Abstract
Purpose
Regenerex is a novel porous titanium construct with a three-dimensional porous structure and biomechanical characteristics close to that of normal trabecular bone. The aim of this study was to evaluate the adaptive bone remodeling of the proximal tibia after uncemented total knee arthroplasty (TKA) using a tibial tray with this novel coating compared to a well-proven standard porous coated (PPS) tibial tray.
Materials
Sixty patients scheduled for TKA were randomized to receive either a Regenerex (n = 31) or a PPS tibial component (n = 29). Changes in bone mineral density (BMD) of the proximal tibia were measured at three, six, 12 and 24 months by dual-energy X-ray absorptiometry (DEXA).
Results
In the lateral region (ROI 3), a significant increase in BMD was seen in both groups at three, six, and 12 months after surgery. The relative increase at 12 months was 8.1 % (P = 0.007) for the PPS group and 6.5 % (P = 0.002) for the Regenerex group. Positive values were retained at 24 months in both groups. At 24 months BMD in the distal region below the central stem (ROI 1) had decreased in the PPS group by 3.4 % (P = 0.005) and in the Regenerex group by 2.4 % (P = 0.17). In the medial region (ROI 2) BMD remained unchanged at all follow-up evaluations in both groups. There were no significant differences between the two groups (P = 0.45) in any ROI at any follow-up evaluation.
Conclusion
The significant increase in BMD of the lateral proximal tibia plateau with very limited changes medially and distally seen in both implants suggests that the novel porous titanium construct Regenerex and the PPS implant have a pronounced beneficial effect with regard to maintaining periprosthetic BMD in all regions of interest investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After total knee arthroplasty (TKA), adaptive bone remodeling of both the proximal tibia and distal femur occurs, and several studies have shown that this process results mainly in a reduction in bone mineral density (BMD) [1–5]. Stress shielding as a consequence of altered biomechanical loading at the bone–implant interface, bone reaction to surgical trauma, and immobilization of the extremity are considered to be the major causes of this bone loss [6–9]. A correlation between BMD and migration of the tibial component after cementless TKA has been reported, along with less continuous migration in knees with a high BMD [4, 10]. Newer techniques are currently being developed that are based on histologic evidence and clinical outcome because it is clear that porous surfaces support tissue ingrowth [11–14]. Uncemented implants rely on bone ingrowth and remodeling around the implant for secure fixation and long-term implant survival [12, 15]. Pores are necessary for bone tissue formation because they allow migration and proliferation of osteoblasts and mesenchymal cells, as well as vascularization [11, 13, 14]. In addition, a porous surface improves mechanical interlocking between the implant and the surrounding bone, providing mechanical stability.
Regenerex is a novel porous titanium construct imitating normal trabecular bone. It has large pore size and an interconnecting porous structure with biomechanical characteristics, such as compressive strength and elastic modulus that are very close to those of normal trabecular bone. It is believed that these characteristics will facilitate bone ingrowth and secure better fixation to the host bone, thus increasing implant survival.
We conducted a study to quantitatively measure the adaptive bone remodeling of the proximal tibia after TKA using dual energy X-ray absorptiometry (DEXA) and, using a prospective, randomized design, to evaluate the remodeling pattern induced after insertion of an uncemented tibial component coated with the Regenerex versus a porous plasma-sprayed (PPS) coated implant. We hypothesized that implant fixation with the use of the novel titanium construct would minimize the decrease in BMD usually seen after TKA. Less loss of BMD is expected because of the reported beneficial biocompatible characteristics of the material and the high potential for bone ingrowth that it offers. At the time of our study, no published DEXA data were available for Regenerex or PPS-coated tibial components.
Materials and methods
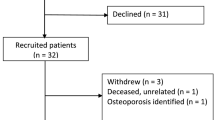
After giving their written and oral consent, 61 patients scheduled for uncemented TKA because of osteoarthritis were enrolled in our prospective, randomized study to receive one of two tibial components, each with a different coating (Table 1): Vanguard PPS and Vanguard Regenerex Primary Tibial Tray, both produced by Biomet (Warsaw, Indiana, USA) (Fig. 1). Initially, 66 patients (representing 61 procedures) who underwent surgery between September 1, 2010 and July 1, 2011, and who met the inclusion criteria, were offered to participate in the study. The procedures were performed at the Hørsholm Knee Clinic of Hillerød Hospital (n = 41) or at Gentofte Hospital (n = 20). Of the 66 patients, 64 consent ed; two refused to participate because of the requirement for time-consuming follow-up. Three patients were excluded for logistical reasons; they lived too far away to participate in follow-up. One patient who was allocated to receive a PPS implant was found during surgery to have a large bone defect and was excluded from the study. Randomization was performed with the use of closed envelopes (block randomization with ten in each block) opened in the operating room. Because one patient did not receive the allocated intervention, additional envelopes were prepared for randomization, which is how we ended up with 31 Regenerex implants and 29 PPS implants (Fig. 2).
One patient was lost to follow-up because of early infection and revision with soft-tissue debridement and removal of the polyethylene insert. Bacterial cultures produced negative findings. One patient sustained a periprostetic fracture above the femoral component, which was treated with open reduction and internal fixation. This patient was excluded from further DEXA analyses because of the known relationship between fracture and decrease in BMD [16]. For the same reason, the patient who sustained an ipsilateral proximal femur fracture was excluded from the study after three months, leaving 57 patients for further analysis (Fig. 2).
Conventional radiographs (standing and weight bearing) were obtained pre-operatively and post-operatively with the aim of evaluating knee alignment. All patients were evaluated using the Knee Society Score (KSS) [17] pre-operatively and at three, six, 12 and 24 months after surgery. Physiotherapy began on the day of surgery, and patients were mobilized with full weight bearing using crutches.
The study was approved by the Scientific Ethical Committee of Copenhagen (H-3-2009-007) and the Danish Data Protection Agency (J. nr. 2009-41-3737). The study was registered at ClinicalTrials.gov (NCT01936415).
The prostheses used are a part of the Vanguard total knee system (Fig. 1). The main difference between the implants is the titanium coating of the tibial tray. They both have a central stem, with the Regenerex implant having an additional four peripheral pegs. Both implants retain the posterior cruciate ligament, are modular with uncemented porous coated femoral components, and use cemented patellar resurfacing. The Regenerex implant has a porosity of 70–80 %, with an interconnecting pore structure and an average pore size of 300 μm. The PPS implant has a porous surface with a non-interconnecting pore structure of randomized size.
Measurement of bone mineral density
Measurements of BMD (g/cm²) were performed with DEXA, using a Norland XR-46 bone densitometer (Norland Corp., Fort Atkinson, WI, USA). Using the research scan option (scan speed of 45 mm/s), we performed scans in the coronal plane of the proximal tibia in close relation to the tibial component (pixel size, 0.5 × 0.5 mm) and bilaterally of the distal tibia and fibula, just above the ankle joint (pixel size, 0.5 × 0.5 mm). All scans were performed by the same laboratory technician with patients lying flat on their back, with the knee extended and with the ankle in a neutral position pointing straight up. Post-operative scans were performed within the first week after surgery and at follow-up examinations at three, six, 12, and 24 months after surgery. Custom-made software was used for analysis of the DEXA scans. The software allows measurements of BMD in close relation to orthopaedic implants, by exclusion of pixels considered by the software to be metal. The software allows a variable metal exclusion threshold to be set by the physician. On the computerized scan plots, three regions of interest (ROI) were selected for measurements of BMD of the proximal tibia: an area below the central stem (ROI 1), the medial tibial condyle (ROI 2), and the lateral tibial condyle (ROI 3) (Fig. 3). In the distal tibia and fibula, one ROI was selected at 1 cm above the joint line.
Bone mineral density (BMD) measurement in the proximal part of the tibia for the Regenerex group (a) and the porous plasma-sprayed implant (b). Region of interest (ROI) 1 is the distal region, ROI 2 is the medial region, and ROI 3 is the lateral region. The results of BMD measurements at 24 months are displayed for all three ROIs
Statistics and sample size calculation
In the study we intended to measure a significant difference in changes in BMD between the two groups of 7.5 % (minimal relevant difference). On the basis of findings in an earlier study by Petersen et al. [18] in which BMD was measured in the proximal tibia in patients with primary uncemented TKA, we estimated a SD (7.53 %) of the changes in BMD that could be used for sample size calculation in our study. The calculation (type I error = 5 %; type II error = 15) revealed that a sample size of 20 in each group would be required. To make allowance for dropouts, we decided to include 30 participants in each group. Analysis in both groups showed that the DEXA data were normally distributed. The unpaired t-test was used to assess differences between the two groups at three, six, 12 and 24 months after surgery. For comparison of changes in BMD over time in the two groups, we used the paired t-test. Comparison of clinical data between the groups was done using the Mann–Whitney U test. Statistical analyses were done using SPSS software (version 20.0; IBM, Armonk, NY, USA); P values < 0.05 were considered significant. The precision of BMD measurements was determined from double scans of the proximal tibia in ten patients. Double scans were done consecutively on the same day, with full repositioning of the patient and a break of five minutes between scans. The coefficient of variation (CV) was calculated to evaluate the precision of the BMD measurements in the various ROI:
Results
By the two-year follow-up examination, the knee score and function score for each study participant had improved significantly over preoperative scores. In the Regenerex group, the average knee score increased from 43 points (range, 22–68 points) to 95 points (range, 55–100 points; P = 0.001) and the function score improved from 52 points (range, 18–66 points) to 94 points (range, 50–100 points; P = 0.001). In the PPS group, the average knee score improved from 46 points (range, 22–68 points) to 93 points (range, 62–100 points; P = 0.002) and the function score improved from 50 points (range, 22–68 points) to 95 points (range, 40–100 points; P = 0.001). No significant differences were found between the two groups (Table 2). No significant change was seen between 12 and 24 months. There were no differences between the two groups in terms of preoperative demographic parameters or preoperative BMD (Table 1). For knees with pre-operative varus (n = 50) or valgus (n = 10) alignment, neutral or normal alignment (mean, 183°; range, 180–187°) was restored as a consequence of TKA.
Results of dual energy x-ray absorptiometry
The precision of BMD measurements expressed by CV was as follows: CVROI 1 = 2.3 % (range, 0.1–6.2 %), CVROI 2 = 1.3 % (range, 0.3–3.1 %), and CVROI 3 = 1.8 % (range, 0.2–4.5 %).
In the distal region below the central stem (ROI 1), there were only small and statistically insignificant variations in BMD during the first year after surgery in both groups. At 24 months after surgery, BMD had decreased in the PPS group by 3.4 % (P = 0.005) and in the Regenerex group by 2.4 % (P = 0.17) (Table 3, Fig. 4). In the medial region (ROI 2), BMD remained almost unchanged at all follow-up evaluations both in the Regenerex group and the PPS group (Table 3, Fig. 5) with a small increase. In the lateral region (ROI 3), a significant increase in BMD was seen in both groups at three, six, and 12 months after surgery. The relative increase at 12 months was 8.1 % (P = 0.007) for the PPS group and 6.5 % (P = 0.002) for the Regenerex group. Positive values by 5.5 % (P = 0.02) and 3.3 % (P = 0.09) in the PPS and Regenerex groups, respectively, were retained at 24 months, but remained only significant in the PPS group (Table 3, Fig. 6). There were no significant differences between the two groups (P = 0.45) in any ROI at any follow-up evaluation, and both groups showed very similar periprostetic bone remodeling (Table 3).
In both groups we observed an early and significant decrease in BMD in the distal tibia of operated legs within the first six months reaching 3.3 % (P = 0.0005) in the PPS group and 4.8 % (P = 0.007) in the Regenerex group. This decrease was not seen in contralateral legs (unpaired t-test; P = 0.01) and no further decrease was seen beyond six months in the operated legs. There were no significant differences between the two groups.
Discussion
At the time of our study, no published clinical DEXA data were available regarding prospective quantitative measurement of adaptive bone remodeling around the novel titanium construct or the PPS tibial component. Most previous studies have demonstrated a decrease in BMD in the proximal part of tibia after TKA [3, 5, 19, 20]. The decrease is most obvious in the first six months, with a magnitude as high as 23 % [21]. Li and Nilsson [19] reported a temporary decrease of 13 % during the initial three months after surgery. Levitz et al. [3] observed only small changes in BMD within the initial year after surgery but found a statistically significant decrease of 36.4 % at eight years. Hvid et al. [20] found an 11 % decrease in BMD at two years, whereas Petersen et al. [5] reported a statistically significant decrease of 22 % by three years after TKA.
In our study, the BMD in the lateral region (ROI 3) had increased significantly in both groups at three, six, and 12 months after surgery. At 24 months the increase was significant in the PPS group whereas the net increase was not significant in the Regenerex group. During the operation, alignment is restored, altering the biomechanical load from the medial to the lateral condyle (Table 2). An increased load stimulates bone formation [8]. Hvid et al. [20] measured trabecular bone remodeling of the proximal tibia after TKA with a cemented non-metal-backed tibial component in 18 patients (nine with arthritis and nine with rheumatoid arthritis). Measurements were performed within the first post-operative week and then two years after surgery. During the observation period, the mean bone density had decreased significantly in the tibial condyles that had a higher pre-operative load, whereas the density in the condyles with less pre-operative load was unchanged. Petersen et al. [18] randomized 18 patients to receive either tibial components coated with hydroxyapatite (HA) or components without an HA coating and found an increase in BMD of the lateral ROI of 6.1 % at two years; the presence of an HA coating had no effect on BMD. Petersen et al. [5] measured BMD in 25 uncemented TKAs and found that they had a small temporary increase in BMD of 2–7 % in the tibial condyles with increased load. We consider the adaptive bone remodeling pattern in these studies to be closely in agreement with our results, but the present study is without the often-described decrease in the post-operatively less-loaded tibial condyle.
It has been reported that BMD and migration of the tibia component after TKA are correlated. Less continuous migration of the tibial component has been observed in knees with a higher BMD [4, 10]. However, Regnér et al. [22] and Li and Nilsson [23] found no such relationship. Numerous studies using radiostereometric analysis have shown that the largest migration of uncemented tibial components in TKA occurs during the initial three months after surgery [24–26], a period that coincides with the observed early general loss in BMD. Ryd et al. [27] stated that the cause of late-detected aseptic loosening in TKA could be traced back to factors in the early post-operative period. The overall increase in peri-prosthetic BMD found in this study is believed to have a positive influence on the migration pattern for both prostheses. Not surprisingly did both implants show an initially significant decrease in BMD in the operated distal tibia as a consequence of immobilization and the surgical trauma. As the patients mobilized the loss of BMD eased off and no further decrease was seen beyond six months.
Even through there are no previous DEXA studies evaluating the changes in BMD of tibial components with the Biomet PPS coating, several studies have shown good long-term results using the uncemented Anatomic Graduated Component (AGC, Biomet, Warsaw) knee arthroplasty system. Ritter and Meneghini [28] reported findings for 73 uncemented TKAs (AGC) with 96.8 % survivorship at 20 years for the tibial component. Schrøder et al. [29] reported ten-year results for 114 AGC porous-plasma-coated TKAs with a success rate of 97.2 % of the tibial component. Only two tibial components in that study were revised because of aseptic loosening. Erikson et al. [30] found a 97 % survivorship at 20 years for AGC components. Meding et al. [31] retrospectively reviewed 2,321 PPS femoral stems and found stem survival at 15 years to be 99 %. The increase in BMD in our study supports these excellent clinical long-term results, indicating a well-integrated and well-fixated PPS tibial component.
The Regenerex group in our study had an overall increase in BMD and a significant increase in the lateral region at three, six, and 12 months and the net increases were, although not statistically significant, retained at 24 months. This is consistent with our hypothesis that the novel titanium construct would be able to minimize the expected post-operative decrease in BMD. A relatively new and similar construct made of the metal tantalum has been investigated as a trabecular metal-coated surface in uncemented TKA. This construct is also highly porous, with an interconnecting pore structure imitating that of normal trabecular bone. Two groups have reported BMD measurements in comparing an uncemented trabecular metal-coated tibial component with a cemented component. Both groups showed a significant decrease in BMD in the distal, the medial, and the lateral ROIs, but in the lateral ROI the trabecular implant showed a significantly smaller decrease in BMD [32]. At five years after surgery, this result was maintained [33].
In our study, the two trays differ not only in the materials involved. In addition, the Regenerex implant has an additional four small pegs underneath the baseplate, which theoretically could influence initial stability, and thereby bone ingrowth. Sumner et al. [34] evaluated the effects of pegs and screws on bone ingrowth in a canine study comparing three groups with different fixation of the tibial component, one with four pegs and screws versus a group without screws and a third group with screws only. Findings from that study indicated that pegs provided no added benefit in a circumstance where sufficient initial fixation was obtained with screws. Nilsson et al. [35] randomized 85 TKAs into three groups to evaluate the migration of the tibial component using radiostereometric analysis—one group with cemented implants and two groups with uncemented HA-coated implants. The migration between the two uncemented components, whether fixed with screws or fixed without screws, was identical, and there were no significant differences in external or internal rotation of the tibial components between the two groups. In our study, both implants have an identical 4-cm-long three-flanged central stem for fixation and initial stabilization. It is our belief that the additional four minor pegs added to the Regenerex baseplate do not provide any clinically relevant advantage in fixation or bone ingrowth.
Our findings suggest that the novel porous titanium construct and the PPS implant used as an ingrowth surface for tibial components in uncemented TKA has a beneficial effect with regard to maintaining peri-prosthetic BMD in the proximal tibia. In this study we compared a novel trabecular construct to a well-proven PPS coated component, which have shown excellent clinical results, and found that the Regenerex component did no better at 24 months of follow-up.
References
Bohr HH, Lund B (1987) Bone mineral density of the proximal tibia following uncemented arthroplasty. J Arthroplasty 2(4):309–312
Soininvaara TA, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM, Kröger HP (2004) Periprosthetic tibial bone mineral density changes after total knee arthroplasty: one-year follow-up study of 69 patients. Acta Orthop Scand 75(5):600–605
Levitz CL, Lotke PA, Karp JS (1995) Long-term changes in bone mineral density following total knee replacement. Clin Orthop Relat Res 321:68–72
Petersen MM, Nielsen PT, Lebech A, Toksvig-Larsen S, Lund B (1999) Preoperative bone mineral density of the proximal tibia and migration of the tibial component after uncemented total knee arthroplasty. J Arthroplasty 14(1):77–81
Petersen MM, Nielsen PT, Lauritzen JB, Lund B (1995) Changes in bone mineral density of the proximal tibia after uncemented total knee arthroplasty. A 3-year follow-up of 25 knees. Acta Orthop Scand 66(6):513–516
Donaldson CL, Hulley SB, Vogel JM, Hattner RS, Bayers JH, McMillan DE (1970) Effect of prolonged bed rest on bone mineral. Metabolism 19(12):1071–1084
Krolner B, Toft B (1983) Vertebral bone loss: an unheeded side effect of therapeutic bed rest. Clin Sci (Lond) 64(5):537–540
Lanyon LE (1983) Functional strain as a determinant for bone remodeling. Calcif Tissue Int 36(Suppl 1):S56–S61
Järvinen M, Kannus P (1997) Injury of an extremity as a risk factor for the development of osteoporosis. J Bone Joint Surg Am 79(2):263–276
Li MG, Nilsson KG (2000) The effect of the preoperative bone quality on the fixation of the tibial component in total knee arthroplasty. J Arthroplasty 15(6):744–753
Bobyn JD, Pilliar RM, Cameron HU, Weatherly GC (1980) The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin Orthop Relat Res 150:263–270
Cameron HU, Pilliar RM, MacNab I (1973) The effect of movement on the bonding of porous metal to bone. J Biomed Mater Res 7(4):301–311
Dabrowski B, Swieszkowski W, Godlinski D, Kurzydlowski KJ (2010) Highly porous titanium scaffolds for orthopaedic applications. J Biomed Mater Res B Appl Biomater 95(1):53–61
Karageorgiou V, Kaplan D (2005) Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26(27):5474–5491
Søballe K, Hansen ES, B-Rasmussen H, Jørgensen PH, Bunger C (1992) Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res 10(2):285–299
Andersson SM, Nilsson BE (1979) Changes in bone mineral content following tibia shaft fractures. Clin Orthop Relat Res 144:226–229
Insall JN, Dorr LD, Scott RD, Scott WN (1989) Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 248:13–14
Petersen MM, Gehrchen PM, Ostgaard SE, Nielsen PK, Lund B (2005) Effect of hydroxyapatite-coated tibial components on changes in bone mineral density of the proximal tibia after uncemented total knee arthroplasty: a prospective randomized study using dual-energy X-ray absorptiometry. J Arthroplasty 20(4):516–520
Li MG, Nilsson KG (2000) Changes in bone mineral density at the proximal tibia after total knee arthroplasty: a 2-year follow-up of 28 knees using dual energy X-ray absorptiometry. J Orthop Res 18(1):40–47
Hvid I, Bentzen SM, Jørgensen J (1988) Remodeling of the tibial plateau after knee replacement. CT bone densitometry. Acta Orthop Scand 59(5):567–573
Saari T, Uvehammer J, Carlsson L, Regnér L, Kärrholm J (2007) Joint area constraint had no influence on bone loss in proximal tibia 5 years after total knee replacement. J Orthop Res 25(6):798–803
Regnér LR, Carlsson LV, Kärrholm JN, Hansson TH, Herberts PG, Swanpalmer J (1999) Bone mineral and migratory patterns in uncemented total knee arthroplasties: a randomized 5-year follow-up study of 38 knees. Acta Orthop Scand 70(6):603–608
Li MG, Nilsson KG (2001) No relationship between postoperative changes in bone density at the proximal tibia and the migration of the tibial component 2 years after total knee arthroplasty. J Arthroplasty 16(7):893–900
Henricson A, Linder L, Nilsson KG (2008) A trabecular metal tibial component in total knee replacement in patients younger than 60 years: a two-year radiostereophotogrammetric analysis. J Bone Joint Surg (Br) 90(12):1585–1593
Onsten I, Nordqvist A, Carlsson AS, Besjakov J, Shott S (1998) Hydroxyapatite augmentation of the porous coating improves fixation of tibial components. A randomised RSA study in 116 patients. J Bone Joint Surg (Br) 80(3):417–425
Ryd L (1986) Micromotion in knee arthroplasty. A roentgen stereophotogrammetric analysis of tibial component fixation. Acta Orthop Scand Suppl 220:1–80
Ryd L, Albrektsson BE, Carlsson L, Dansgård F, Herberts P, Lindstrand A, Regnér L, Toksvig-Larsen S (1995) Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg (Br) 77:377
Ritter MA, Meneghini RM (2010) Twenty-year survivorship of cementless anatomic graduated component total knee arthroplasty. J Arthroplasty 77(3):377–383
Schroder HM, Berthelsen A, Hassani G, Hansen EB, Solgaard S (2001) Cementless porous-coated total knee arthroplasty: 10-year results in a consecutive series. J Arthroplasty 25(4):507–513
Eriksen J, Christensen J, Solgaard S, Schrøder H (2009) The cementless AGC 2000 knee prosthesis: 20-year results in a consecutive series. Acta Orthop Belg 75(2):225–233
Meding JB, Galley MR, Ritter MA (2010) High survival of uncemented proximally porous-coated titanium alloy femoral stems in osteoporotic bone. Clin Orthop Relat Res 468:441–447
Minoda Y, Kobayashi A, Iwaki H, Ikebuchi M, Inori F, Takaoka K (2010) Comparison of bone mineral density between porous tantalum and cemented tibial total knee arthroplasty components. J Bone Joint Surg Am 92(3):700–706
Minoda Y, Kobayashi A, Ikebuchi M, Iwaki H, Inori F, Nakamura H (2013) Porous tantalum tibial component prevents periprosthetic loss of bone mineral density after total knee arthroplasty for five years-a matched cohort study. J Arthroplasty 28:1760
Sumner DR, Turner TM, Dawson D, Rosenberg AG, Urban RM, Galante JO (1994) Effect of pegs and screws on bone ingrowth in cementless total knee arthroplasty. Clin Orthop Relat Res 309:150–155
Nilsson KG, Henricson A, Norgren B, Dalén T (2006) Uncemented HA-coated implant is the optimum fixation for TKA in the young patient. Clin Orthop Relat Res 448:129139
Acknowledgments
Financial support was received from Biomet (Warsaw, IA, USA), the Maggie og Svend ritzches Memorial Fund, and from Hillerød Hospital (research grant).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winther, N., Jensen, C., Petersen, M. et al. Changes in bone mineral density of the proximal tibia after uncemented total knee arthroplasty. A prospective randomized study. International Orthopaedics (SICOT) 40, 285–294 (2016). https://doi.org/10.1007/s00264-015-2852-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2852-1