Abstract
Purpose
The purpose of this study was to determine if a new titanium cup with increased porosity resulted in different periacetabular bone loss and migration compared to a porous coated cup.
Methods
Fifty-one patients with primary hip osteoarthritis were randomized to either a cup with porous titanium construct backside (porous titanium group, n = 25) or a conventional porous coated titanium cup (control group, n = 26). The primary outcome variable was change in periacetabular bone mineral density two years after surgery measured with dual energy X-ray absorptiometry (DXA). Secondary outcomes were implant fixation measured with radiostereometry (RSA) and clinical outcome scores.
Results
The pattern of bone remodelling was similar in the two groups with almost complete restoration to baseline values. BMD diminished in the two proximal zones and increased in the two distal zones. After minimal migration up to six months all implants in both groups became stable. We found no difference between the two groups in clinical outcome scores.
Conclusions
In this prospective, randomized, controlled trial on a new porous titanium cup we found, compared to the control group, no clinically relevant differences regarding periacetabular bone preservation, implant fixation or clinical outcome up to two years postoperatively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periacetabular bone loss is an important factor influencing long-term stability and survival of the acetabular implants in total hip arthroplasty (THA) [1, 2]. Both periacetabular adaptive bone remodelling, known as stress shielding, and osteolysis, due to wear debris, could negatively influence periacetabular bone mass. By combining clinically-proven titanium and a new three-dimensional (3D) porous construct shell backside with an enhanced interconnecting pore structure implant, the manufacturers of this implant claim that they can enhance osseointegration and reduce adaptive periacetabular bone resorption [3]. No in-vivo results regarding this on humans have been published. Design rationales for the acetabular shell with the 3D porous titanium backside are to increase friction, porosity and compressive strength. These features are said to increase initial stability and to enhance bone ingrowths into the titanium construct.
In this study, we compare a new acetabular implant with a porous titanium backside to a clinically well-proven titanium cup with a porous coated backside.
Patients and methods
Trial design
This was a prospective randomized controlled trial between October 2009 and August 2013 at the Orthopaedic Department at Danderyd Hospital, in collaboration with the Department of Clinical Sciences at Karolinska Institute in Stockholm. The CONSORT statements were followed [4]. The design and performance of the clinical trial were approved by the local ethics committee and the local committee for protection against radiation. The trial was initiated, designed and performed as an academic investigation.
Participants
We recruited patients with primary osteoarthritis, 40–70 years of age, scheduled for THA. We included patients with bone stock suitable for uncemented cups, i.e., no large structural defects in the acetabulum and a type A or B femur according to the classification of Dorr et al. [5]. No previous hip surgery on the affected side was allowed, along with no regular intake of corticosteroids, bisphosphonates or cytostatic drugs six months prior to surgery. A body mass index (BMI) above 35 was set as an exclusion criterion. All patients gave their oral and written informed consent to participate in the study.
Implants and surgery
Patients in the porous titanium group received an acetabular shell with a backside of a 3D porous titanium (Regenerex™-shell, E1™-liner, Biomet, USA). The implant has a 1.5-mm thick trabecular-like porous titanium construct backside surface with an enhanced interconnecting porosity of 67 % and a mean pore size of 300 μm. The control group received a porous coated titanium shell (Pinnacle™-shell, Marathon™-liner, Depuy Johnson & Johnson, USA). This implant has a porous coating of sintered titanium beads with a mean pore size of 250 μm. Both liners were of highly cross-linked polyethylene (HXLPE). On the femoral side, we used a 32-mm cobalt-chrome head and matching uncemented implants from either of the manufacturers (Bimetric™, Biomet, USA and Proxima™, Depuy Johnson & Johnson, USA). Surgery was performed by five senior surgeons using a posterolateral approach [6]. Local infiltration analgesia with ropivacaine, ketorolac and epinephrine was administered perioperatively. Postoperatively, crutches were used during the initial weeks according to each patient's preference. Full weight-bearing was allowed as tolerated. Rehabilitation was supervised by a physiotherapist at the ward and thereafter in a day-clinic setting during the first few weeks.
Endpoints and evaluation
The primary endpoint was change in periacetabular bone mineral density 24 months after surgery. The secondary endpoints were cup migration and the functional clinical result. Follow-up was performed continuously through the study period at six weeks (RSA only) and at three, six, 12 and 24 months postoperatively.
Bone densitometry analysis
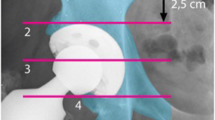
Bone mineral density was measured in four zones defined by Wilkinson et al. [7] and subsequently modified by Laursen et al. [8] (Fig. 1). The change in bone mineral density in each zone was calculated by dividing the bone mineral density value from each examination by the baseline value measured two days postoperatively. The ratio was expressed as a percentage of the baseline value. Each patient's individual regions of interest (ROI) were saved and used for subsequent examinations to reduce measurement errors.
After 12 months of follow-up, we performed duplicated examinations with repositioning of the patient to calculate the precision of the DXA measurements. Precision was calculated as the coefficient of variation (CV) and was 2.9 %, 4.4 %, 5.6 % and 4.5 % in zones 1–4, respectively. We scanned the lumbar spine and the opposite hip to collect each patient's pre-operative general bone mass and classified it according to the World Health Organization (WHO) osteoporosis classification. After 24 months of follow-up, we re-scanned the lumbar spine. By comparing these values, we calculated each patient's loss in general bone mass over time. DXA-scanning was performed with a machine from General Electric Healthcare, Lunar Prodigy Advance (GE Healthcare, Pittsburg, US). The software used was enCore version 13.31.016. Quality controls of the DXA equipment were performed according to the manufacturer's guidelines. No deviation from ordinary high-quality functioning was found during the study period.
Radiostereometry
Migration of the acetabular shell was evaluated with radiostereometry (RSA) [10]. UmRSA 6.0 computer software from RSA Biomedical AB, Sweden, was used together with a uniplanar calibration cage 43 from the same manufacturer. The digital calibrated stereo radiographs (Bucky Diagnostic™, Philips, Netherlands) were taken using one fixed and one mobile roentgen source. The periacetabular iliac bone was marked with up to nine one-mm diameter tantalum spheres that were well distributed around the acetabulum to form a rigid body segment. The cup segment was measured with a markerless technique because both types of implanted acetabular shells were hemispherical. This is an edge-detecting ellipse algorithm that outlines the outer diameter and the opening diameter of the metal shell [9]. Perioperatively, we inserted tantalum marker beads in the peripheral rim of the polyethylene liners as well. The markerless algorithm was used in conjunction with between one and three consistently visible liner beads to form a rigid body cup segment, which was used to evaluate three-dimensional translations and rotations in six degrees of freedom in relation to the surrounding periacetabular pelvic bone. We followed the published guidelines for radiostereometric analysis [10]. Mean error of body fitting <0.3 mm and condition number <137 were set as the cut-off limits to be included in the RSA analysis. After 12 months of follow-up, we performed duplicate RSA examinations in all the patients with complete repositioning of the X-ray tubes and the calibration cage. We calculated the precision as the 95 % confidence interval (CI) (SD 1.96) of the difference between these examinations. For translation along the x-(transverse), y-(vertical) and z-(anteroposterior [AP]) axes, this was 0.19, 0.16 and 0.29 mm, respectively. For rotation about the x-(flexion/extension), y-(ante-/retroversion) and z-(varus/valgus) axes, the values were 1.1°, 1.1° and 0.5°, respectively. The precision for our RSA setting was similar to that previously reported [11].
Clinical outcome
The clinical result was evaluated with a self-administered score protocol at each follow-up. Hip specific outcome score was the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [12]. Health-related quality of life was measured with EQ-5D [13].
Sample size and power analysis
We conducted a power analysis (two-sided, p = 0.05) before the study start and tested the null hypothesis that the mean change in bone mineral density would be equal in both groups. We assumed that ROI 1 and 2, proximal to the cup, were the most interesting zones because compromised bone stock and focal osteolysis in this region would be of great importance should a later cup revision be necessary. We also assumed that a mean difference of 10 % (standard deviation of 10 % [7, 14]) in bone mineral density in ROI 1 and 2 would be the smallest difference of clinical relevance. We calculated that a total of 44 patients (22 in each group) would have a power of 90 % to yield a statistically significant result. We planned to include 50 patients to accommodate drop outs.
Randomization
Pre-operative general bone mass is an important factor influencing periprosthetic bone loss after hip arthroplasty [15–17]. Therefore, we stratified the randomization for age and sex, factors known to influence general bone mass [17, 18]. To obtain an approximately equal number of patients in the two age strata, the age ranges were set to 40–59 and 60–70 years of age. Each stratum contained several blocks of four. Patients were randomly assigned at a 1:1 ratio to the porous titanium group or control group. Randomization was carried out with the use of sequentially numbered, opaque, sealed envelopes. A research nurse generated the random allocation sequence. None of the surgeons involved in recruiting and operating on the patients were involved in the randomization process. Neither patients nor surgeons were blinded during the study.
Statistical methods
Subjects with missing bone mineral density data or missing migration data at any of the follow-up visits were analysed by carrying the last observation forward. This was done for two single follow-ups in the porous titanium group and two in the control group. BMD- and stem-migration data were tested for normality and homogeneity using the Kolmogorov-Smirnov and Levene's tests. Because both sets of data were normally distributed, we used the unpaired Student t-test for between-group comparisons. Correlation between changes in bone mineral density and migration and other factors known to influence BMD, i.e., age, sex, BMI [14, 19] and preoperative general bone mass, was analysed with a multivariate linear regression analysis. We used the non-parametric Mann–Whitney U-test for between-group comparisons of clinical score data because they are ordinal data levels.
Results
Participant flow and baseline data
We included a 51st patient because one patient died of causes unrelated to surgery five months after inclusion. One patient randomized to the porous titanium group received a control group cup instead due to technical error during surgery. This patient stayed in the designated group for analysis according to intention-to-treat. Baseline data were similar in the two groups (Table 1, Fig. 2).
Complications
One patient in the control cup group underwent a stem revision three weeks postoperatively due to a calcar femoral fracture. One patient in the porous titanium group suffered from increasing hip pain starting several months after surgery. Radiolucent lines were visible along the stem but not behind the cup. During revision surgery, a low virulent deep periprosthetic infection could be verified. These two patients have been excluded from the analysis of the clinical results. We observed no dislocation and no thromboembolic event.
Bone remodelling
Comparison of bone remodelling in the periacetabular region as an entity, i.e., zones 1–4, showed that bone mineral density was almost completely restored to baseline values after 24 months in both groups. The difference of −1.5 % was non-significant (95 % CI 2.8 to −5.9, p = 0.483; Fig. 3). The pattern of bone remodelling was also similar in the two groups, with diminishing BMD in the two proximal zones and increasing BMD in the two distal ones. However, the extent of change in BMD differed in individual zones (Table 2).
The results of the primary endpoint was unchanged after factors known to influence BMD, i.e., age, sex, BMI and pre-operative general bone mass, were included as covariates in a multivariate linear regression analysis. We found a statistically significant association (p = 0.007) between BMI and bone remodelling in zone 1 after 24 months. High BMI was correlated with less demineralization. No significant loss in general bone mass after 24 months was found in any of the patients when we rescanned the lumbar spine.
Implant migration
There was an initial migration of the implants in both groups up to six months postoperatively. After this, all implants were stable, i.e. there were no micromotions above the precision of RSA. We observed no radiographic signs of loosening. However, the migration pattern differed slightly between the two groups (Table 3). The porous titanium group migrated 0.14 mm (95 % CI −0.28 to −0.0005, p = 0.049) more proximally than the control group (Fig. 4). The differences in migration in all other axes were statistically non-significant at every follow-up visit. In the linear regression analysis, none of the studied covariates affected migration up to 24 months.
Clinical results
We recorded improvements in WOMAC score in both groups two years after surgery, increasing from median (range) 43 (5–72) preoperatively to 95 (47–100) in the control group and from 47 (15–70) to 94 (70–100) in the porous titanium group (p = 0.610). The improvement in health-related quality of life was also similar; EQ-5D increased from 0.69 (median) preoperatively to 1.00 (median) at two-year follow-up in both groups.
Discussion
In this randomized controlled trial comparing periacetabular bone remodelling and component fixation in two acetabular implants with differing properties regarding shell backside finish we found no clinically relevant differences between the implants and thus no sign of enhanced osseointegration nor reduced adaptive periacetabular bone resorption. Both implants conserved the periacetabular bone, but the pattern of bone remodelling differed slightly. Micromotions in the acetabular implants were also small. Although the proximal migration for the porous titanium group was slightly larger, after an initial “bedding in” process in both types of shells, no continuous migration was observed. Thus, in the short two-year time perspective and with the number of patients available in this study, we could not see any clinically relevant differences between bone remodelling or implant fixation between the two cups.
Bone remodelling
Several studies reporting periacetabular bone remodelling with DXA [14, 20, 21] have demonstrated a pronounced reduction in BMD in the proximal regions and a minor reduction, or a gain, in BMD in the distal regions. We observed the same pattern in this study. Initially, after insertion of a press-fit cup, the load will primarily be transferred to the rim of the acetabulum [22]. Before bone ingrowth has occurred, rim loading will protect the load transfer to the backside area of the shell. This could contribute to the initial decrease in BMD in the two proximal zones. The effect of rim loading on bone mineral density, with a slower decrease in cortical bone and a more pronounced decrease in cancellous bone, cannot be distinguished with the DXA method used in this study.
In the porous titanium group, the cup seemed to save more bone in the zones behind the shell, i.e., zones 2 and 3. It is possible that the increased porosity and the trabecular-like geometry of the backside coating in this cup enables a deeper and more evenly distributed bone ingrowth than the control groups porous-coated surface. This could explain the trend of higher BMD-values measured in these zones. If shell fixation is more pronounced in zones 2 and 3, this might result in less compression forces transferred to the proximal periacetabular bone, which could contribute to the larger reduction in BMD in zone 1 observed in the porous titanium group.
A possible explanation for the unanticipated phenomenon that bone preservation is more pronounced in the distal two zones than in the proximal two is that a well-fixed shell will induce traction forces acting on the periacetabular bone distally, and traction forces are a strong stimulus for an increase in bone mineral density. Another plausible explanation for the increase in BMD in zone 3 is that the acetabular floor is medialized through reaming during surgery. The native bone underlying the acetabular fossa will be loaded by the absolute proximity of the implanted metal shell. This will contribute to a change in load pattern in this zone. If bone ingrowth is more pronounced in the porous titanium group, this could explain the trend of higher BMD in zone 3.
Migration
Excessive early migration and continuous migration of a joint replacement implant can predict later implant loosening [23, 24]. Prediction of a threshold when an implant might be at risk of later loosening has been suggested in several studies [23, 25]. For an uncemented implant, it is not dichotomized if a minimum of micromotion occurs initially before the osseointegration process has started, as long as the implant becomes stabilized after such a “bedding in” process. In our study, the initial rotation around the x- and y-axes was slightly larger compared to what others have reported [14, 26, 27]. This finding may depend on the fact that in this study, we under-reamed the acetabulum by 1 mm instead of the more common 2 mm, and only in one case did we use one screw for additional primary stability of the shell. Despite this, both types of implants achieved good fixation, and we observed no continuous migration of the components. The only statistically significant difference in migration between the groups was that the porous titanium group, with its rougher backside surface, migrated more proximally than the control group (Fig. 3). This proximal migration occurred during the first six months. Although the amount of migration was above the level suggested by Pijls et al. [25], after three months, none of the cups had a migration in any direction above the detection resolution of RSA. Thus, all were firmly fixed in bone. It is possible that the larger proximal migration in the porous titanium group is an effect of the higher friction coefficient of this cup. As a result, we may not have been able to impact the shell all the way in during surgery to achieve absolute proximity to the acetabular bone as often as we did with the control group's shells. On plain postoperative radiographs however, this hypothetical difference in seating of the implants was not visible. We also found no radiolucent lines in any of the implants of the study, thus questioning the clinical importance of this difference in migration.
Clinical outcomes
Patients in both groups reported excellent improvements in clinical scores with no statistically significant differences between the groups. This indicated that both types of cups functioned very well from a clinical perspective. In the majority of comparative total joint replacement studies evaluating clinical outcome, the improvement is of such a magnitude that it is difficult to find a difference between groups related to implant matters. The difference between implants might be of great importance in the long run, especially when younger patients undergo operation, but the clinical difference related to implant factors in the short term tends to be overshadowed by the great improvement that is normally observed after any type of modern total joint replacement surgery.
Strengths and weaknesses
To the best of our knowledge, this is the first randomized controlled study comparing this new porous titanium shell with a porous-surface implant in uncemented THA. We were able to follow all of the patients, with none lost to follow-up. Our randomization was successful with similar baseline characteristics of the subjects. Highly accurate methods used to evaluate bone mineral density (DXA), implant micromotion (RSA) and polyethylene wear (RSA) were other strengths of this study. In addition, the study was performed by an independent academic centre with no relation to the manufacturers of the implants.
The study was not blinded, but the analysis of the effect was performed according to the intention-to-treat principle. Weaknesses in the study design were the differences regarding different stems and the fact that the stems were from different manufacturers. There are a number of variables in this study, including stem type and design of the cup shell. These variables may interact and thus confound the outcomes of the study. Ideally, the design of the shell should be used as a single variable to study bone remodelling (and probably cup migration). This is a well-known problem and dilemma when planning and conducting randomized studies comparing different joint implants. To what extent such confounding variables influence the outcomes is obviously difficult to estimate.
Conclusions
In conclusion, the cups used for both the porous titanium group and the control group conserved the periacetabular bone after 24 months, even though the pattern of periacetabular bone remodelling differed between the two groups. Fixation of the shell used in the porous titanium group is, up to two years, as good as the well-proven and clinically well-functioning cup used for the control group.
References
Thanner J, Karrholm J, Malchau H, Herberts P (1999) Poor outcome of the PCA and Harris-Galante hip prostheses. Randomized study of 171 arthroplasties with 9-year follow-up. Acta Orthop Scand 70(2):155–162
Hallan G, Lie SA, Havelin LI (2006) High wear rates and extensive osteolysis in 3 types of uncemented total hip arthroplasty: a review of the PCA, the Harris Galante and the Profile/Tri-Lock Plus arthroplasties with a minimum of 12 years median follow-up in 96 hips. Acta Orthop 77(4):575–584. doi:10.1080/17453670610012638
Lim L, Bobyn JD, Bobyn KM, Lefebvre LP, Tanzer M (2012) The Otto Aufranc Award: Demineralized bone matrix around porous implants promotes rapid gap healing and bone ingrowth. Clin Orthop Relat Res 470(2):357–365. doi:10.1007/s11999-011-2011-y
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340:c332
Dorr LD, Faugere MC, Mackel AM, Gruen TA, Bognar B, Malluche HH (1993) Structural and cellular assessment of bone quality of proximal femur. Bone 14(3):231–242
Moore AT (1957) The self-locking metal hip prosthesis. J Bone Joint Surg Am 39-A(4):811–827
Wilkinson JM, Peel NF, Elson RA, Stockley I, Eastell R (2001) Measuring bone mineral density of the pelvis and proximal femur after total hip arthroplasty. J Bone Joint Surg (Br) 83(2):283–288
Laursen MB, Nielsen PT, Soballe K (2005) DXA scanning of acetabulum in patients with cementless total hip arthroplasty. J Clin Densitom 8(4):476–483
Borlin N, Rohrl SM, Bragdon CR (2006) RSA wear measurements with or without markers in total hip arthroplasty. J Biomech 39(9):1641–1650
Valstar ER, Gill R, Ryd L, Flivik G, Börlin N, Kärrholm J (2005) Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop 76(4):563–572. doi:10.1080/17453670510041574
Lazarinis S, Milbrink J, Mattsson P, Mallmin H, Hailer NP (2014) Bone loss around a stable, partly threaded hydroxyapatite-coated cup: a prospective cohort study using RSA and DXA. Hip Int 24(2):155–166. doi:10.5301/hipint.5000104
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12):1833–1840
Rabin R, de Charro F (2001) EQ-5D: a measure of health status from the EuroQol Group. Ann Med 33(5):337–343
Baad-Hansen T, Kold S, Nielsen PT, Laursen MB, Christensen PH, Soballe K (2011) Comparison of trabecular metal cups and titanium fiber-mesh cups in primary hip arthroplasty: a randomized RSA and bone mineral densitometry study of 50 hips. Acta Orthop 82(2):155–160. doi:10.3109/17453674.2011.572251
Rahmy AI, Gosens T, Blake GM, Tonino A, Fogelman I (2004) Periprosthetic bone remodelling of two types of uncemented femoral implant with proximal hydroxyapatite coating: a 3-year follow-up study addressing the influence of prosthesis design and preoperative bone density on periprosthetic bone loss. Osteoporos Int 15(4):281–289
Skoldenberg OG, Salemyr MO, Boden HS, Ahl TE, Adolphson PY (2011) The effect of weekly risedronate on periprosthetic bone resorption following total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am 93(20):1857–1864. doi:10.2106/JBJS.J.01646
Alm JJ, Makinen TJ, Lankinen P, Moritz N, Vahlberg T, Aro HT (2009) Female patients with low systemic BMD are prone to bone loss in Gruen zone 7 after cementless total hip arthroplasty. Acta Orthop 80 (5):531–537. doi:10.3109/17453670903316801 [pii] 10.3109/17453670903316801
Brodner W, Bitzan P, Lomoschitz F, Krepler P, Jankovsky R, Lehr S, Kainberger F, Gottsauner-Wolf F (2004) Changes in bone mineral density in the proximal femur after cementless total hip arthroplasty a five-year longitudinal study. J Bone Joint Surg Br 86(1):20–26
Laursen MB, Nielsen PT, Soballe K (2007) Bone remodelling around HA-coated acetabular cups : a DEXA study with a 3-year follow-up in a randomised trial. Int Orthop 31(2):199–204. doi:10.1007/s00264-006-0148-1
Penny JO, Brixen K, Varmarken JE, Ovesen O, Overgaard S (2012) Changes in bone mineral density of the acetabulum, femoral neck and femoral shaft, after hip resurfacing and total hip replacement: Two-year results from a randomised study. J Bone Joint Surg (Br) 94(8):1036–1044
Digas G, Kärrholm J, Thanner J (2006) Different loss of BMD using uncemented press-fit and whole polyethylene cups fixed with cement: repeated DXA studies in 96 hips randomized to 3 types of fixation. Acta Orthop 77(2):218–226
Schmidt R, Kress AM, Nowak M, Forst R, Nowak TE, Mueller LA (2012) Periacetabular cortical and cancellous bone mineral density loss after press-fit cup fixation: a prospective 7-year follow-up. J Arthroplasty 27(7):1358–1363. doi:10.1016/j.arth.2011.09.031
Karrholm J, Borssen B, Lowenhielm G, Snorrason F (1994) Does early micromotion of femoral stem prostheses matter? 4–7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg (Br) 76(6):912–917
Karrholm J (2012) Radiostereometric analysis of early implant migration—a valuable tool to ensure proper introduction of new implants. Acta Orthop 83(6):551–552. doi:10.3109/17453674.2012.745352
Pijls BG, Nieuwenhuijse MJ, Fiocco M, Plevier JW, Middeldorp S, Nelissen RG, Valstar ER (2012) Early proximal migration of cups is associated with late revision in THA: a systematic review and meta-analysis of 26 RSA studies and 49 survival studies. Acta Orthop 83(6):583–591. doi:10.3109/17453674.2012.745353
Wolf O, Mattsson P, Milbrink J, Larsson S, Mallmin H (2012) The effects of different weight-bearing regimes on press-fit cup stability: a randomised study with five years of follow-up using radiostereometry. Int Orthop 36(4):735–740. doi:10.1007/s00264-011-1413-5
Zhou ZK, Li MG, Borlin N, Wood DJ, Nivbrant B (2006) No increased migration in cups with ceramic-on-ceramic bearing: an RSA study. Clin Orthop Relat Res 448:39–45. doi:10.1097/01.blo.0000223999.10389.c9
Acknowledgments
We gratefully acknowledge help in funding from these foundations: Ulla and Gustaf Ugglas Stiftelse, Åke Wiberg Stiftelse, Loo and Hans Ostermans Stiftelse, Sven Norén Foundation, and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salemyr, M., Muren, O., Eisler, T. et al. Porous titanium construct cup compared to porous coated titanium cup in total hip arthroplasty. A randomised controlled trial. International Orthopaedics (SICOT) 39, 823–832 (2015). https://doi.org/10.1007/s00264-014-2571-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2571-z