Abstract
Purpose
Osteogenesis imperfecta is a serious genetic disorder that results from improper type I collagen production. We aimed to evaluate whether bone marrow stromal cells (BMSC) delivered locally into femurs were able to engraft, differentiate into osteoblasts, and contribute to formation of normal bone matrix in the osteogenesis imperfect murine (oim) model.
Methods
Donor BMSCs from bone-specific reporter mice (Col2.3GFP) were expanded in vitro and transplanted into the femoral intramedullary cavity of oim mice. Engraftment was evaluated after four weeks.
Results
We detected differentiation of donor BMSCs into Col2.3GFP+ osteoblasts and osteocytes in cortical and trabecular bone of transplanted oim femurs. New bone formation was detected by deposition of dynamic label in the proximity to the Col2.3GFP+ osteoblasts, and new bone showed more organized collagen structure and expression of type I α2 collagen. Col2.3GFP cells were not found in the contralateral femur indicating that transplanted osteogenic cells did not disseminate by circulation. No osteogenic engraftment was observed following intravenous transplantation of BMSCs. BMSC cultures derived from transplanted femurs showed numerous Col2.3GFP+ colonies, indicating the presence of donor progenitor cells. Secondary transplantation of cells recovered from recipient femurs and expanded in vitro also showed Col2.3GFP+ osteoblasts and osteocytes confirming the persistence of donor stem/progenitor cells.
Conclusion
We show that BMSCs delivered locally in oim femurs are able to engraft, differentiate into osteoblasts and osteocytes and maintain their progenitor potential in vivo. This suggests that local delivery is a promising approach for introduction of autologous MSC in which mutations have been corrected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteogenesis imperfecta (OI) is a genetic disorder caused by reduced or improper type I collagen production that results in bone fragility. Due to the early onset of disease symptoms and the requirement for multispecialty approaches, OI is one of the most expensive genetic disorders to treat. At present, the standard of treatment to ameliorate the frequent fractures and pain has been the use of bisphosphonates [1]. They act by inhibiting osteoclast function and bone resorption, but do not correct the primary cause of the disease. The underlying mutation is still responsible for continuous generation of mutated collagen molecules.
Recent studies have been directed at silencing or correcting the mutation in mesenchymal stem/progenitor cells (MSC) derived from patients with OI [2]. The introduction of normal collagen-producing MSCs to affected subjects will potentially provide an autologous source of healthy bone-forming cells. While this approach appears promising, multiple studies suggest that systemic introduction of MSCs, while showing therapeutic effects in numerous conditions, results in very low long-term functional engraftment [3]. Systemic introduction of mesenchymal lineage cells, either via parabiosis, bone marrow transplantation or direct intravenous injection in mice have shown engraftment of cells on the bone surface, but these cells lack the critical attributes of an osteoblast [4–6]. Therefore, further development of techniques for reintroducing engineered cells back into the patient is required. There have been a number studies attempting intravenous transplantation of MSCs into mouse models of OI, many of which have shown improvements in collagen production and biomechanical properties, however engraftment is consistently low [7–11]. Trials have also been performed in a small number of children with severe OI, but engraftment was also low, and it is unclear whether the transient clinical improvements were directly due to effects of the introduced cells [12–14]. It is also unclear in many of these studies whether the introduced cells are differentiating into osteoblast lineage cells and directly forming bone.
To address the issue of evaluating engraftment of osteoprogenitors we have previously developed and characterized markers that allow specific identification of cells at mature stages of the osteoblast lineage, such as visual markers under the control of a bone-directed collagen type I promoter (Col2.3GFP) [15]. For these studies we utilized the osteogenesis imperfecta murine (oim) mouse, which lacks expression of functional type I α2 collagen chains and instead produce an α1 homotrimer. We have evaluated the engraftment of wild type Col2.3GFP BMSCs delivered locally into femurs. Our results demonstrate that BMSCs delivered locally can engraft and contribute to formation of wild type-like bone within oim animals, and establish a pool of wild type osteoprogenitors in the local marrow environment.
Materials and methods
Mice
All animal procedures were approved by an institutional animal care and use committee. oim mice were obtained from Jackson Labs stock # 001815 (Bar Harbor, ME) and were genotyped as described [16]. Col2.3GFP mice in a C57Bl6 background were previously described [15].
Bone marrow stromal cell (BMSC) culture
BMSC for transplantation were prepared from eight- to ten-week old Col2.3GFP transgenic mice. Bone marrow was flushed from the femora and tibiae, and cells were seeded at a density of 4 × 105/cm2 and expanded in 5 % O2 in minimum essential medium alpha (αMEM), 10 % fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. After seven days, adherent cells were harvested in 2.5 % trypsin for transplantation.
To assess engraftment of osteoblast precursors, bone marrow from one femur was seeded per well of a 6-well plate in αMEM 10 % FBS. After seven days, cultures were supplemented with 50 μg/mL ascorbic acid and 8 mM β-glycerophosphate to induce osteogenic differentiation. Presence of Col2.3GFP + cells was evaluated by using an Observer Z1 fluorescence microscope (Carl Zeiss, Thornwood, NY).
Cell transplantation
In preparation for transplantation, two- to three-month-old oim mice were exposed to a lethal dose of 900 cGy total body gamma irradiation using a 137Cs source. Recipient mice were anaesthetized with ketamine (130 mg/kg) and xylazine (11 mg/kg). All animals received retroorbital infusion of 5 × 106 wild type non-transgenic whole bone marrow cells. Local transplantations were performed in the right femur bone marrow cavity. The knee was flexed to 90° and the distal end of the femur was drawn anteriorly. A 26-gauge needle was inserted into the joint surface through the patellar tendon and into the medullary space and 106 cells in 15 μL serum free αMEM were delivered. Systemic transplantation was performed by infusion of the cells into the retroorbital sinus. Mice were sacrificed four or 12 weeks after transplantation. In some cases, xylenol orange (XO) was injected to label mineralizing surfaces.
Histological analysis
Bones were fixed for three to five days in 10 % formalin, decalcified in 14 % EDTA (where applicable), incubated in 30 % sucrose overnight and embedded in cryomatrix (Thermo Fischer Scientific, Waltham, MA). Sections of 7 μm were obtained using a Leica cryostat (Wetzler, Germany) and tape transfer system (Section-lab, Hiroshima, Japan). Fluorescent images were obtained as previously described [17], and sections were stained with hematoxylin and reimaged.
Immunostaining
Decalcified bone sections were immunostained for the presence of collagen I α2 using an antibody kindly provided by Dr. Charlotte Phillips, University of Missouri–Columbia [18]. After permeablizing in PBS 0.1 % triton X, and blocking with Powerblock (Biogenex, San Ramon, CA), primary antibody was diluted 1:400 and applied at 4 °C overnight, followed by incubation with donkey anti-rabbit secondary conjugated to Cy3. Sections were mounted with 4',6-diamidino-2-phenylindole (DAPI) and fluorescent imaging was performed using standardized parameters on a 20x objective as described above.
Two photon imaging
Formalin fixed 50-μm sections were imaged using the Prairie Ultima IV multi-photon microscope with a 20X/0.95 W Olympus water immersion objective. The Col2.3GFP signal and second harmonic generation (SHG) for collagen were visualized at an excitation wavelength of 900 nm and bandpass filters of 435–485 nm (SHG) and 500–550 nm (Col2.3GFP).
Results
Locally transplanted cells contribute to new bone formation in oim animals
In this study we sought to establish whether transplanted wild type cells could contribute to the osteoblast lineage and form normal wild type-like bone in oim mice. In order to identify engrafted donor osteoblasts, we cultured BMSCs from Col2.3GFP mice. These animals have a GFP reporter that is activated specifically in mature osteoblasts and osteocytes. Bone marrow was cultured in 5 % oxygen to expand mesenchymal progenitor cells, and primary adherent cells (negative for Col2.3GFP reporter at the time of transplantation) were transplanted into the femoral bone marrow cavity. Since the transplanted cells were from mice with a different genetic background to oim, recipients were irradiated prior to transplantation. One month after transplantation, cell engraftment was assessed histologically. This demonstrated engraftment of Col2.3GFP+ donor-derived osteoblasts and osteocytes in both cortical and trabecular bone of the transplanted femur (Fig. 1). Labeling of mineralizing surfaces showed that Col2.3GFP+ cells on the bone surface were associated with areas of active mineralization confirming their osteoblastic phenotype (Fig. 2a–c). Col2.3GFP+ cells were still apparent in transplanted bones three months after transplantation (data not shown). oim mice produce a defective bone matrix because the type I collagen lacks the α2 chain. Immunostaining indicated that bone associated with engrafted osteoblasts contained α2 collagen (Fig. 3a), similar to wild type bone (Fig. 3c), while control oim bone was negative for α2 collagen (Fig. 3d). Second harmonic generation detected a change in collagen structure. Donor derived bone containing Col2.3GFP+ osteocytes showed a brighter collagen signal indicative of better collagen organization (Fig. 3f–h).
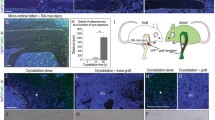
Locally transplanted BMSCs become osteoblasts and osteocytes in vivo. BMSCs (106) derived from Col2.3GFP mice were transplanted into oim mice by direct intrabone injection. (a–b) One month after transplantation, engraftment of osteogenic cells was assessed by the presence of donor-derived Col2.3GFP expression in the transplanted distal femur. Col2.3GFP+ osteoblasts are present on the bone surface and Col2.3GFP+ osteocytes are within the newly formed bone matrix. (c–d) High power images show recipient cortical bone (white line), while newly deposited bone with GFP+ osteocytes is indicated by the red line. Data are indicative of histology of 15 transplanted mice. M muscle, BM bone marrow
Transplanted cells are located in areas of active bone formation. oim mice underwent transplantation as described. Mineralizing surfaces were labeled with xylenol orange (red) the day before transplantation and one month later, the day before sacrifice. (a) Histology of a locally transplanted femur indicates that Col2.3GFP+ are closely associated with mineralization label. Higher power images show this in trabecular (b) and cortical (c) bone. (d) Histological analysis of the contralateral untransplanted femur did not show evidence of Col2.3GFP+ cells. (e) Histological analysis of femurs one month after systemic transplantation via retroorbital sinus showed similar absence of engrafted donor cells
Detection of normal collagen production in transplanted bones. (a–e) Bone sections were immunostained for the presence of type I α2 collagen (shown in red). Images of cortical bone are shown from a transplanted femur (a), the contralateral untransplanted femur (b), as well as sections from untreated wild type (c), and oim (d) mice. A negative control on a wild type section is also shown (e). Donor cells, where present, are indicated in green and the area of α2 collagen staining in the transplanted bone is marked by asterisks. The width of the cortical bone is indicated by the white lines and nuclei are stained blue with DAPI. Second harmonic generation was used to analyse collagen structure (f–h). In a transplanted femur (f), original bone shows low signal (dashed blue line), while newly formed donor-derived bone embedded with Col2.3GFP+ cells is brighter, indicative of better collagen organization (solid blue line). Images from oim (g) and wild type (h) bone are shown for reference. The red scale bars represent 50 μm. bm bone marrow
Donor osteoprogenitors engraft after transplantation
In order to determine whether osteoprogenitors were present in the bone marrow after local transplantation, BMSC cultures were established from the transplanted femur after one month. Cultures were Col2.3GFP- after seven days (data not shown), consistent with the established expression of this transgene [15]. However, after culture in osteogenic medium for a week (day 14 of culture), Col2.3GFP+ colonies were present (Fig. 4b–c) indicating the persistence of donor cells in the marrow. To further confirm this observation, we performed secondary transplantation using cells expanded from transplanted oim femurs after one month (Fig. 5). BMSCs from these cultures were also Col2.3GFP- at the time of transplantation (data not shown). After secondary transplantation, Col2.3GFP+ osteoblasts were detected in trabecular and cortical bone (Fig. 5b–d), although this appeared to occur at a lower frequency than we observed in primary transplantations.
Donor osteoprogenitor cells remain in the bone marrow one month after transplantation. BMSCs were established from femurs of transplanted oim mice one month after transplantation (n = 9). Cells were grown to confluence, then osteogenic differentiation was induced. By day 14 of culture, there were no Col2.3GFP+ cells evident in cultures from an untransplanted contralateral femur (a), but cultures from transplanted limbs showed numerous Col2.3GFP+ colonies (b, c). Images are composite scans of the center of a 6-well dish from representative cultures
Donor cells are still present after secondary transplantation. (a) Secondary transplantation was performed in oim animals as indicated in the experimental design (n = 2). Histology of a distal femur after secondary transplantation indicates the presence of Col2.3GFP+ cells one month after transplantation (b), on both trabecular (c) and cortical (d) bone surfaces
Absence of systemic engraftment of osteogenic cells
We also assessed engraftment in the contralateral non-transplanted limb. We never observed Col2.3GFP+ donor cells in locally transplanted animals at one or three months after transplantation (Fig. 2d). There was also no evidence of α2 collagen expression in bone from contralateral limbs (Fig. 3b). In addition, we attempted systemic intravenous transplantation of BMSC via the retro-orbital sinus. We also failed to observe engraftment in the femurs under these conditions (Fig. 2e). When bone marrow was flushed from contralateral untransplanted limbs and cultured under osteogenic conditions, there was no evidence of the presence of donor osteoprogenitor cells indicated by the absence of Col2.3GFP+ colonies (Fig. 4a). Similar results were seen with cultures from limbs of systemically transplanted animals (data not shown).
Discussion
Through increases in bone mass, reductions in fracture rate and improved growth, bisphosphonate therapy has improved the quality of life for OI patients. However, side effects can include decreased bone remodeling rate, reduction in growth plate cartilage resorption, and a delay in the healing of osteotomy sites [1, 19]. In addition, this type of therapy cannot correct the primary defect of inadequate collagen production. In theory, introduction of normal osteoprogenitors into affected subjects would provide a healthy population of bone-forming cells. The selective advantage of wild type cells over cells bearing a mutation has been observed in studies of patients that are mosaic for OI mutations. The presence of 40 % normal cells minimizes the effects from the mutant population, preventing the appearance of the clinical phenotype [20]. These data indicate that transplantation procedures would be successful even with partial engraftment of normal MSCs.
There are two main factors to consider in transplantation protocols: the source and preparation of donor cells, and the method by which the donor cells are introduced. Previous studies utilizing transplantation of BMSCs in murine models of disease have shown a low success rate due to low engraftment. Initial experiments indicated that BMSCs introduced via intraperitoneal injection were capable of engraftment, and showed some improvement in bone mineral content, and this has been confirmed in a recent study which demonstrated 1.5 % engraftment on the bone surface [10, 11]. Studies in children showed similarly low levels of engraftment, and it is unclear whether transient improvements in their growth velocity were due to active participation of the transplanted cells in bone formation, or a consequence of immunosuppressive treatments employed to prepare them for transplant affecting bone resorption [12–14]. Transplantation of uncultured CD3-depleted bone marrow also failed to demonstrate any observed clinical efficacy more than three months after transplantation [11]. The most effective transplantation results have been achieved during the fetal period in both mice and humans [7–9, 21]. The percentage of engrafted cells ranged from 1 % to 5 %. Jones et al. [22] recently showed large improvements in engraftment frequency (from 5 % to 14 %) when human fetal blood-derived MSCs were preconditioned with stromal cell-derived factor 1 and implanted intraperitoneally in E13.5-15 oim embryos suggesting that source and preparation of the donor cells is also critical to the success of engraftment. One case report of MSC transplantation in utero for a patient diagnosed with severe OI showed better than expected growth and fracture rates, and no immune rejection with engraftment of up to 7.4 %, depending on the method of evaluation, at nine months of age [21]. Prenatal transplantation is not a realistic treatment option for all patients, particularly those with less severe forms of the disease that are unlikely to be diagnosed in utero, so it is important to develop effective methods of transplantation postnatally.
Many of the previous studies did not show conclusive evidence that the transplanted cells differentiated into the osteogenic lineage. Therefore, in the present study, we utilized donor cells from Col2.3GFP mice to visualize mature osteoblast lineage cells and assess functional engraftment. Primary mouse BMSCs were used as a donor population, and these cells are well established to be capable of bone formation both in vitro and in vivo [6, 15]. While we did not directly assess the proportion of osteoprogenitors in these cultures in this study, previous work has indicated that similar cultures consist of 30–40 % osteoprogenitor cells [23]. Our data indicate that locally transplanted BMSCs are capable of differentiation into matrix producing osteoblasts and osteocytes and production of normal collagen matrix containing the α2 chain of type I collagen. Unlike a previous study using local transplantation, we did not use a collagen gel carrier to deliver cells [24], but demonstrated effective engraftment delivering cells in serum-free culture medium. We noted significant contribution to both cortical and trabecular bone formation in all the transplanted limbs. It is notable that the distribution of cells is variable, both within a bone and between animals with some areas having around 100 % donor osteoblasts and others with none, possibly due to the location and contact of the needle during transplantation.
In contrast, we observed consistent absence of donor-derived osteoblasts in contralateral untransplanted bones suggesting that bone marrow stromal progenitor cells do not exhibit the ability to circulate following direct intra-bone marrow transplantation. Furthermore, systemic intravenous transplantation of BMSC did not yield any Col2.3GFP positive osteoblasts in bone. The controversy on whether mesenchymal progenitor cells or more mature osteoblasts can circulate still persists, and a number of reports provide evidence for this phenomenon [25]. However, the criteria to define the engrafted donor cells as osteoblasts have not been clearly defined. Some reports consider localization on the bone surface and the expression of ubiquitous GFP marker to be sufficient evidence of osteoblast engraftment. However, due to heterogeneity of the donor cells in many of these protocols, the majority of the donor cells on the bone surface appear to be of hematopoietic origin [4–6]. We propose that it would be necessary to utilize mature-osteoblast lineage markers such as osteocalcin or Col2.3GFP expression to confirm the osteogenic potential of donor cells. In addition, donor cells should be in a close contact with new bone formation that can be assessed by deposition of a dynamic bone label (such as calcein, xylenol orange).
A key aspect of mesenchymal progenitor transplantation is to provide sufficient cells at an undifferentiated stage, so that they can self-renew and generate more osteoblasts over a prolonged time period. This characteristic of the “stemness” of the transplanted cells can be assessed by evaluating the ability of the transplanted cells to generate osteogenic colonies some time after transplantation and by evaluating the ability to self replicate by a secondary transplantation experiment. In this study we demonstrated by these methods that donor osteoprogenitors were established in the bone marrow one month after transplantation.
To summarize, our results indicate that BMSCs delivered locally to oim femurs are able to engraft, differentiate into osteoblasts and osteocytes and maintain their progenitor potential in vivo. The use of a bone specific marker to trace the transplanted cells greatly simplifies assessment of functional engraftment. Our work also suggests that mesenchymal progenitor cells do not engraft by systemic circulation. In further studies, simultaneous inclusion of a mesenchymal progenitor or ubiquitous marker would assist with tracking the cells, and further studies are required to evaluate the effect of local transplantation on bone mass and mechanical parameters. This source of donor cells also provides a good model in which to test different methods of preparing and transplanting cells that may be more clinically relevant than local transplantation in a condition like OI.
References
Rauch F, Glorieux FH (2004) Osteogenesis imperfecta. Lancet 363:1377–1385
Chamberlain JR, Schwarze U, Wang PR, Hirata RK, Hankenson KD, Pace JM, Underwood RA, Song KM, Sussman M, Byers PH, Russell DW (2004) Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science 303:1198–1201
Prockop DJ, Oh JY (2012) Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem 113:1460–1469
Boban I, Jacquin C, Prior K, Barisic-Dujmovic T, Maye P, Clark SH, Aguila HL (2006) The 3.6 kb DNA fragment from the rat Col1a1 gene promoter drives the expression of genes in both osteoblast and osteoclast lineage cells. Bone 39:1302–1312
Boban I, Barisic-Dujmovic T, Clark SH (2010) Parabiosis model does not show presence of circulating osteoprogenitor cells. Genesis 48:171–182
Wang LP, Liu YL, Kalajzic Z, Jiang X, Rowe DW (2005) Heterogeneity of engrafted bone-lining cells after systemic and local transplantation. Blood 106:3650–3657
Vanleene M, Saldanha Z, Cloyd KL, Jell G, Bou-Gharios G, Bassett JHD, Williams GR, Fisk NM, Oyen ML, Stevens MM, Guillot PV, Shefelbine SJ (2011) Transplantation of human fetal blood stem cells in the osteogenesis imperfecta mouse leads to improvement in multiscale tissue properties. Blood 117:1053–1060
Guillot PV, Abass O, Bassett JH, Shefelbine SJ, Bou-Gharios G, Chan J, Kurata H, Williams GR, Polak J, Fisk NM (2008) Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood 111:1717–1725
Panaroni C, Gioia R, Lupi A, Besio R, Goldstein SA, Kreider J, Leikin S, Vera JC, Mertz EL, Perilli E, Baruffaldi F, Villa I, Farina A, Casasco M, Cetta G, Rossi A, Frattini A, Marini JC, Vezzoni P, Forlino A (2009) In utero transplantation of adult bone marrow decreases perinatal lethality and rescues the bone phenotype in the knockin murine model for classical, dominant osteogenesis imperfecta. Blood 114:459–468
Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ (1998) Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA 95:1142–1147
Otsuru S, Gordon PL, Shimono K, Jethva R, Marino R, Phillips CL, Hofmann TJ, Veronesi E, Dominici M, Iwamoto M, Horwitz EM (2012) Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 120:1933–1941
Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5:309–313
Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK (2001) Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 97:1227–1231
Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T (2002) Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA 99:8932–8937
Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D (2002) Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res 17:15–25
Saban J, King D (1996) PCR genotyping of oim mutant mice. Biotechniques 21(190):192
Roguljic H, Matthews BG, Yang W, Cvija H, Mina M, Kalajzic I (2013) In vivo identification of periodontal progenitor cells. J Dent Res 92:709–715
Roberts-Pilgrim AM, Makareeva E, Myles MH, Besch-Williford CL, Brodeur AC, Walker AL, Leikin S, Franklin CL, Phillips CL (2011) Deficient degradation of homotrimeric type I collagen, alpha 1 (I)(3) glomerulopathy in oim mice. Mol Genet Metab 104:373–382
Uveges TE, Kozloff KM, Ty JM, Ledgard F, Raggio CL, Gronowicz G, Goldstein SA, Marini JC (2009) Alendronate treatment of the brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J Bone Miner Res 24:849–859
Cabral WA, Marini JC (2004) High proportion of mutant osteoblasts is compatible with normal skeletal function in mosaic carriers of osteogenesis imperfecta. Am J Hum Genet 74:752–760
Le Blanc K, Gotherstrom C, Ringden O, Hassan M, McMahon R, Horwitz E, Anneren G, Axelsson O, Nunn J, Ewald U, Norden-Lindeberg S, Jansson M, Dalton A, Astrom E, Westgren M (2005) Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 79:1607–1614
Jones GN, Moschidou D, Lay K, Abdulrazzak H, Vanleene M, Shefelbine SJ, Polak J, de Coppi P, Fisk NM, Guillot PV (2012) Upregulating CXCR4 in human fetal mesenchymal stem cells enhances engraftment and bone mechanics in a mouse model of osteogenesis imperfecta. Stem Cells Transl Med 1:70–78
Kalajzic Z, Li H, Wang LP, Jiang X, Lamothe K, Adams DJ, Aguila HL, Rowe DW, Kalajzic I (2008) Use of an alpha-smooth muscle actin GFP reporter to identify an osteoprogenitor population. Bone 43:501–510
Li F, Wang X, Niyibizi C (2010) Bone marrow stromal cells contribute to bone formation following infusion into femoral cavities of a mouse model of osteogenesis imperfecta. Bone 47:546–555
Pignolo RJ, Kassem M (2011) Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res 26:1685–1693
Acknowledgments
This work has been supported by NIH/NIAMS grant AR055607 to I.K and a Fellows Research Award by Connecticut Children’s Medical Center to P.P. We would like to thank Dr. Charlotte Phillips, University of Missouri–Columbia for providing the collagen type I α2 chain antibody.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pauley, P., Matthews, B.G., Wang, L. et al. Local transplantation is an effective method for cell delivery in the osteogenesis imperfecta murine model. International Orthopaedics (SICOT) 38, 1955–1962 (2014). https://doi.org/10.1007/s00264-013-2249-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-013-2249-y