm with the hip flexed at 30-45°, abducted and externally rotated. In a cadaveric study [25], the sciatic nerve was put under greater tension and risk for laceration during the first ischial cut as the hip was flexed. Reducing hip flexion resulted in relaxation of the sciatic nerve that was further improved by abduction. The authors showed that the short external rotators of the hip are a barrier protecting the nerve during the first incomplete ischial cut and the posterior column osteotomy. It may be that our neuromonitoring findings reflect not a direct contact of chisel to the nerve but rather a proximity to the nerve with protection of this soft tissue, as we had no cases of definitive nerve damage. These previous reports and the findings of this study support positioning of the hip with minimal flexion to avoid tension in the anterior soft tissue envelope and slight abduction to reduce tension of the nerve during the first incomplete ischial osteotomy and at the lower end of the posterior column osteotomy at the ilium-ischial junction (Fig. 5a and b).
a Schematic illustration showing the lateral portion of the first ischial osteotomy. Note the proximity of the sciatic nerve and the direction of the chisel toward the nerve. During this part of the osteotomy, the hip should not exceed 30° of flexion and be positioned in slight abduction and external rotation. The chisel should aim medially pointing to the opposite shoulder. b Schematic illustration of the final step of the posterior column osteotomy. Note the chisel is placed at the corner between the posterior column and incomplete ischial osteotomy. The chisel points laterally toward the nerve and during this osteotomy; there should be no tension on retractors placed in the quadrilateral surface and the hip should be slightly flexed, abducted, and externally rotated to avoid tension on the sciatic nerve. Ilustrations by Nathan Billington - Childrens Hospital Colorado Medical Animation and Ilustration Surgeon in Chief Program
In our preliminary experience, neuromonitoring has allowed for identification of surgical steps and maneuvers that place the sciatic nerve at higher risk of injury and for corrective actions to take place. As we gained more experience with PAO, we were able to incorporate this knowledge to our surgical routine. We currently do not exceed more than 30° of hip flexion during the ischial osteotomy and abduct the hip slightly when cutting the lateral cortex of the ischium. Aiming the chisel medially also reduces the risk of sciatic nerve injury during the incomplete ischial cut [12, 24]. We currently start the posterior column osteotomy with a radiolucent reverse Hohmann placed at quadrilateral surface distal to the sciatic notch. Once we have confirmed the appropriate path of the chisel using fluoroscopy, we remove the retractor and continue with the posterior column osteotomy with palpation of the medial tip of the chisel to avoid violating the lateral cortex abruptly. Finally, to complete the distal corner of the posterior column osteotomy, at the ilium-ischial junction, we place the hip in slight flexion, abduction, and external rotation to avoid any tension of the sciatic nerve. Although we cannot recommend routine neuromonitoring for PAO, we suggest the use of the multimodal technique described in this study to control the sensory and motor pathways if neuromonitoring is chosen. Patients with multiple previous hip surgeries, including pelvic and femoral osteotomies, may have change in the native anatomy that could place the nerve under tension due to scar tissue and may benefit from nerve monitoring. However, future research is necessary to identify selective cases that could benefit from neuromonitoring by investigating patient-specific factors that may increase the risk of sciatic nerve injury during PAO and to determine whether neuromonitoring can improve PAO outcome by reducing the risk of nerve injury.
References
Ganz R, Klaue K, Vinh TS, Mast JW (1988) A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res 232:26–36
Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H (2008) Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop 32(5):611–617. doi:10.1007/s00264-007-0372-3
Clohisy JC, Nunley RM, Curry MC, Schoenecker PL (2007) Periacetabular osteotomy for the treatment of acetabular dysplasia associated with major aspherical femoral head deformities. J Bone Joint Surg Am 89(7):1417–1423. doi:10.2106/JBJS.F.00493
Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW (2009) Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res 467(8):2041–2052. doi:10.1007/s11999-009-0842-6
Crockarell J Jr, Trousdale RT, Cabanela ME, Berry DJ (1999) Early experience and results with the periacetabular osteotomy. The Mayo Clinic experience Clin Orthop Relat Res 363:45–53
Davey JP, Santore RF (1999) Complications of periacetabular osteotomy. Clin Orthop Relat Res 363:33–37
Peters CL, Erickson JA, Hines JL (2006) Early results of the Bernese periacetabular osteotomy: the learning curve at an academic medical center. J Bone Joint Surg Am 88(9):1920–1926. doi:10.2106/JBJS.E.00515
Zaltz I, Baca G, Kim YJ, Schoenecker P, Trousdale R, Sierra R, Sucato D, Sink E, Beaule P, Millis MB, Podeszwa D, Clohisy JC (2014) Complications associated with the periacetabular osteotomy: a prospective multicenter study. J Bone Joint Surg Am 96(23):1967–1974. doi:10.2106/JBJS.N.00113
Sierra RJ, Beaule P, Zaltz I, Millis MB, Clohisy JC, Trousdale RT, group A (2012) Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res 470(8):2209–2219. doi:10.1007/s11999-012-2409-1
Haidukewych GJ, Scaduto J, Herscovici D Jr, Sanders RW, DiPasquale T (2002) Iatrogenic nerve injury in acetabular fracture surgery: a comparison of monitored and unmonitored procedures. J Orthop Trauma 16(5):297–301
Helfet DL, Anand N, Malkani AL, Heise C, Quinn TJ, Green DS, Burga S (1997) Intraoperative monitoring of motor pathways during operative fixation of acute acetabular fractures. J Orthop Trauma 11(1):2–6
Brown DM, McGinnis WC, Mesghali H (2002) Neurophysiologic intraoperative monitoring during revision total hip arthroplasty. J Bone Joint Surg Am 84-A(Suppl 2):56–61
Fee D, Sabet A (2008) Monitoring during total hip arthroplasty. Intraoperative monitoring of neural function. Elsevier, Amsterdam
Sutter M, Hersche O, Leunig M, Guggi T, Dvorak J, Eggspuehler A (2012) Use of multimodal intra-operative monitoring in averting nerve injury during complex hip surgery. J Bone Joint Surg Br 94(2):179–184. doi:10.1302/0301-620X.94B2.28019
Vitale MG, Skaggs DL, Pace GI, Wright ML, Matsumoto H, Anderson RCE, Brockmeyer DL, Dormans JP, Emans JB, Erickson MA, Flynn JM, Glotzbecker MP, Ibrahim KN, Lewis SJ, Luhmann SJ, Mendiratta A, Richards BS III, Sanders JO, Shah SA, Smith JT, Song KM, Sponseller PD, Sucato DJ, Roye DP, Lenke LG (2014) Best practices in intraoperative neuromonitoring in spine deformity surgery: development of an intraoperative checklist to optimize response. Spine Deformity 2(5):333–339. doi:10.1016/j.jspd.2014.05.003
Pring ME, Trousdale RT, Cabanela ME, Harper CM (2002) Intraoperative electromyographic monitoring during periacetabular osteotomy. Clin Orthop Relat Res 400:158–164
Toleikis JR, American Society of Neurophysiological M (2005) Intraoperative monitoring using somatosensory evoked potentials. A position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput 19(3):241–258. doi:10.1007/s10877-005-4397-0
Francis L, Mohamed M, Patino M, McAuliffe J (2012) Intraoperative neuromonitoring in pediatric surgery. Int Anesthesiol Clin 50(4):130–143. doi:10.1097/AIA.0b013e31826f32ae
Sloan TB, Toleikis JR, Toleikis SC, Koht A (2015) Intraoperative neurophysiological monitoring during spine surgery with total intravenous anesthesia or balanced anesthesia with 3% desflurane. J Clin Monit Comput 29(1):77–85. doi:10.1007/s10877-014-9571-9
Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M (2010) Intermediate to long-term results following the bernese periacetabular osteotomy and predictors of clinical outcome: surgical technique. J Bone Joint Surg Am 92(Suppl 1 Pt 2):115–129. doi:10.2106/JBJS.J.00646
Macdonald DB, Skinner S, Shils J, Yingling C, American Society of Neurophysiological M (2013) Intraoperative motor evoked potential monitoring—a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol: Off J Int Fed Clin Neurophysiol 124(12):2291–2316. doi:10.1016/j.clinph.2013.07.025
Nelson KR, Vasconez HC (1995) Nerve transection without neurotonic discharges during intraoperative electromyographic monitoring. Muscle Nerve 18(2):236–238. doi:10.1002/mus.880180215
Lewallen DG (1998) Neurovascular injury associated with hip arthroplasty. Instr Course Lect 47:275–283
Ogata K, Naito M (1986) Blood flow of peripheral nerve effects of dissection, stretching and compression. J Hand Surg 11(1):10–14
Kalhor M, Gharehdaghi J, Schoeniger R, Ganz R (2015) Reducing the risk of nerve injury during Bernese periacetabular osteotomy: a cadaveric study. Bone Joint J 97-B(5):636–641. doi:10.1302/0301-620X.97B5.35084
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No source of funding was used in this study. Eduardo Novais, Travis Heare, Lauryn Kestel, Patricia Oliver, Willy Boucharel, Jason Koerner, and Kim Strupp declare they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Novais, E.N., Heare, T., Kestel, L. et al. Multimodal nerve monitoring during periacetabular osteotomy identifies surgical steps associated with risk of injury. International Orthopaedics (SICOT) 41, 1543–1551 (2017). https://doi.org/10.1007/s00264-016-3394-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-016-3394-x