Abstract
Purpose
The reported success rates of debridement, antibiotics, and implant retention (DAIR) for prosthetic joint infections (PJIs) vary widely. Several risk factors have been described for treatment failure, but they vary between studies. The purpose of this study was to evaluate the predictors of DAIR failure in PJI treatment and to assess the efficacy of rifampin combined with ciprofloxacin versus rifampin combined with other antibiotics in staphylococcal PJIs.
Methods
Patients with PJI that underwent DAIR for the first time between February 2001 and August 2009 were identified retrospectively in the hospital’s patient databases. A total of 113 PJI cases with early postoperative or acute haematogenous PJI were followed for up to two years from the start of treatment.
Results
In univariate analysis, variables significantly associated with treatment failure were acute haematogenous infections (p = 0.022), leucocyte count at admission > 10 × 109/l (p < 0.01), pain in the joint (p < 0.01), and ineffective empirical antibiotics (p < 0.01). In a multivariate Cox model, leucocyte count > 10 × 109/l and ineffective empirical antibiotics were significant risk factors for failure. Compared to rifampin-ciprofloxacin, the hazard ratio (HR) for treatment failure was significantly increased in the rifampin-other antibiotics group (HR 6.0, 95 % CI 1.5−28.8, p = 0.014) and the group treated without rifampin (HR 14.4, 95 % CI 3.1−66.9, p < 0.01).
Conclusions
Rifampin-ciprofloxacin combination therapy was significantly more effective than rifampin combined with other antibiotics. Effective empirical antibiotics are essential for successful PJI treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prosthetic joint infection (PJI) is one of the most devastating complications of hip or knee arthroplasty. PJI occurs in approximately 0.3–2.2 % of primary arthroplasties and in up to 5.9 % of revision arthroplasties [1–4]. Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend considering debridement, antibiotics, and implant retention (DAIR) for PJIs when the patient has a well-fixed prosthesis without a sinus tract and is within approximately 30 days of prosthesis implantation or less than three weeks from the onset of infectious symptoms [5]. Reported success rates for DAIR vary widely (14–100 %) [6–8]. Several risk factors for treatment failure have been described, including the duration of symptoms, comorbidities, microbiology, and bacterial resistance, but they vary among studies [8–14].
Rifampin is an antimicrobial agent with bactericidal activity against Staphylococcus species. Rifampin achieves high intracellular levels and can penetrate biofilms and kill organisms in the sessile phase of growth [15–18]. Rifampin combination therapy has also been associated with high treatment success rates in PJIs caused by Staphylococcus aureus [12, 13]. In the recent IDSA guidelines, the use of rifampin combination therapy is recommended for the treatment of staphylococcal PJIs [5].
The purpose of this retrospective study was to evaluate the predictors of DAIR treatment failure for PJIs and to identify factors that can be influenced in order to improve success rates in the treatment of PJIs. We also assessed the efficacy of rifampin combined with ciprofloxacin compared to rifampin combined with other antibiotics in staphylococcal PJIs.
Materials and methods
This study was conducted at Oulu University Hospital, a tertiary-level centre with 900 beds that serves northern Finland. Approximately 700 hip and knee primary arthroplasties and 300 revisions are carried out in the hospital annually. PJIs were treated in the hospital by a clinical team of infectious disease physicians and orthopaedic surgeons. Patients with PJI who underwent DAIR for the first time between February 2001 and August 2009 were identified retrospectively in the hospital’s patient databases using the tenth Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10), code T84.5 (infection and inflammatory reaction due to internal joint prosthesis). We excluded patients who were referred directly to a two-stage exchange, patients with late chronic PJI, patients lost to follow-up, and patients who died of non-infectious causes before completing the treatment. Data were collected from the medical records by a senior orthopaedic surgeon (A-PP) and a medical student.
We recorded age, gender, co-morbidities (e.g., diabetes or rheumatoid arthritis), body mass index (BMI), American Society of Anaesthesiologists (ASA) score, type of implant (i.e., hip or knee prosthesis), time of a prior surgery (implantation of primary prosthesis or revision operation), symptoms, symptom onset, microbiological culture results, C-reactive protein, leucocyte count, blood cultures, duration of antimicrobial therapy, time of surgical therapy, and outcome. The institutional ethics committee approved the study.
A PJI was defined as the detection of the same microorganism growing in two or more cultures of synovial fluid or periprosthetic tissue [5, 6]. The treatment was considered DAIR when the first operation after suspecting an infection was debridement; if the first operation was prosthesis removal the patient was excluded from this study. PJIs were classified using the McPherson staging system, which classifies infections into three categories: early postoperative (< 4 postoperative weeks), acute haematogenous (< 4 week symptom duration), and late chronic (> 4 weeks symptom duration) [19]. Patients with late chronic infections were excluded from the present study.
Treatment was considered successful when the original prosthesis was retained, the patient had no symptoms or signs of infection (pain, swelling, erythema, fever, wound discharge, or loosening of the prosthesis) [20], and the C-reactive protein and sedimentation rate were normal at the end of follow-up. Treatment was considered to have failed if the patient was referred to two-stage exchange surgery at any time during treatment or follow-up, the patient had symptoms or signs of PJI after the end of antibiotic treatment, or the patient was referred to permanent suppressive antibiotic treatment. The follow-up was restricted to two years for the analysis. The outcome of the joint at the end of the follow-up was classified as prosthesis retention, resection arthroplasty of the hip, arthrodesis of the knee, or amputation.
The normal procedure in cases of suspected PJI was surgical debridement, the collection of multiple tissue specimens (4–6 samples) for microbiological cultures, and to start antimicrobial treatment. The exposed tissue surfaces were irrigated with sterile saline using pulsed lavage and any modular prosthesis components were exchanged when possible. Wounds were closed primarily and no drains were used. The recommended empirical antibiotics in our hospital were a combination of vancomycin (1 g twice daily) and cefuroxime (1.5 g three times daily). The antibiotic treatment was modified based on the results of the bacterial cultures, which routinely included enrichment culture and their sensitivities. In the group of patients treated with rifampin combinations, rifampin was started at a dose of 300 mg, 450 mg if body weight > 70 kg, twice daily when the results of the microbiological examinations and bacterial sensitivities were available.
The antibiotic treatment duration in DAIR was modified in April 2006. Before that time, DAIR treatment included antibiotics for six months for total knee arthroplasty (TKA) PJIs and for three months for total hip arthroplasty (THA) PJIs. After April 2006, the duration of antibiotic treatment was reduced to three months for TKA PJIs and two months for THA PJIs as described in our earlier study [4]. We did not find any significant differences between these treatment durations in that study.
Statistical analysis was performed using SPSS for Windows (IBM SPSS Statistics for Windows, Version 21.0; Armonk, NY). Summary measures are presented as the mean with standard deviation (SD) or as the median with 25th–75th percentile. Categorical variables were compared using the chi-squared test or Fisher’s exact test when necessary, and continuous variables were compared using the Student’s t-test. A multivariate Cox proportional hazards model was built to detect possible risk factors for treatment failure. The follow-up was restricted to two years. Variables with p < 0.3 in the univariate analysis were added one by one into the Cox model. A variable was left in the multivariate model if p < 0.05 or the variable’s impact on the –2log likelihood function was significant. Hazard ratios (HRs) and 95 % confidence intervals (CIs) are presented based on the Cox model. The assumption of proportional hazards was visually assessed from survival curves. Two-tailed p-values are presented.
Results
We identified 197 patients with PJI throughout the study period. Forty-eight percent (95/197) of the patients had previously undergone arthroplastic surgery at Oulu University Hospital. The overall incidence of infection was 1.9 % (61/3198) in primary arthroplasties and 2.4 % (34/1439) in revision arthroplasties. Eighty-four patients were ineligible for the following reasons: 56 patients were treated directly with a two-stage exchange, 23 patients had a late chronic infection, four patients were lost to follow-up, and one patient died from non-infectious causes before completing the antibiotic treatment. Thus, 113 cases were included in the final analysis, of which six patients with a follow-up time < two years and without failure were treated as censored cases in the survival analyses.
The clinical characteristics of patients are presented in Table 1. Table 2 summarizes the microbiological characteristics. The median time from the start of treatment to failure was 24 days (25th–75th percentile, 12–76 days), and 74.4 % (32/43) of the failure cases experienced failure before the end of the antibiotic treatment.
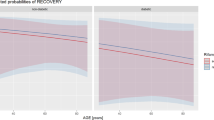
Variables significantly associated with failure in the univariate analysis were acute haematogenous infections, leucocyte count > 10 × 109/l, pain in the joint, and ineffective empirical antibiotics. In the multivariate Cox model adjusted for antibiotic treatment (i.e., rifampin-ciprofloxacin combination vs. rifampin-other antibiotic treatment vs. combination without rifampin therapy), leucocyte count > 10 × 109/l (HR 3.7, 95 % CI 1.9–7.3, p < 0.01; Fig. 1) and ineffective empirical antibiotics (HR 3.2, 95 % CI 1.4−7.1, p = 0.006; Fig. 2) increased the risk of treatment failure. Among the 13 patients who received ineffective empirical antibiotics, ten experienced treatment failure (76.9 %) and three experienced treatment success. Among the ten cases of failure, three infections were caused by methicillin-resistant coagulase-negative Staphylococci; in these cases, empirical antibiotic therapy did not include vancomycin, which violated our empirical treatment recommendation. Three infections were caused by cefuroxime-resistant Gram-negative rods, and four were polymicrobial infections (at least one microbe resistant to empirical antibiotics). For the three cases that were successfully treated, one infection was caused by cefuroxime-resistant Serratia species, one was caused by methicillin-resistant coagulase-negative Staphylococci (empirical antibiotic therapy did not include vancomycin), and one was caused by Enterococcus faecalis (empirical antibiotic therapy did not include vancomycin).
Our cohort included 66 cases of staphylococcal (S. aureus and coagulase-negative Staphylococci) infection. Among these cases, 23 received rifampin-ciprofloxacin combination therapy and 29 received rifampin combined with another antibiotic agent due to in vitro bacterial resistance to ciprofloxacin, allergies, or interactions with the patient’s other medications. The remaining 14 cases were treated without rifampin therapy due to in vitro bacterial resistance to rifampin, allergies, or drug interactions. Of 52 cases with rifampin treatment, 14 (26.9 %) did not complete the initially scheduled treatment due to adverse reactions (e.g., nausea or vomiting) or allergic reactions (e.g., rash). The HR for treatment failure was increased in the rifampin-other antibiotics group (HR 6.0, 95 % CI 1.5–28.8, p = 0.014) and the group treated without rifampin (HR 14.4, 95 % CI 3.1−66.9, p < 0.01) compared to the rifampin-ciprofloxacin group (Fig. 3).
Discussion
The aim of this study was to evaluate the predictors of DAIR treatment failure and to assess the efficacy of rifampin combination treatment in staphylococcal PJIs. In the multivariate Cox model, high leucocyte count and ineffective empirical antibiotics significantly increased the risk of treatment failure. Moreover, rifampin combination therapy, especially rifampin and ciprofloxacin, was significantly associated with successful treatment of staphylococcal PJIs with DAIR.
To the best of our knowledge, this study is the first to find that a high leucocyte count is associated with DAIR failure. This association is probably due to more severe infections, which are more likely to fail treatment, being more likely to induce a pronounced acute phase response. Some previous studies found that the erythrocyte sedimentation rate [9] or very high (> 220 mg/l) C-reactive protein levels [14] predict treatment failure. In our series, the median C-reactive protein level was 2.5-times higher in the failure group due to large variations in C-reactive protein values, but the difference between the failure and success groups was not significant. Several other factors have been found to predict treatment failure; symptoms being present for less than one week was associated with success in two studies [7, 9]. The factors associated with poor outcomes in DAIR-treated PJIs have also included ASA score > 2 [13], the need for a second debridement, and the presence of a sinus tract [10]. However, the significance of these parameters varies between studies, probably due to different study populations and study designs.
Staphylococcus species (S. aureus and coagulase-negative Staphylococcus species) are the most common pathogens causing PJIs [12, 20]. Rifampicin is very efficient against biofilm-associated and stationary-phase Staphylococci [16, 17]. Several studies have shown that rifampin combination therapy is associated with better success rates in S. aureus PJIs treated with DAIR [12, 13, 21–23]. Quinolones have good bioavailability, antimicrobial activity, and tolerability [6]. Rifampin-quinolone combination therapy has been suggested as the treatment of choice in S. aureus PJIs [6, 13]. Similarly, our study found that rifampin combination therapy achieves significantly better success rates for staphylococcal PJIs than treatment without rifampin. The success rate with rifampin-ciprofloxacin combination therapy was also superior to the success rates of rifampin combined with other antimicrobial agents. These results emphasize the importance of combining rifampin, especially with fluoroquinolones, in DAIR treatment of staphylococcal PJIs. However, many significant drug interactions of rifampin must be taken into consideration, as well as the possible side effects of both rifampin (e.g., nausea, vomiting, stomach ache, rash, hepatitis,) and fluoroquinolone (e.g., tendinopathy). Twenty-seven percent of our rifampin treatment group did not complete the scheduled treatment due to side effects. Notably, the increasing resistance of Staphylococci to quinolones may reduce the possibility of using quinolones in DAIR treatment [6]. Therefore, new antibiotic combinations for treating staphylococcal PJIs must be studied in the future. Lora-Tamayo et al. [24] treated 18 acute PJIs caused by fluoroquinolone-resistant Staphylococci with high doses of daptomycin (10 mg/kg/d) plus rifampin. The success rates in their study were comparable to previous rifampin-based combinations used for these infections. In another study, 19 % of 211 Gram-negative bacteria isolates were ciprofloxacin-resistant [25]. In that study, ciprofloxacin treatment exhibited an independent protective effect in susceptible Gram-negative PJIs treated with DAIR.
In earlier studies, the factors associated with poor outcomes in DAIR-treated PJIs included S. aureus infection [8, 10, 11, 26, 27], coagulase-negative Staphylococcus infection [9, 11], and polymicrobial infection [12]. S. aureus PJIs have been shown to have worse outcomes than PJIs caused by Streptococcus species [27, 28]. Infections by methicillin-resistant S. aureus (MRSA) have been suggested to have a worse prognosis than infections by methicillin-susceptible S. aureus (MSSA), but this finding is controversial [12]. Lora-Tamayo et al. [12] found no difference in the success rates of MRSA-PJI and MSSA-PJI. In our cohort, treatment failure was not associated with causative microbe. However, in agreement with Senneville et al. [13], our study demonstrated the importance of adequate empirical antibiotics. Instructions concerning empirical antibiotics were not always followed in our study, and we observed failure in 77 % of cases not treated with effective empirical antibiotics. Hospitals require proper instructions concerning the use of empirical antibiotics in suspected PJI. Compliance with these instructions should also be systematically monitored. Bacterial resistance in the hospital should be monitored, and the guidelines must be modified according to resistance patterns.
This study has a few limitations. First, it was a retrospective single centre study. Although the study population was quite large, in some subgroups the number of cases may have been insufficient to show a significant difference.
In summary, high leucocyte count and ineffective empirical antibiotics were identified as independent risk factors for treatment failure. Empirical antibiotics are a factor that can be influenced by proper instructions and by monitoring compliance with the instructions. Our study also showed that higher success rates are achieved with rifampin combination therapy in staphylococcal PJIs. Rifampin-quinolone therapy appeared to be significantly more effective than rifampin combined with other antibiotics. Therefore, special attention should be paid to the choice of antibiotics in PJIs.
References
Bongartz T, Halligan CS, Osmon DR, Reinalda MS, Bamlet WR, Crowson CS, Hanssen AD, Matteson EL (2008) Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum 59:1713–1720. doi:10.1002/art.24060
Pulido L, Ghanem E, Joshi A, Purtill J, Parvizi J (2008) Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 466:1710–1715. doi:10.1007/s11999-008-0209-4
Phillips JE, Crane TP, Noy M, Elliott TSJ, Grimer RJ (2006) The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg (Br) 88:943–948. doi:10.1302/0301-620X.88B7.17150
Puhto A-P, Puhto T, Syrjala H (2012) Short-course antibiotics for prosthetic joint infections treated with prosthesis retention. Clin Microbiol Infect 18:1143–1148. doi:10.1111/j.1469-0691.2011.03693.x
Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR (2013) Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi:10.1093/cid/cis803
Zimmerli W, Trampuz A, Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi:10.1056/NEJMra040181
Marculescu C, Cantey JE (2008) Polymicrobial prosthetic joint infections: risk factors and outcome. Clin Orthop Relat Res 466:1397–1404. doi:10.1007/s11999-008-0230-7
Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, Gundle R, Berendt A (2009) One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother 63:1264–1271. doi:10.1093/jac/dkp107
Kuiper JWP, Vos SJ, Saouti R, Vergroesen DA, Graat HCA, Debets-Ossenkopp Y, Peters EJG, Nolte PA (2013) Prosthetic joint-associated infections treated with DAIR (debridement, antibiotics, irrigation, and retention): analysis of risk factors and local antibiotic carriers in 91 patients. Acta Orthop 84:380–386. doi:10.3109/17453674.2013.823589
Marculescu C, Berbari E, Hanssen A, Steckelberg J, Harmsen S, Mandrekar J, Osmon D (2006) Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis 42:471–478. doi:10.1086/499234
Buller LT, Sabry FY, Easton RW, Klika AK, Barsoum WK (2012) The preoperative prediction of success following irrigation and debridement with polyethylene exchange for hip and knee prosthetic joint infections. J Arthroplasty 27:857–864.e1-4. doi:10.1016/j.arth.2012.01.003
Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sánchez-Somolinos M et al (2013) A large multicenter study of methicillin–susceptible and methicillin–resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 56:182–194. doi:10.1093/cid/cis746
Senneville E, Joulie D, Legout L, Valette M, Dezèque H et al (2011) Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis 53:334–340. doi:10.1093/cid/cir402
Vilchez F, Martínez-Pastor JC, García-Ramiro S, Bori G, Maculé F, Sierra J, Font L, Mensa J, Soriano A (2011) Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect 17:439–444. doi:10.1111/j.1469-0691.2010.03244.x
Blaser J, Vergères P, Widmer AF, Zimmerli W (1995) In vivo verification of in vitro model of antibiotic treatment of device-related infection. Antimicrob Agents Chemother 39:1134–1139. doi:10.1128/AAC.39.5.1134
Widmer AF, Frei R, Rajacic Z, Zimmerli W (1990) Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J Infect Dis 162:96–102. doi:10.1093/infdis/162.1.96
Zimmerli W, Frei R, Widmer AF, Rajacic Z (1994) Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J Antimicrob Chemother 33:959–967. doi:10.1093/jac/33.5.959
Zimmerli W (2014) Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med 276:111–119. doi:10.1111/joim.12233
McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis M (2002) Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res 403:8–15
Del Pozo JL, Patel R (2009) Infection associated with prosthetic joints. N Engl J Med 361:787–794. doi:10.1056/NEJMcp0905029
Laffer RR, Graber P, Ochsner PE, Zimmerli W (2006) Outcome of prosthetic knee-associated infection: Evaluation of 40 consecutive episodes at a single centre. Clin Microbiol Infect 12:433–439. doi:10.1111/j.1469-0691.2006.01378.x
Berdal J-E, Skråmm I, Mowinckel P, Gulbrandsen P, Bjørnholt JV (2005) Use of rifampicin and ciprofloxacin combination therapy after surgical debridement in the treatment of early manifestation prosthetic joint infections. Clin Microbiol Infect 11:843–845. doi:10.1111/j.1469-0691.2005.01230.x
Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE (1998) Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: A randomized controlled trial. Foreign Body infection (FBI) study group. JAMA 279:1537–1541. doi:10.1001/jama.279.19.1537
Lora-Tamayo J, Parra-Ruiz J, Rodríguez-Pardo D, Barberán J, Ribera A, Tornero E, Pigrau C, Mensa J, Ariza J, Soriano A (2014) High doses of daptomycin (10 mg/kg/d) plus rifampin for the treatment of staphylococcal prosthetic joint infection managed with implant retention: a comparative study. Diagn Microbiol Infect Dis 80:66–71. doi:10.1016/j.diagmicrobio.2014.05.022
Rodríguez-Pardo D, Pigrau C, Lora-Tamayo J, Soriano A, del Toro MD et al (2014) Gram-negative prosthetic joint infection: outcome of a debridement, antibiotics and implant retention approach. A large multicentre study. Clin Microbiol Infect 20:O911–O919. doi:10.1111/1469-0691.12649
Choi H, von Knoch F, Kandil A, Zurakowski D, Moore S, Malchau H (2012) Retention treatment after periprosthetic total hip arthroplasty infection. Int Orthop 36:723–729. doi:10.1007/s00264-011-1324-5
Zürcher-Pfund L, Uçkay I, Legout L, Gamulin A, Vaudaux P, Peter R (2013) Pathogen-driven decision for implant retention in the management of infected total knee prostheses. Int Orthop 37:1471–1475. doi:10.1007/s00264-013-1923-4
Betz M, Abrassart S, Vaudaux P, Gjika E, Schindler M, Billières J, Zenelaj B, Suvà D, Peter R, Uçkay I (2015) Increased risk of joint failure in hip prostheses infected with staphylococcus aureus treated with debridement, antibiotics and implant retention compared to streptococcus. Int Orthop 39:397–401. doi:10.1007/s00264-014-2510-z
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puhto, AP., Puhto, T., Niinimäki, T. et al. Predictors of treatment outcome in prosthetic joint infections treated with prosthesis retention. International Orthopaedics (SICOT) 39, 1785–1791 (2015). https://doi.org/10.1007/s00264-015-2819-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2819-2