Abstract
Purpose
Augmented reality (AR) enables superimposition of virtual images onto the real world. The aim of this study is to present a novel AR-based navigation system for sacroiliac screw insertion and to evaluate its feasibility and accuracy in cadaveric experiments.
Methods
Six cadavers with intact pelvises were employed in our study. They were CT scanned and the pelvis and vessels were segmented into 3D models. The ideal trajectory of the sacroiliac screw was planned and represented visually as a cylinder. For the intervention, the head mounted display created a real-time AR environment by superimposing the virtual 3D models onto the surgeon’s field of view. The screws were drilled into the pelvis as guided by the trajectory represented by the cylinder. Following the intervention, a repeat CT scan was performed to evaluate the accuracy of the system, by assessing the screw positions and the deviations between the planned trajectories and inserted screws.
Results
Post-operative CT images showed that all 12 screws were correctly placed with no perforation. The mean deviation between the planned trajectories and the inserted screws was 2.7 ± 1.2 mm at the bony entry point, 3.7 ± 1.1 mm at the screw tip, and the mean angular deviation between the two trajectories was 2.9° ± 1.1°. The mean deviation at the nerve root tunnels region on the sagittal plane was 3.6 ± 1.0 mm.
Conclusions
This study suggests an intuitive approach for guiding screw placement by way of AR-based navigation. This approach was feasible and accurate. It may serve as a valuable tool for assisting percutaneous sacroiliac screw insertion in live surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous sacroiliac screw fixation is a generally accepted method for the treatment of instability of the pelvis posterior ring [1]. Compared with open reduction and internal fixation techniques, percutaneous sacroiliac screwing is much less invasive with a lower incidence of post-operative wound infection [2]. The traditional method of achieving correct screw placement is to insert it under fluoroscopic guidance. However, multiple views are required, including anteroposterior, lateral, inlet and outlet views, with the surgeon and patient exposed to excessive radiation [3]. Moreover, there are inherent errors in fluoroscopic imaging due to factors such as obesity and bowel gas, and these are compounded when images are obtained sequentially in order to cover all four planes (anteroposterior, lateral, inlet and outlet views) [4]. The rates of screw malpositioning are high, with incidences ranging from 2 to 15 % according to previous reports [5, 6]. Incorrect placement of the sacroiliac screw may cause critical complications, including perforation of the sacral canal, neuroforamina, and iliac vessels [7]. Over the past few decades, various navigation systems were developed capable of facilitating sacroiliac screw insertion, such as two-dimensional (2D) fluoroscopy [8, 9] and three-dimensional (3D) fluoroscopy [7, 9, 10], CT-based [11], and CT-3D-fluoroscopy navigation systems [12]. Compared with traditional techniques, the use of these navigation systems led to an improvement over the number of screw outliers and less radiation exposure.
However, the drawback of current image-based navigation systems is that they rely on external monitors for visualisation of navigation data. The surgeon is required to constantly shift his view between the surgical site and the monitor while simultaneously manoeuvring surgical tools. There is a steep learning curve with new surgeons having to adapt to these unfamiliar hand-eye coordination settings, and on top of that, distraction and loss of concentration may have a negative bearing on the surgical outcome [13]. Hence, these navigation systems are still not perfect and require further refinement.
In recent times, innovation and the rapid development of computing technology led to the creation of augmented reality (AR) systems. In present day AR, a head-mounted display (HMD) is worn, and virtual images are overlaid onto the wearer’s field of view. The wearer sees virtual images that are updated in real time, in synchrony with head movements and those of surrounding objects. Core internal structures can also be visualized. As the wearer has no need to learn new hand-eye co-ordination skills, the benefit of an AR system can easily be appreciated.
Based on these advantages, AR is becoming a promising technology in medical applications and has already been trialed in various settings, including neurosurgery [14], laparoscopic surgery [15], oral and maxillofacial surgery [13, 16, 17], and spine surgery [18]. However the application of AR assisted sacroiliac screw insertion is not yet studied. Hence, the aim of our study was to evaluate the feasibility and accuracy of AR assisted sacroiliac screw insertion, using cadavers for a pilot study.
Materials and methods
Six fresh frozen cadaveric specimens (mean age = 55, range 38–69) with intact pelvises and no obvious deformity or pathology on CT images were enrolled in the study after approval by the institutional ethics committee. Twelve drillings were performed under AR navigation with one screw inserted into each side of the sacroiliac joint, aiming towards the S1 vertebra. All drillings were performed by an experienced orthopaedic surgeon. Before the experiment, he was allowed to acclimatize to the AR navigation system until he was at ease with it.
AR navigation system
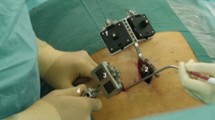
The hardware architecture consisted of an optical see-through HMD (nVisor ST60, NVIS, USA), an optical tracking system (Polaris Spectra, NDI, Canada), a high-performance graphical workstation (HP, USA), and an LCD monitor (G2200W, BenQ, Taiwan) (Fig. 1). The HMD with high resolution micro displays (1280 × 1024 24-bit colour pixels per eye) was connected to the workstation and provided the surgeon superimposed virtual images onto the real world in real time. The Polaris Spectra captured the position of infrared-reflecting (IR) markers attached to reference of frames fixed onto the various movable objects such as the drill, pelvis and HMD.
The AR navigation system was programmed in Microsoft Visual C++ under the platform of the Integrated Development Environment (IDE) VS2008. Several well-known open source toolkits were employed (such as VTK, CTK, ITK, QT et al.).
Data acquisition and pre-operative plan
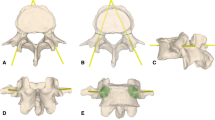
Five tiny titanium fiducial screws were pre-operatively fixed along the iliac crest on the side of the surgical region through small skin incisions as landmarks. Also barium sulfate suspension (50 % W/V, 600 ml) was injected into pelvis vessels through the aortaventralis. CT scans of the pelvises were performed three to five days later (GE Light speed 64 CT machine, USA). The acquired CT data was saved in DICOM (Digital Imaging and Communications in Medicine) format and then imported into the planning software Mimics (Materialise, Belgium). 3D models of the pelvis and vessels were reconstructed by way of selecting the appropriate Hounsfield units, some manual editing, and an automation function for segmentation called “region growing”. Following this, virtual planning of the ideal sacroiliac screw trajectory was performed, with it passing midway within the cortical margins, through the sacroiliac joint towards the S1 vertebra. The planned needle trajectory was then represented as a cylinder (Fig. 2).
Experiment setup
Calibration of surgical instruments
Three rigid reference frames were secured to the bony pelvis, surgical drill and the HMD. Calibration of the drill was performed first, in such a way that the spatial relationship between the drill and the reference frame fixed to it was registered, allowing movement of the reference frame to represent the drill. Consequently, movement of the drill could be visualized in real time using the navigation system.
Registration
The next process was registration of the virtual co-ordinate system to that of the cadaver co-ordinate system (as represented by the reference frame fixed to it). The registration was performed using the point-to-point registration method. The co-ordinates of fiducial landmarks in the virtual co-ordinate system were obtained through an image recognition technique, while their co-ordinates under the cadaver co-ordinate system were acquired by using a positioning probe. Then the system accomplished the registration process using the SVD algorithm which is based on the obtained co-ordinates of the homologous fiducial landmarks.
Calibration of the HMD
Following registration, the virtual images on the HMD were then matched with the surgical site. The next step was calibration of the HMD in relation to the position of the pelvis. This calibration was done by way of a transformation matrix once the two reference frames were affixed to the cadaver and HMD [19]. When this was done, the position and orientation of the virtual images on the HMD corresponded to that of the real world, in perfect synchrony with movement of the surgeon’s head, and that of the cadaver.
Experiment procedure
With the HMD, 3D models of the needle, the planned trajectory, pelvis bones and surrounding vessels were displayed as an overlay onto the surgical scene in real time. The surgeon could select which virtual models to see or hide. Under guidance of the navigation system, the surgeon inserted a 3 mm-diameter needle by matching its entry point, axis and tip with the planned trajectory while avoiding the vessels (Fig. 3). Then a cannulated 6.5-mm-diameter partial threaded screw (Synthes, Switzerland) was inserted. The times required for the experiment setup and procedure were also recorded.
Post-operative analysis of accuracy
Post-operative CT scans of the pelvises were performed and the data was imported into Mimics. 3D models of the inserted screws and pelvises were reconstructed. Analysis of screw positions on the CT images were performed by two senior orthopaedic surgeons. Screw localisation was graded according to a validated classification method for correct pedicle screw placement [20]: grade 0: no perforation, grade 1: perforation < 2 mm, grade 2: perforation between 2 and 4 mm, and grade 3: perforation > 4 mm.
The pre-operative and post-operative CT datasets were matched based on the position of the fiducial screws. The planned trajectories were then superimposed on the post-operative CT images in order to evaluate the accuracy of the AR navigation system. The distance between the centres of the planned trajectory and the inserted screw at the level of the nerve root tunnel region on the sagittal plane was measured (Fig. 4). Following that, 3D models of the screw and the planned trajectory were imported into 3-matic software (Materialise, Belgium). The distances between the planned and actual bony entry points, tips, as well as angular deviation were measured (Fig. 5).
Results
Percutaneous sacroiliac screw insertion using the AR navigation system was successfully completed in all 12 cadaver trials. Post-operative CT images showed all 12 screws were correctly placed with no perforation (grade: 0). There was no disagreement between the two raters regarding the screw perforation grade (100 % agreement).
The mean deviation between the planned and the inserted screw positions was 2.7 ± 1.2 mm (range 1.3–5.5) at the bony entry point of the screw and 3.7 ± 1.1 mm (range 1.1–5.2) at the tip of the screw. The mean angular deviation was 2.9° ± 1.1° (range 1.6–4.8°). The mean distance between the centres of the planned trajectory and the inserted screw at the level of the nerve root tunnel region on the sagittal plane was 3.6 ± 1.0 mm (range 1.4–4.7). The mean time required for the experiment setup was 13.6 ± 2.2 mins (SD) and for the experiment procedure 11.1 ± 2.0 (SD) mins (Table 1).
Discussion
Sacroiliac screw placement is a difficult procedure requiring a high degree of precision and a narrow margin of error. Various computer navigation systems were developed over the years and positive results were reported [7, 21, 22]. However, in these systems, the surgeon needs to adapt to a new hand-eye co-ordinate system, combining the visual frame of reference with that of the navigation system as images are displayed on external monitors. S/he is also required to alternate attention between the monitor and the surgical site during the navigation task. Consequently, many surgeons find it difficult to precisely correlate the images on the monitor to the operative site. AR navigation aims to address all of these problems and shorten the learning curve. Additionally, the perforation of adjacent vessels is a major complication in sacroiliac screw insertion [23]. AR allows visualisation of internal deep-tissue structures during the surgery, which is of great significance in minimally invasive procedures. In our study, we managed to do this, with clear visualization of the surrounding vessels during cadaveric surgery. So the surgeon could precisely drill the needle into the sacroiliac joint while avoiding the adjacent vessels.
There are generally two types of AR navigation hardware available today, “optical see-through” and “video see-through” [24]. Optical see-through HMDs project virtual images on a transparent screen, which allows the wearer to see the outside world unhindered. Video see-through HMDs are not transparent, so the real world has to be captured by a video camera, while the virtual images are merged along with the video feed. Optical see-through has the advantage of allowing the wearer to have a real-world view through the transparent display, which allows native hand-eye coordination and proprioception of the surrounding objects. Video see-through systems have been trialed in spinal, oral and maxillofacial surgeries [13, 16, 18]. However the quality and reproducibility of images captured by a camera cannot be comparable to direct human vision, and for this reason we believe that optical see-through HMDs are superior.
Vigh et al. reported the accuracy of a video see-through HMD-based AR navigation system for oral implantology, with a mean distance error of 1.24 mm at the entry point, 2.68 mm at the end point and mean angular deviation of 4.68° between the planned implant and drilled borehole [13]. In our study, the mean distance error was 2.7 mm at entry point, 3.7 mm at the end point and the mean angular deviation was 2.9°.
In addition, checking the screw position near the nerve root tunnel on the sagittal plane perpendicular to the screw axis is considered to be the most important since the safe bony corridor for the sacroiliac screw gets narrowest at the area around the nerve root tunnels [12]. Our results showed a mean deviation of 3.6 mm and maximum of 4.7 mm around the nerve root tunnels with no screw perforation, which is lower in accuracy compared with a CT-3D-fluoroscopy navigation study by Takao et al. (mean deviation of 1.8 mm) [12]. We presume our level of accuracy is acceptable assuming the preoperative planned trajectory is kept more than 5 mm away from the outer cortical margin on the sagittal plane near the nerve root tunnel area. However future studies will be needed to assess the accuracy of improved AR navigation systems with current navigation systems for sacroiliac screw placement, preferably in a randomised controlled trial setting.
The overall deviation is dependent on the compounding of errors produced from the very start, during CT data acquisition, right through to the surgical procedure. From our experience, the following measures could be taken in order to further improve the accuracy. Firstly, the software could be improved further to decrease the inherent error of the system; point-to-point registration combined with surface registration methods could be applied for better registration accuracy; the number of fiducial markers could be increased, while avoiding near-collinear configurations and ensuring their centroids to be as near as possible to the target; surgeons could familiarise themselves more with the AR system before the procedure; and a needle with more rigidity and yield strength could be used to avoid bending during the drilling procedure.
Our study is not without limitations. The first limitation is the limited number of cases in our study. Therefore future studies with larger sample sizes are required to further assess the approach. However, despite the small sample size, we believe the positive results from our pilot study are encouraging and may spur future studies of AR navigation in orthopaedic surgery. The second limitation is that we have not employed sacroiliac joint dislocation/fracture models in our study. Thus our preliminary results are only applicable in non or minimally displaced sacroiliac lesions or after stable closed reduction of the sacroiliac joint. The feasibility of AR navigation in sacroiliac joint dislocation/fracture and the effect of the reduction process have not been evaluated in our pilot study, but will be in our future study. The third limitation is that blood vessels could shift or deform under external forces during surgery, leading to false portrayal of their locations [25]. The critical vessels in danger of perforation during percutaneous sacroiliac insertion are branches of the internal iliac artery (superior gluteal artery, etc.); however, the spatial relation of these deep vessels to the pelvic bones are relatively stable. Thus they are less susceptible to deformation after stable reduction of the sacroiliac joint is achieved. As the pelvises in our study have no known instability, we consider deformation of critical blood vessels as negligible. Nonetheless, in order to address the issue of potential vessel deformation during the surgical reduction process, we are working on a non-rigid registration method to allow for simulation of such deformations in real-time, for implementation into our AR navigation system in the future. The fourth limitation of our prototype setup is a slight latency during movement of the surgeon’s head. This could potentially be improved using faster computer systems for image data processing. The final limitation is the surgeon’s adaptability to our wired and heavy prototype headgear, which may result in fatigue and posture-related injuries during the surgery. However, new hardware is constantly being developed, such as the lighter wireless Google Glass and Microsoft Hololens.
In conclusion, we developed and tested a novel prototype AR navigation system for percutaneous sacroiliac screw insertion in a pilot cadaveric study. Our optical see-through setup allows superimposition of virtual 3D models of the planned screw trajectory along with internal anatomical structures, directly onto the surgeon’s field of view, in synchronisation with head movements. Our results demonstrated that the approach was feasible and accurate for guiding sacroiliac screw placement. Future studies are necessary to evaluate the application of this technology in pathological models, e.g., sacroiliac dislocation/fracture.
References
Shuler TE, Boone DC, Gruen GS, Peitzman AB (1995) Percutaneous iliosacral screw fixation: early treatment for unstable posterior pelvic ring disruptions. J Trauma 38:453–458
Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA (1995) Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma 9:207–214
Yu X, Tang M, Zhou Z, Peng X, Wu T, Sun Y (2013) Minimally invasive treatment for pubic ramus fractures combined with a sacroiliac joint complex injury. Int Orthop 37:1547–1554. doi:10.1007/s00264-013-1954-x
Peng KT, Li YY, Hsu WH, Wu MH, Yang JT, Hsu CH, Huang TJ (2013) Intraoperative computed tomography with integrated navigation in percutaneous iliosacral screwing. Injury 44:203–208. doi:10.1016/j.injury.2012.09.017
Hinsche AF, Giannoudis PV, Smith RM (2002) Fluoroscopy-based multiplanar image guidance for insertion of sacroiliac screws. Clin Orthop Relat Res 135–144
Templeman D, Schmidt A, Freese J, Weisman I (1996) Proximity of iliosacral screws to neurovascular structures after internal fixation. Clin Orthop Relat Res 194–198
Xu P, Wang H, Liu ZY, Mu WD, Xu SH, Wang LB, Chen C, Cavanaugh JM (2013) An evaluation of three-dimensional image-guided technologies in percutaneous pelvic and acetabular lag screw placement. J Surg Res 185:338–346. doi:10.1016/j.jss.2013.05.074
Grossterlinden L, Rueger J, Catala-Lehnen P, Rupprecht M, Lehmann W, Rucker A, Briem D (2011) Factors influencing the accuracy of iliosacral screw placement in trauma patients. Int Orthop 35:1391–1396. doi:10.1007/s00264-010-1092-7
Gras F, Marintschev I, Klos K, Muckley T, Hofmann GO, Kahler DM (2012) Screw placement for acetabular fractures: which navigation modality (2-dimensional vs. 3-dimensional) should be used? An experimental study. J Orthop Trauma 26:466–473. doi:10.1097/Bot.0b013e318234d443
Wahnert D, Raschke MJ, Fuchs T (2013) Cement augmentation of the navigated iliosacral screw in the treatment of insufficiency fractures of the sacrum. A new method using modified implants. Int Orthop 37:1147–1150. doi:10.1007/s00264-013-1875-8
Arand M, Kinzl L, Gebhard F (2004) Computer-guidance in percutaneous screw stabilization of the iliosacral joint. Clin Orthop Relat Res 201–207. doi:10.1097/01.blo.0000128644.46013.08
Takao M, Nishii T, Sakai T, Yoshikawa H, Sugano N (2014) Iliosacral screw insertion using CT-3D-fluoroscopy matching navigation. Inj Int J Care Inj 45:988–994. doi:10.1016/j.injury.2014.01.015
Vigh B, Muller S, Ristow O, Deppe H, Holdstock S, den Hollander J, Navab N, Steiner T, Hohlweg-Majert B (2014) The use of a head-mounted display in oral implantology: a feasibility study. Int J Comput Assist Radiol Surg 9:71–78. doi:10.1007/s11548-013-0912-9
Besharati Tabrizi L, Mahvash M (2015) Augmented reality-guided neurosurgery: accuracy and intraoperative application of an image projection technique. J Neurosurg 1–6. doi:10.3171/2014.9.JNS141001
Kang X, Azizian M, Wilson E, Wu K, Martin AD, Kane TD, Peters CA, Cleary K, Shekhar R (2014) Stereoscopic augmented reality for laparoscopic surgery. Surg Endosc 28:2227–2235. doi:10.1007/s00464-014-3433-x
Qu M, Hou Y, Xu Y, Shen C, Zhu M, Xie L, Wang H, Zhang Y, Chai G (2015) Precise positioning of an intraoral distractor using augmented reality in patients with hemifacial microsomia. J Cranio-Maxillofac Surg: Off Publ Eur Assoc Cranio-Maxillofac Surg 43:106–112. doi:10.1016/j.jcms.2014.10.019
Suenaga H, Hoang Tran H, Liao H, Masamune K, Dohi T, Hoshi K, Mori Y, Takato T (2013) Real-time in situ three-dimensional integral videography and surgical navigation using augmented reality: a pilot study. Int J Oral Sci 5:98–102. doi:10.1038/ijos.2013.26
Abe Y, Sato S, Kato K, Hyakumachi T, Yanagibashi Y, Ito M, Abumi K (2013) A novel 3D guidance system using augmented reality for percutaneous vertebroplasty: technical note. J Neurosurg Spine 19:492–501. doi:10.3171/2013.7.spine12917
Chen X, Xu L, Wang Y, Wang H, Wang F, Zeng X, Wang Q, Egger J (2015) Development of a surgical navigation system based on augmented reality using an optical see-through head-mounted display. J Biomed Inform 55:124–131. doi:10.1016/j.jbi.2015.04.003
Smith HE, Yuan PS, Sasso R, Papadopolous S, Vaccaro AR (2006) An evaluation of image-guided technologies in the placement of percutaneous iliosacral screws. Spine 31:234–238
Wong JM, Bewsher S, Yew J, Bucknill A, de Steiger R (2015) Fluoroscopically assisted computer navigation enables accurate percutaneous screw placement for pelvic and acetabular fracture fixation. Injury 46:1064–1068. doi:10.1016/j.injury.2015.01.038
Zwingmann J, Konrad G, Kotter E, Sudkamp NP, Oberst M (2009) Computer-navigated iliosacral screw insertion reduces malposition rate and radiation exposure. Clin Orthop Relat Res 467:1833–1838. doi:10.1007/s11999-008-0632-6
Routt ML Jr, Simonian PT, Mills WJ (1997) Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma 11:584–589
Rolland JP, Fuchs H (2000) Optical versus video see-through mead-mounted displays in medical visualization. Presence Teleop Virt 9:287–309. doi:10.1162/105474600566808
Shuhaiber JH (2004) Augmented reality in surgery. Arch Surg 139:170–174. doi:10.1001/archsurg.139.2.170
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant numbers: 81272002, 81171429, and 81511130089), Biomedical Engineering Cross of Shanghai Jiao Tong University (Grant number: YG2012MS18) and the School of Medicine, Shanghai Jiao Tong University (Medical Education Grant).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Human and animal studies
This study did not engage in the use of animals or living persons.
Additional information
Huixiang Wang and Fang Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, H., Wang, F., Leong, A.P.Y. et al. Precision insertion of percutaneous sacroiliac screws using a novel augmented reality-based navigation system: a pilot study. International Orthopaedics (SICOT) 40, 1941–1947 (2016). https://doi.org/10.1007/s00264-015-3028-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-3028-8