Abstract
Purpose
Percutaneous vertebroplasty is a widely used vertebral augmentation technique. It is a minimally invasive and low-risk procedure, but has some disadvantages with a relatively high number of bone cement leaks and adjacent vertebral fractures. The aim of this cadaveric study was to determine the minimum percentage of cement fill volume in vertebroplasty needed to restore vertebral stiffness and adjacent intradiscal pressure.
Methods
Thirteen thoracolumbar spine mobile segments were loaded to induce a vertebral fracture. After fracture vertebroplasty was performed, four times in the same fractured vertebra. The injected cement volume was 5 % of the fractured vertebral volume to reach 5, 10, 15 and 20 % of cement fill. Biomechanical testing was performed before the fracture, after the fracture and after each cement injection.
Results
After vertebral fracture compressive stiffness was reduced to 47 % of the pre-fracture value and was partially restored to 61 % after 10 % cement fill. With vertebroplasty intradiscal pressure gradually increased, depending on specimen position, from 48 to a total of 71 % at 15 % of cement fill.
Conclusions
Compressive stiffness and intradiscal pressure increase with the percentage of cement fill. Fifteen per cent of cement fill was the limit beyond which no substantial increase in compressive stiffness or intradiscal pressure could be detected and is the minimum volume of cement we recommend for vertebroplasty. In the average thoracolumbar vertebra this means 4–6 ml of cement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Percutaneous vertebroplasty is the simplest of all vertebral augmentation techniques. It can be performed under local anaesthesia on an outpatient basis inflicting little intraoperative trauma to the patient with significant pain relief [1–6]. The exact reason for pain in osteoporotic vertebral compression fractures (OVCF) remains elusive with proposed mechanisms being lowering of the intradiscal pressure (IDP) producing pain from the posterior annulus [7] and the micromovement of fractured trabeculae. Vertebral augmentation with bone cement stabilises the fractured vertebral body and at least partially restores IDP, thus acting on both proposed pain sources [8].
The most important problems associated with vertebroplasty are cement leakage and adjacent vertebral fractures. Clinical trials have reported a relatively high number of bone cement leaks with vertebroplasty, ranging from 22 to 82 % [9, 10]. Clinical consequences, however, occur only rarely, despite these high rates of cement leaks [11]. The most important factor affecting the leak frequency is the correlation of injected volume and vertebral size: with higher percentages of injected cement leading to increased rates of cement leaks [12]. Another concern with vertebroplasty is the incidence of subsequent adjacent vertebral fractures [13–15]. Reasons for adjacent fractures are thought to be due to either the abnormal loading of the non-augmented vertebral bodies causing elevated IDP generated by increased stiffness of the augmented vertebra [13–16] or simply systemic bone weakening in the osteoporotic spine not related to vertebroplasty [17]. Stiffness of the augmented vertebra is influenced by the volume of bone cement injected into the vertebra [18, 19], the position of the injected cement and the biomechanical characteristics of the cement [20, 21].
Biomechanical [8, 18] and clinical studies [22, 23] suggest that too high a volume of bone cement and consequently high IDP and elevated vertebral stiffness are the reasons for complications and even failure of vertebroplasty. To avoid both the aforementioned complications, smaller volumes of cement have been recommended; however, studies done on single vertebral bodies have shown that very small volumes cannot restore strength and stiffness of the fractured vertebra to a sufficient level leading to procedure failure with persisting pain [18, 24].
The aim of our biomechanical cadaveric study was to determine the minimum amount of cement required in vertebroplasty, measuring vertebral stiffness and adjacent IDP in a functional spine unit. It was hoped that our biomechanical study would enable us to provide a clinical guideline outlining the minimum cement fill for successful vertebroplasty.
Methods
Cadaveric specimens
Eight thoracolumbar spines were removed from cadavers aged 72–83 years (average 77 years) (Table 1). In the selected spines there was no history of malignant disease, traumatic vertebral fractures or evidence of previous surgical procedures. Each spine was dissected to provide one or whenever possible two functional spinal units (FSU), and each FSU consisted of two adjacent vertebrae with intervening intervertebral disc and ligaments. Specimens with large osteophytes, which could interfere with disc stress measurements, were discarded. A total of 13 FSU, nine male and four female, between T9 and L4 were obtained (Table 1). Each FSU was sealed in a plastic bag and stored at −20 °C until testing. FSU were thawed at 4 °C before testing.
Vertebral volume and bone mineral density (BMD)
Each FSU was submitted to a computed tomography (CT) scan before and after vertebral fracture. To obtain the volume of vertebral bodies, a dedicated three-dimensional (3D) model was aligned with the vertebral body in the CT image [25]. BMD was measured with dual-energy X-ray absorptiometry. Osteoporosis was defined as 2.5 standard deviations below the mean of a young healthy reference population of the same gender and corresponded to a BMD <0.75 g/m2.
Vertebral fracture and vertebroplasty
Each FSU was secured in two cups of dental plaster (type IV super hard, Heraeus) and loaded in a computer-controlled hydraulic testing machine (Zwick Roell). Each FSU was positioned in 2° of flexion to simulate a forward stooped posture. The FSU was then compressed at a speed of 200 N/s until one of the vertebral bodies fractured. The yield point was identified by the first nonlinear deformation in a real-time load-deformation graph and the load at yield point was indicated as the yield strength. The fracture was confirmed by a CT scan.
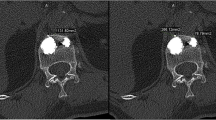
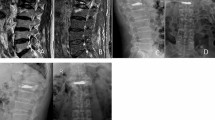
After fracture, vertebroplasty was done using Jamshidi needles (11G) introduced bipedicularly taking special care not to injure the pedicle wall. High-viscosity bone cement polymethylmethacrylate (PMMA, Confidence Spinal Cement System®, DePuy Spine, Raynham, MA, USA) was used to provide better control of cement expansion. Bone cement was injected in increments of 5 % of predetermined fractured vertebral volume to a final injected volume of 20 % of fractured vertebral body volume. The fractured vertebrae were filled in the following way: first bone cement of 5 % of fractured vertebral body volume was injected through one pedicle and the second 5 % (combined 10 %) via the other pedicle. The manoeuvre was repeated for the third (combined 15 %) and fourth (combined 20 % of fractured vertebral volume) injections. Placement of needles and injection of bone cement was monitored closely using alternatively sagittal, frontal and axial plane radiography to show that the needle tips were in the anterolateral part (for the first and second injections) and posterolateral part of the vertebral body (third and fourth injections) as far as possible from lateral borders of vertebrae and vertebral endplates (Fig. 1). Leakage was quantified by collecting the fragments of cement that had leaked and measuring their volume by immersing them in a preset volume of water.
Mechanical testing
Biomechanical testing was performed before and after the fracture and one hour after each cement injection. Compressive stiffness and IDP were measured. Before each mechanical testing, a preliminary creep test (0.5 kN compression applied for one hour) was performed to stimulate the diurnal change in vertebral disc content and height that occurs in vivo [26]. Each FSU was positioned in the testing machine and compressed at a speed of 600 N/s up to a maximum load of 1.3 kN. Compressive stiffness was defined as a slope of the load-deformation curve at 1 kN.
A miniature fibre-optic pressure transducer (Samba Sensors AB, Västra Frölunda, Sweden) 0.36 mm in diameter was used to measure pressure in the nucleus. A plastic cannula was introduced first to the annulus/nucleus border followed by a transducer advanced into the nucleus. Afterwards, an FSU was subjected to a compressive force of 1 kN and IDP was measured as the average pressure in the time span of one minute after 1 kN compressive force was reached. Measurements were done consecutively in a neutral position, 2° of extension (to simulate an erect standing posture) and in 6° of flexion to stimulate slightly stooped posture.
Statistical analysis
Repeated measures analysis of variance (ANOVA) was used to compare measurements across six consecutive time points (pre- and post-fracture, after 5, 10, 15 and 20 % vertebroplasty). Where a significant main effect was found, the paired t test was used to identify where the differences arose. In all tests P < 0.05 was considered significant.
Results
Vertebral volume
The average vertebral body volume before vertebral fracture, measured by the CT method, was 39 ± 5.7 ml and was reduced after fracture to 36.6 ± 5.6 ml. The volume loss was 1.9 ± 1.7 ml (4.7 % on average).
Vertebral fracture and vertebroplasty
In 6/13 FSU CT images showed vertebral fracture in the upper endplate of the lower vertebra and in the other 7/13 FSU in the lower endplate of the upper vertebra. The average force to produce a vertebral fracture was 4.2 ± 2.5 kN. The height loss was 0.77 ± 0.37 mm.
Cement leakage was detected in 5/52 injections (three times during the first injection, once in the third and once in the fourth injection) in three vertebrae on an average of 0.07 ± 0.03 ml per fill. X-ray images showed cement placement adjacent to the vertebral endplate in 10/52 injections (four after the first and two after the second, third and fourth injections). There was no significant difference in compressive stiffness P = 0.96 and IDP P = 0.23 between the group with cement placement adjacent to endplates compared to the group where cement was nonadjacent to the vertebral endplate.
Compressive stiffness
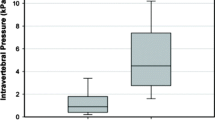
Compressive stiffness was reduced after vertebral fracture to 47 % compared to the pre-fracture level. After vertebroplasty, compressive stiffness increased statistically significantly after the first injection to 55 % (P < 0.01) and after the second injection to 61 % (P < 0.001), while there was no statistically significant increase after the third and fourth injections (Table 2, Fig. 2).
Intradiscal pressure
After vertebral fracture IDP in the neutral position was reduced to 56 % (P < 0.001) of the pre-fracture level, to 58 % in flexion (P < 0.001) and to 48 % in extension (P < 0.001). IDP increased after the first injection in the neutral position to 64 % (P < 0.05), remained the same after the second injection and again increased to 71 % (P < 0.001) after the third. After the fourth injection there was no statistically significant increase of IDP. The results were similar in the flexion position: after the first injection IDP increased to 65 % (P < 0.05), remained the same after the second injection and increased to 69 % (P < 0.001) after the third. After the fourth injection IDP increased to 71 %; this increase was not statistically significant. In extension, there was significant increase in IDP only after the forth injection of cement, with IDP reaching 58 % of the pre-fracture level (P < 0.05) (Table 2, Fig. 3).
Discussion
The main aim of vertebroplasty is to stabilise the fracture and alleviate pain with minimal risk of complications. Although widely used, there are still no clear clinical guidelines for the quantity of cement to be used. Early clinical trials proposed that filling of the vertebral body should be stopped only when half or all of the vertebral body was filled or when extravasation occurs. In current clinical practice, however, the risk of extravasation [12, 19, 27] precluded use of bigger filling volumes [23]. It is also our clinical practice to inject no more than 5 ml of bone cement per vertebra, except for the three lowermost lumbar levels. The aim of this study was to find out the minimum cement volume needed for successful vertebroplasty. A biomechanical study was performed on an FSU model, since this goal cannot be reached easily in an in vivo study.
The biomechanical properties of the FSU in our study were only partially restored after vertebroplasty which is in accordance with the biomechanical study in which inability to completely restore vertebral body stiffness and IDP by vertebroplasty was reported [8]. The failure to restore IDP to pre-fracture level could be explained by disc space anatomy, which presumably remains affected even after vertebroplasty [8]. On the other hand, inability to restore FSU compressive stiffness to pre-fracture level after vertebroplasty does not accord with observations of complete restoration of compressive stiffness reported in biomechanical studies performed on a single vertebral body [18, 19, 24]. Intuitively the difference between the single vertebral body model and the FSU model is not surprising as the stiffness of the FSU depends on factors other than vertebral body stiffness alone [28].
It has been suggested from biomechanical studies of vertebral body strength and stiffness that the amount of cement needed to relieve pain clinically may approximate the amount of cement needed to restore the vertebral body’s pre-fracture mechanical properties [18, 19]. On the other hand, this in vitro finding was not proven clinically [23]. Fracture stabilisation with associated clinical improvement can occur without complete restoration of the pre-fracture biomechanical properties, i.e. of the strength and stiffness of the vertebral body. In the same study, it was suggested that once a certain amount of cement fill has been reached, little or no further gain in terms of pain and medication use reduction resulted from any additional amount of cement used [23]. The results of our study indicate that no further change in biomechanical properties of FSU is observed with volume fills beyond 15 % of fractured vertebral body volume, which corresponds to 4–6 ml of bone cement. This is the same amount of cement fill volume needed to restore biomechanical properties in a finite element study on a single vertebral body [18]. Our result is also in accordance with other clinical studies where the relationship between pain relief after vertebroplasty and volume of the injected bone cement expressed in percentage of fractured vertebral body volume was assessed [22, 29]. The suggested optimal cement fill volume varies from 11 % of fractured vertebral body volume in a study by Jin et al. [22] to 24 % in the study by Nieuwenhuijse et al. [29].
In previous studies, the same volume of cement was used irrespective of vertebral body size. Consequently, percentage of fill in the same group was diverse [8, 24]. The advantage of our study is that the size of the vertebral body was taken into account and that an FSU model was used. Individual cement volumes calculated on percentage of fractured vertebral body volume determined by a CT-based mathematical model [25] were applied. Our study characteristic might be important in order to provide a useful clinical guide regarding the need to know the minimum amount of cement volume needed for successful vertebroplasty in an individual case.
There are weak points to our study. Frozen cadaveric material was used which could to some extent alter biomechanical properties of the FSU. The data presented were collected using a single FSU which is obviously different when compared to an in vivo whole spine situation. We were forced to accept all the negative consequences of this difference, since we were unable to perform an adequate clinical study. Vertebroplasty was done consecutively four times and four small plugs of cement were placed, which differs from clinical practice where one or two bigger plugs are made. This could also have had a potential effect on the results; however, the study of Rohlmann et al. has shown that the number and symmetry of cement plugs have virtually no effect on the maximum stresses of the augmented vertebral body [27]. Biomechanical properties of the vertebral body also depend on the location of bone cement [20], i.e. whether it is placed adjacent or nonadjacent to the vertebral body endplate. Despite a meticulous technique and the high-viscosity PMMA cement used, ten of 52 injections resulted in cement being placed adjacent to the vertebral body endplate, which was not our intention. In contrast to biomechanical studies [20], where effects of vertebroplasty were dependent on the cement location within the vertebral body, no differences in biomechanical properties of the ten vertebroplasties with cement adjacent to the vertebral body endplate compared to the other 42 fills with cement nonadjacent to the endplate were noted in our study. Since our two compared groups, i.e. adjacent and nonadjacent, are not homogeneous with respect to filled cement volume, the direct clinical application of the fact that no difference was noted seems limited. In future this issue could be a matter for a separate study.
Conclusions
According to our study model, vertebroplasty with PMMA cement fill cannot restore IDP and compressive stiffness of an FSU to pre-fracture level with cement fills up to 20 % of the vertebral body volume. Fifteen per cent of cement fill was the limit beyond which no substantial increase in compressive stiffness and IDP was detected. We recommend that cement fill for vertebroplasty should be 15 % of fractured vertebral body volume which in concordance with average thoracolumbar vertebral body volumes for men and women [30] corresponds to 4–6 ml of cement fill depending on the vertebral level. In spite of the fact that our study was purely in vitro, we suggest that this rule can be applied clinically.
References
Heini PF, Wälchli B, Berlemann U (2000) Percutaneous transpedicular vertebroplasty with PMMA: operative technique and early results. A prospective study for the treatment of osteoporotic compression fractures. Eur Spine J 9(5):445–450
Chang X, Lv YF, Chen B, Li HY, Han XB, Yang K, Zhang W, Zhou Y, Li CQ (2014) Vertebroplasty versus kyphoplasty in osteoporotic vertebral compression fracture: a meta-analysis of prospective comparative studies. Int Orthop Sep 27
Voormolen MH, Mali WP, Lohle PN et al (2007) Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. Am J Neuroradiol 28(3):555–556
Dong R, Chen L, Tang T, Gu Y, Luo Z, Shi Q, Li X, Zhou Q, Yang H (2013) Pain reduction following vertebroplasty and kyphoplasty. Int Orthop 37(1):83–87
Han S, Wan S, Ning L, Tong Y, Zhang J, Fan S (2011) Percutaneous vertebroplasty versus balloon kyphoplasty for treatment of osteoporotic vertebral compression fracture: a meta-analysis of randomised and non-randomised controlled trials. Int Orthop 35(9):1349–1358
Dong R, Chen L, Gu Y, Han G, Yang H, Tang T, Xiaoqing C (2009) Improvement in respiratory function after vertebroplasty and kyphoplasty. Int Orthop 33(6):1689–1694
García-Cosamalón J, del Valle ME, Calavia MG et al (2010) Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat 217(1):1–15
Luo J, Daines L, Charalambous A, Adams MA, Annesley-Williams DJ, Dolan P (2009) Vertebroplasty: only small cement volumes are required to normalize stress distributions on the vertebral bodies. Spine 34(26):2865–2873
Bhatia C, Barzilay Y, Krishna M, Friesem T, Pollock R (2006) Cement leakage in percutaneous vertebroplasty: effect of preinjection gelfoam embolization. Spine 31(8):915–919
Martin DJ, Rad AE, Kallmes DF (2012) Prevalence of extravertebral cement leakage after vertebroplasty: procedural documentation versus CT detection. Acta Radiol 53(5):569–572
Krueger A, Bliemel C, Zettl R, Ruchholtz S (2009) Management of pulmonary cement embolism after percutaneous vertebroplasty and kyphoplasty: a systematic review of the literature. Eur Spine J 18(9):1257–1265
Ryu KS, Park CK, Kim MC, Kang JK (2002) Dose-dependent epidural leakage of polymethylmethacrylate after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. J Neurosurg 96(1):56–61
Trout AT, Kallmes DF, Kaufmann TJ (2006) New fractures after vertebroplasty: adjacent fractures occur significantly sooner. Am J Neuroradiol 27(1):217–223
Trout AT, Kallmes DF, Layton KF, Thielen KR, Hentz JG (2006) Vertebral endplate fractures: an indicator of the abnormal forces generated in the spine after vertebroplasty. J Bone Miner Res 21(11):1797–1802
Uppin AA, Hirsch JA, Centenera LV, Pfiefer BA, Pazianos AG, Choi IS (2003) Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology 226(1):119–124
Polikeit A, Nolte LP, Ferguson SJ (2003) The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit: finite-element analysis. Spine 28(10):991–996
Rho YJ, Choe WJ, Chun YI (2011) Risk factors predicting the new symptomatic vertebral compression fractures after percutaneous vertebroplasty or kyphoplasty. Eur Spine J 21(5):905–911
Liebschner MA, Rosenberg WS, Keaveny TM (2001) Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine 26(14):1547–1554
Belkoff SM, Mathis JM, Jasper LE, Deramond H (2001) The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine 26(14):1537–1541
Kinzl M, Benneker LM, Boger A, Zysset PK, Pahr DH (2012) The effect of standard and low-modulus cement augmentation on the stiffness, strength, and endplate pressure distribution in vertebroplasty. Eur Spine J 21(5):920–929
Belkoff SM, Mathis JM, Jasper LE (2002) Ex vivo biomechanical comparison of hydroxyapatite and polymethylmethacrylate cements for use with vertebroplasty. AJNR Am J Neuroradiol 23(10):1647–1651
Jin YJ, Yoon SH, Park KW, Chung SK, Kim KJ, Yeom JS, Kim HJ (2011) The volumetric analysis of cement in vertebroplasty: relationship with clinical outcome and complications. Spine 36(12):E761–E772
Kaufmann TJ, Trout AT, Kallmes DF (2006) The effects of cement volume on clinical outcomes of percutaneous vertebroplasty. Am J Neuroradiol 27(9):1933–1937
Molloy S, Mathis JM, Belkoff SM (2003) The effect of vertebral body percentage fill on mechanical behavior during percutaneous vertebroplasty. Spine 28(14):1549–1554
Stern D, Likar B, Pernuš F, Vrtovec T (2011) Parametric modelling and segmentation of vertebral bodies in 3D CT and MR spine images. Phys Med Biol 56(23):7505–7522
O’Connell GD, Jacobs NT, Sen S, Vresilovic EJ, Elliott DM (2011) Axial creep loading and unloaded recovery of the human intervertebral disc and the effect of degeneration. J Mech Behav Biomed Mater 4(7):933–942
Rohlmann A, Boustani HN, Bergmann G, Zander T (2010) A probabilistic finite element analysis of the stresses in the augmented vertebral body after vertebroplasty. Eur Spine J 19(9):1585–1595
Berlemann D, Ferguson SJ, Nolte LP, Heini PF (2002) Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J Bone Joint Surg Br 84(5):748–752
Nieuwenhuijse MJ, Bollen L, van Erkel AR, Dijkstra PD (2012) Optimal intravertebral cement volume in percutaneous vertebroplasty for painful osteoporotic vertebral compression fractures. Spine 37(20):1747–1755
Limthongkul W, Karaikovic EE, Savage JW, Markovic A (2010) Volumetric analysis of thoracic and lumbar vertebral bodies. Spine J 10(2):153–158
Acknowledgments
The authors kindly thank DePuy Spine, Raynham, MA, USA, for donating the Confidence Spinal Cement System® for vertebroplasty.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinčič, D., Brojan, M., Kosel, F. et al. Minimum cement volume for vertebroplasty. International Orthopaedics (SICOT) 39, 727–733 (2015). https://doi.org/10.1007/s00264-014-2620-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2620-7