Abstract
Purpose
Autologous iliac crest bone graft (ICBG) is the gold standard material for spinal fusion. Bone graft substitutes, such as recombinant human bone morphogenic protein 2 (rhBMP-2) have been developed to promote spinal fusion and address morbidity issues related to ICBG harvesting. The objective of this study was to compare bone fusion rates after anterior lumbar interbody fusion (ALIF) between ICBG and rhBMP-2 by examining thin-cut computed tomography (CT) images at the one year follow-up.
Methods
Fifty one patients (62 levels) who underwent single- or two-level ALIF via the video-assisted minimally invasive anterior approach in our institution were assessed. Radiolucent cages were inserted in all cases. Each cage has a middle beam delimiting two chambers. Grafting was performed as follows: one chamber was filled with autologous ICBG, and the other chamber was filled with 6 mg of rhBMP-2. Thin-cut CT-scan multiplanar reconstruction analyses were performed to assess the rate and quality of bone fusion at one year of follow-up.
Results
Fusion was observed in 55 levels (88.7 %), with significant differences in fusion rates with rhBMP-2 and ICBG (71 % vs. 88.7 %) (P=0.001). Osteogenesis in the rhBMP-2 chamber had a centripetal pattern in all cases, leaving a central void in 97.7 % of cases representing 38.3 % of the surface of its chamber (range 0–80.3 %). In ICBG chambers, graft resorption was present in 44.4 %, representing 9.8 % of the chamber surface (range 0–52.2 %).
Conclusion
RhBMP-2 was inferior to ICBG in terms of rate and quality of bone fusion in one- or two-level ALIF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, autologous iliac crest bone graft (ICBG) has been the gold standard material for spinal fusion due to its osteogenic, osteoinductive and osteoconductive characteristics. However, major or minor complications are associated with iliac crest bone harvest, with rates ranging from 1 % to 39 % and including haematoma, infection, prolonged chronic pain and sensory deficit [1–4]. The development of recombinant human bone morphogenetic protein-2 (rhBMP-2) was of promise because of its great osteoinductive capabilities and lack of donor-site morbidity. However, BMP has been associated with a variety of unique complications in the ventral cervical and lumbar spines [5], and a recent systematic review by Carragee et al. [6] suggested that there was level I and II evidence of increased risk of complications and adverse events to patients receiving rhBMP-2 in spinal fusion. After anterior interbody lumbar fusion (ALIF), rates of implant displacement, subsidence, infection, urogenital events and retrograde ejaculation were higher in patients receiving rhBMP-2 than in controls. Under criticism for sponsored studies for rhBMP-2, an independent data review was sponsored by the industry; in the Yale Open Data Access (YODA) project, two independent groups, using different methodologies, reviewed complete patient data from industry-sponsored clinical trials and found no appreciable clinical benefit to using rhBMP-2 instead of autograft in spinal fusion [7, 8]. However, to our knowledge, there is no previous report comparing fusion rates between ICBG and rhBMP-2 in the same cohort of patient. The main purpose of this retrospective comparative study was to determine whether bone fusion rates differ between ICBG and rhBMP-2 by examining computed tomography (CT) images at one year follow-up and assessing fusion quality. Data obtained on all patients were entered into a database on admission, and those records were updated whenever the patient returned for follow-up. Radiographic results and clinical details were retrospectively reviewed.
Materials and methods
Patients
All patients who underwent single- or two-level ALIF via a video-assisted, minimally invasive anterior approach in our institution between September 2008 and September 2009 were included in this series. Indications comprised the following groups: degenerative disc disease, degenerative lumbar scoliosis and degenerative and isthmic spondylolisthesis. Radiolucent polyetheretherketone (PEEK) ROI-A cages (LDR Medical, Troyes, France) were inserted in all cases. Each cage has a middle beam delimiting two chambers, as showed in Fig. 1. Grafting was performed as follows: one chamber was filled with autologous ICBG and the other with 6 mg rhBMP-2 (InductOs®, Medtronic Wyeth Pharmaceuticals, Maidenhead, Berkshire, UK). Body mass index (BMI) and smoking habit, which may influence bone fusion, were documented. Exclusion criteria were fusions performed with rhBMP-2 exclusively, or ICBG exclusively because of a contraindication to the use of rhBMP-2, such as a history of tumor or infection.
The ROI-A cage is separated into two chambers by a median beam (a). Analysis of computed tomography (CT) scans was performed comparatively using the same method. In order to correctly assess fusion, sagittal (b), axial (c) and frontal (d) reconstructions of the cage were performed using the multiplanar reconstruction (MPR) mode in Kodak Carestream Picture Archiving and Communications System (PACS) independently to their position in the disc space, using Tantalum markers and the middle beam of the cage as landmarks
Surgical procedure
The patient was positioned in a supine position on a radiolucent operating table. A left retroperitoneal approach was performed with fluoroscopic and videoscopic control through a transverse incision for L5–S1 or through a mini-lumbotomy incision for all other levels. Assessment of implant positioning was performed using Tantalum markers in the cage. After safely retracting the great vessels, a complete anterior discectomy was performed. When indicated, the posterior longitudinal ligament was released in order to remove extruded disc material and/or to regain appropriate intervertebral disc height. Intersomatic space distractors were used to gain as much disc height as possible. After extensive discectomy, endplate cartilage was removed, and the proper-sized implant was determined using trial implants. Particular attention was taken to preserve both endplates during disc preparation and implant insertion. Intersomatic cages filled with autologous ICBG and rhBMP-2 were then inserted under fluoroscopic control in the intervertebral space to maintain the correction and perform fusion. Two VerteBRIDGE® plates were then locked in the cage.
Radiographic assessment
A CT scan was performed in the early postoperative period and at one year follow-up; thin-cut multiplanar reconstruction (MPR) analyses were performed using Kodak Carestream Picture Archiving and Communications System (PACS) (Eastman Kodak/Carestream Health, Rochester, NY, USA). All scans were analysed comparatively using the same method. In order to correctly assess fusion, frontal, sagittal and axial reconstructions of the cage were performed, independently to their position in the disc space, using the Tantalum markers and the middle beam of the cage as landmarks, as show in Fig. 1. On thin-cut scan, two independent, blinded spinal surgeons graded endplate preparation and fusion. Endplate preparation was graded as summarized in Table 1. Fusion was graded at 1-year follow-up as follows:
-
Acquired fusion: trabecular bone continuity between the two vertebrae within and/or out of the cage, at least on one image in sagittal and/or coronary plane
-
Fusion failure: no trabecular bone continuity between the two vertebrae within and/or out of the cage, in both planes
-
Doubtful fusion: trabecular bone continuity between the two vertebrae interrupted by a median thin radiolucent line
The surface of the bone void was measured on the CT-scan section running axially through the cage and compared with the chamber surface and expressed as a percentage for BMP (absence of bone formation) and ICBG (resorption) (Fig. 2). Other documented items were ectopic or heterotopic bone formation, endplate osteolysis, geode formation defined as the presence of well-defined lytic lesion in vertebrae, and cage subsidence or migration. Subsidence was defined as any sinking of the cage into the vertebral plateau (comparison of CT scans done in the early postoperative period and at 1-year follow-up).
The surface of the bone void (right) was measured at 1-year follow-up on the computed tomography (CT) scan section running axially through the cage using the multiplanar reconstruction (MPR) mode in Kodak Carestream Picture Archiving and Communications System (PACS) and compared with the chamber surface (left), with results expressed as percentage
Statistical analysis
The null hypothesis for this study was that the fusion groups (rhBMP-2 and ICBG) were the same. Statistical analysis using SPSS version 18.0 included descriptive statistics, t test and Pearson’s chi-squared test. Statistical significance was set at P < 0.05.
Results
We evaluated 51 patients (62 levels). Single-level ALIF was performed in 40 patients (78.4 %) and two-level ALIF in 11 (21.6 %). Demographic data, surgical indication levels of fusion and grades of endplate preparation are listed in Table 1.
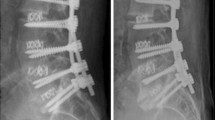
Fusion was observed in 55 levels (88.7 %). However, analysis of CT scans at one year follow-up showed significant differences in fusion rates between rhBMP-2 and ICBG (Fig. 3). The rate of acquired fusion was significantly lower with rhBMP-2 than with ICBG (71 % vs. 88.7 %) (P = 0.001). We found no heterotopic ossifications. Three patients (4.8 %) experienced pseudarthrosis: two underwent two-level fusions for degenerative lumbar scoliosis and one was an L5/S1 isthmic spondylolisthesis. Complementary posterolateral fusion was performed in the first two patients; the last one declined surgery because of good functional result.
A significant effect of smoking was found in the multivariate analysis with respect to fusion with rhBMP-2 (P = 0.04) and ICBG (P = 0.03). No significant effect of smoking was found on the occurrence of geode formation, endplate osteolysis or subsidence. A significant effect of two-level fusion towards higher nonunion rates was found with respect to overall fusion (P = 0.009) with ICBG (P = 0.033) but not with rhBMP-2 (P = 0.21). No significant effect of the indication for fusion was found in terms of fusion occurrence. Corporeal microgeodes and endplate osteolysis were significantly more frequent adjacent to chambers filled with rhBMP-2 than chambers filled with ICBG (respectively, P < 0.001 and P = 0.016) (Fig. 4). The rate of secondary cage subsidence at one year follow-up was 17.7 % (n = 11), but none led to revision surgery. We found no correlation between the rate of endplate osteolysis and subsidence. It was not possible to determine if subsidence occurred more on the BMP side or on the ICBG side. There was no cage migration.
Analysis of the bone bridge in each chamber confirmed that osteogenesis in the rhBMP-2 chamber had a centripetal pattern in all cases, leaving a central void in 97.7 % of cases. In ICBG chambers, resorption—seen as a central bone void—was present in 44.4 % of cases (Table 2). The bone bridge visible at 1-year follow-up was significantly larger in the ICBG chamber than in the rhBMP-2 chamber.
Discussion
Bone graft substitutes, such as BMP, have been developed to address the issues related to morbidity with ICBG harvesting. The rhBMP-2 was introduced commercially in 2002 and increasingly used by surgeons all over the world in on- and off-label indications because it was easy to use and because early reports showed a similar rate of fusion with low rates of complications [5, 6]. Two studies confirm that the use of BMP in spinal fusion procedures has grown dramatically since 2002 in the United States, expanding to off-label indications [9, 10]. In spine surgery, rhBMP-2 (INFUSE®, Medtronic, Memphis, TN, USA) is US Food and Drug Administration (FDA)-approved for single-level use in the ALIF for degenerative disc disease or grade I spondylolisthesis using titanium LT-Cages® (Medtronic) [11]. However, according to an epidemiological study published in 2010, at least 85 % of principal procedures using BMP in the United States were for off-label applications, including but not limited to posterolateral lumbar fusions, uni- or multilevel ALIF with PEEK cages and primary cervical fusions [9]. Recent reviews and the Yale University Open Data Access (YODA) project suggest that complication rates are underrated [5–8]. Mroz et al. [5] state that there is a lack of substantive data or consensus among the reviewed studies to determine the ideal accompanying grafting material or interbody cage (e.g. synthetic, metallic, allograft), carrier, optimal dosing and placement location of BMP-2 in the interbody space. The reason we performed this study was to have a realistic comparison of bone union rates in ALIF procedures between rhBMP-2 and ICBG using each patient as its own control; we used a PEEK cage with two chambers separated by a middle beam, hypothesizing that the beam was limiting local interaction between rhBMP-2 and ICBG.
As a result, we demonstrated that the fusion rate at one year follow-up was significantly higher with ICBG than with rhBMP-2. Previous reports showed that the fusion rate achieved by autologous ICBG was as high as 71.4–100 % [12, 13] . There are few reports that compare rhBMP-2 and ICBG in ALIF, and most of those reports are industry-sponsored, peer-reviewed publications [6–8]. In a preliminary study, Boden et al. [14] showed that all 11 patients who received rhBMP-2 had solid fusions at six months, whereas only two of the three control (ICBG) patients were fused. In a multicenter, prospective, randomized study on 279 patients who underwent ALIF for degenerative lumbar disc disease, Burkus et al. [15, 16] reported that at 12 and 24 months, the investigational group (rhBMP-2) showed higher rates of fusion when compared with the control group (ICBG), with 94.5 % and 88.7 %, respectively. Vaidya et al. [17], in a non-industry-sponsored study, reported that a high rate of fusion (100 %) was achieved with rhBMP-2 but also showed that significant subsidence occurred in more than half of the levels (23 of 37). Pradhan et al. [18] demonstrated that the use of rhBMP-2 did not enhance the fusion rate in stand-alone ALIF with femoral-ring allografts and that there was a trend towards a higher nonunion rate with rhBMP-2, although it was not significant with the numbers available. In the YODA project, the Oregon group found that rhBMP-2 provided little or no benefit over bone graft and may be associated with important harm, making it difficult to identify clear indications for rhBMP-2 [8]. In ALIF, there was no consistent difference between rhBMP-2 and ICBG in fusion rates from six weeks through 24 months after surgery [8]. At 24 months, fusion rates ranged from 60 % to 100 %. However, based on the same data analyzed with a different methodology, the York group concluded that radiographic fusion was 12 % higher with rhBMP-2 compared with ICBG [7].
The relatively low rate of fusion with rhBMP-2 in our study can be related to many factors. First, analysis of bone bridging may be more difficult with rhBMP-2 than with ICBG, as we had significantly more doubtful fusions with rhBMP-2 than with ICBG (Fig. 3). This might be due to the quality of the bone bridge, which is also questionable, as a central void is found in almost all cages. This void represents a mean 37.8 % (range 0–80.3 %) of the cage’s chamber surface (Fig. 5 and Table 2). Second, control CT-scan timing might be an issue. When cancellous autograft is used, full graft integration into the native bony structure is well underway by six months and usually complete by one year [18]. The centripetal osteogenesis with rhBMP-2 is a slower process; however, in the aforementioned studies [14–17], fusion was complete and documented at one year follow-up in almost all cases. Therefore, similar rates should be found in our report presented here. Third, we cannot prove that there was no interaction between rhBMP-2 and ICBG despite the central beam dividing the cage into two chambers. These possible interactions might have slowed the osteogenesis process with rhBMP-2 and might have increased ICBG resorption. Last, we chose a dose of 6 mg of InductOs®, which is higher than recommended with LT-Cages® (2–4 mg). This dose was given because the cages we used were larger, and the larger dose therefore seemed more appropriate. As stated by Mroz et al. [5], there is a lack of evidence from well-designed and executed controlled studies regarding the dose–response relationship between rhBMP-2 dose and fusion and complication rates. Moreover, many studies do not specify the dose of rhBMP-2 placed within the cage, as if such data was not important [5, 6]. However, BMP has a role in regulating bone turnover via coupled osteoblastic and osteoclastic activity [18]. Itoh et al. [19] showed that BMPs induce osteoclast differentiation and survival, which occurs, as with fracture healing, before bone formation by osteoblasts. Poynton and Lane [20] warned that large doses of BMP may lead to local areas of resorption. The correct dosage of rhBMP-2 should certainly be adapted to the distance between vertebral endplates, i.e. to the height and volume of the cage.
L4–L5 anterior lumbar interbody fusion with a radiolucent polyetheretherketone (PEEK) cage. Axial (a), frontal (b) and sagittal (c, d) reconstructions of the cage performed at 1-year follow-up using the multiplanar reconstruction (MPR) mode in Kodak Carestream Picture Archiving and Communications System (PACS); rhBMP-2 is placed within the left chamber (a, b, d) and the iliac crest bone graft in the right chamber (a, b ,c). Corporeal microgeodes (b) demonstrate bone remodeling
The rate of corporeal microgeode development was significantly higher adjacent to rhBMP-2 chambers, even though an important amount of microgeodes was also found adjacent to the ICBG chambers. These geodes reflect bone remodeling and may be due to diffusion of rhBMP-2 in the vertebral body. Localised bone remodeling has already been reported in many studies [5, 6]; Burkus et al. [21] showed that it was completely healed and filled with new trabecular bone by 24 months and that it had no effect on fusion. We also found a significant difference in endplate osteolysis but could not link it to the high rate of cage subsidence (17.7 %). Higher rates of resorption have been noted with BMP, and this is presumed to be a result of enhanced osteoclastic activity due to the BMP [22]. Vaidya et al. [23] reported an 82 % resorption rate in a prospective study that evaluated rhBMP-2 for single- or multilevel lumbar interbody fusion surgery with PEEK cages. The transition from resorption to bone formation occurred between six and nine months after surgery. The degree of resorption varied between patients as well as between vertebral levels in patients who underwent fusion of more than one level. However, to ascertain endplate resorption, it is essential to compare the one year follow-up CT scan to a CT scan performed in the early postoperative period, which is not the case in most reported series. It is probable that some of the so-called endplate resorption is actually an endplate violation related to surgical technique. For instance, in our series, correct endplate preparation (grade 1 and 2) was found in only 82.3 % of cases (Table 1).
We report a 17.7 % rate of cage subsidence but no cage migration. Due to the small number of patients, we could not find a correlation between endplate preparation, endplate osteolysis and cage subsidence. The higher rate of subsidence has been previously reported [6, 8]. These data also suggest a clinically important early osteoclastic effect of rhBMP-2 in bone [6, 22]. The absence of migration is most likely due to cage fixation by the VerteBRIDGE® plates.
The study presented here reports the rates of fusion and radiographic complications in a cohort of patients treated with rhBMP-2 and ICBG. One limitation of the study is that we cannot prove the absence of interaction between ICBG and rhBMP-2 in the cage. Second, assessment of fusion can be subjective; therefore, we performed a double analysis of each early postoperative and one year follow-up CT scan to improve efficiency. Third, this was not a prospective randomized control trial but a retrospective comparative study. Its originality lies in the fact that each patient was his or her own control, thus limiting bias related to patient demographics, smoking habits, surgical indications or levels fused.
In conclusion, we found that rhBMP-2 was inferior to ICBG in terms of fusion rate and quality.
References
Banwart JC, Asher MA, Hassanein RS (1995) Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976) 20(9):1055–1060
Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, Hilibrand AS, Vaccaro AR, Albert TJ (2003) Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 28(2):134–139. doi:10.1097/01.BRS.0000041587.55176.67
Summers BN, Eisenstein SM (1989) Donor site pain from the ilium. A complication of lumbar spine fusion J Bone Joint Surg Br 71(4):677–680
Sasso RC, LeHuec JC, Shaffrey C, Spine Interbody Research G (2005) Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech 18 Suppl:S77-81. doi:00024720-200502001-00011 [pii]
Mroz TE, Wang JC, Hashimoto R, Norvell DC (2010) Complications related to osteobiologics use in spine surgery: a systematic review. Spine (Phila Pa 1976) 35(9 Supp):S86–104. doi:10.1097/BRS.0b013e3181d81ef2
Carragee EJ, Hurwitz EL, Weiner BK (2011) A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 11(6):471–491. doi:10.1016/j.spinee.2011.04.023
Simmonds MC, Brown JV, Heirs MK, Higgins JP, Mannion RJ, Rodgers MA, Stewart LA (2013) Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med 158(12):877–889. doi:10.7326/0003-4819-158-12-201306180-00005
Fu R, Selph S, McDonagh M, Peterson K, Tiwari A, Chou R, Helfand M (2013) Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med 158(12):890–902. doi:10.7326/0003-4819-158-12-201306180-00006
Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD (2010) Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine (Phila Pa 1976) 35(19):1794–1800. doi:10.1097/BRS.0b013e3181ecf6e4
Lad SP, Nathan JK, Boakye M (2011) Trends in the use of bone morphogenetic protein as a substitute to autologous iliac crest bone grafting for spinal fusion procedures in the United States. Spine (Phila Pa 1976) 36(4):E274–281. doi:10.1097/BRS.0b013e3182055a6b
Epstein NE (2011) Pros, cons, and costs of INFUSE in spinal surgery. Surg Neurol Int 2:10. doi:10.4103/2152-7806.76147
Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM (2000) Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976) 25(11):1437–1446
Thalgott JS, Fogarty ME, Giuffre JM, Christenson SD, Epstein AK, Aprill C (2009) A prospective, randomized, blinded, single-site study to evaluate the clinical and radiographic differences between frozen and freeze-dried allograft when used as part of a circumferential anterior lumbar interbody fusion procedure. Spine (Phila Pa 1976) 34(12):1251–1256. doi:10.1097/BRS.0b013e3181a005d7
Boden SD, Zdeblick TA, Sandhu HS, Heim SE (2000) The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine (Phila Pa 1976) 25(3):376–381
Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA (2002) Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 27(21):2396–2408. doi:10.1097/01.BRS.0000030193.26290.DD
Burkus JK, Gornet MF, Dickman CA, Zdeblick TA (2002) Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech 15(5):337–349
Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD (2007) Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br 89(3):342–345. doi:10.1302/0301-620X.89B3.18270
Pradhan BB, Bae HW, Dawson EG, Patel VV, Delamarter RB (2006) Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 31(10):E277–284. doi:10.1097/01.brs.0000216442.12092.01
Itoh K, Udagawa N, Katagiri T, Iemura S, Ueno N, Yasuda H, Higashio K, Quinn JM, Gillespie MT, Martin TJ, Suda T, Takahashi N (2001) Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology 142(8):3656–3662
Poynton AR, Lane JM (2002) Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine (Phila Pa 1976) 27(16 Suppl 1):S40–48
Burkus JK, Sandhu HS, Gornet MF (2006) Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine (Phila Pa 1976) 31(7):775–781. doi:10.1097/01.brs.0000206357.88287.5a
McKay B, Sandhu HS (2002) Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine (Phila Pa 1976) 27(16 Suppl 1):S66–85
Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG (2008) Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech 21(8):557–562. doi:10.1097/BSD.0b013e31815ea897
Conflict of interest and funding source
None of the authors has, currently or in the past, financial activity for the work under consideration.
CH F-L is receiving travel expenses from LDR Medical, Medtronic and DePuy Synthes.
AG declares no conflict of interest.
JA is currently receiving honoraria, royalties, payment for lectures or travel support from Medtronic, DePuy Synthes, LDR Medical.
PH declares no conflict of interest.
AP has received travel support from LDR Medical.
CB has received travel support from LDR Medical, DePuy Synthes and Medtronic
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flouzat-Lachaniette, CH., Ghazanfari, A., Bouthors, C. et al. Bone union rate with recombinant human bone morphogenic protein-2 versus autologous iliac bone in PEEK cages for anterior lumbar interbody fusion. International Orthopaedics (SICOT) 38, 2001–2007 (2014). https://doi.org/10.1007/s00264-014-2301-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2301-6