Abstract
Amputation is a commonly performed procedure during natural disasters and mass casualties related to industrial accidents and military conflicts where large civilian populations are subjected to severe musculoskeletal trauma. Crush injuries and crush syndrome, an often-overwhelming number of casualties, delayed presentations, regional cultural and other factors, all can mandate a surgical approach to amputation that is different than that typically used under non-disaster conditions. The following article will review the subject of amputation during natural disasters and mass casualties with emphasis on a staged approach to minimise post-surgical complications, especially infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last few years, a growing incidence of extreme natural disasters has demonstrated nature’s power to strike, in often-unpredictable ways, in any place on earth. Coincident with this, human societies have caused a growing number of preventable disasters from large-scale industrial accidents, aggressive military actions, and terrorist acts targeting civilians—demonstrating our perverse inability to grow responsibly and resolve differences peacefully.
Regardless of the cause of these disasters, however, more often than not the resulting casualties affect large populations; and the majority of injuries are orthopaedic.
The earthquake that hit Haiti on January 12, 2010, caused between 217,000 and 300,000 deaths, nearly 300,000 injuries, and left about one million homeless—revealing just how unprepared some countries are to deal with mass casualties and disaster-related injuries [1, 28]. It also demonstrated the medical community’s inadequate disaster preparedness and a surprising lack of knowledge about some of the most common injuries and effective surgical procedures to deal with them, e.g. crush injuries and crush syndrome, field amputation, and the delayed presentation of closed and open fractures. Lack of knowledge in managing these injuries appropriately can lead to complications even worse than the original trauma: local and systemic infection, renal and cardiac failure, even death [23].
Principles of disaster trauma management
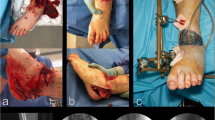
Adhering to general principles of trauma care such as rapid triage, transport, early stabilisation and definitive management is critically important in disaster situations. Battlefield extremity wounds are typically characterised by high-energy injury, extensive soft-tissue damage (Fig. 1), and prolonged injury-to-operation time. During earthquakes and other mass-casualty events, the mechanism of injury is primarily low-energy, prolonged, crushing trauma, with extensive soft tissue damage and late presentation (Fig. 2). Both these varieties of factors lead to an increased risk of infection and higher levels of amputation [9–12].
Extremity injuries should be addressed after the patient’s airway, breathing, and circulation are managed according to the Advanced Trauma Life Support (ATLS) protocol. In the event of exsanguinating haemorrhage from the extremities, haemostasis with the use of tourniquets is the highest priority [2]. Prior to wound management, appropriate resuscitation must be completed and adequate antibiotics and tetanus toxoid administered.
When possible, a multidisciplinary approach to treating such injuries may ultimately improve outcomes and maximise functional rehabilitation. Unfortunately, this is not often possible in a disaster zone.
Crush injuries, crush syndrome, compartment syndrome
Crush injuries are common consequences of earthquakes and are estimated to affect 3–20 % of victims [50]. Because of the different treatment protocols required, it is of major importance that field physicians differentiate between crush injury, crush syndrome, and compartment syndrome, and that the actual injury be correctly identified and treated accordingly.
Crush injury to extremities is caused by direct pressure that damages the soft tissues—skin, muscles, nerves, and blood vessels. Compartment syndrome is caused by the compression of tissues within a confined space (the compartment) and can result in tissue necrosis.
Crush injuries may lead to compartment syndrome with or without any associated skeletal injury [13]. By definition, compartment syndrome is produced when tissue pressure within a limited space rises to the point where the circulation and function of the tissues in that space are compromised [14–16]. Treatment of acute compartment syndrome injuries using fasciotomy, for up to six hours, can reverse ischaemic changes and release compartment pressure, producing satisfactory outcomes [16].
When an injured extremity is exposed to substantial crushing force for a prolonged period of time—and the volume of the compressed, crushed soft tissue is substantial—irreversible changes, including muscle cell death and systemic manifestations, can take place. This condition, crush syndrome—or mechanical muscle crush injury—was reported by British physician Eric Bywaters during the London Blitz [17, 18]. Syndrome victims had massive muscle damage and subsequently died from renal failure. During natural disasters, crush syndrome is a very common diagnosis [19, 21, 26–28].
Crush syndrome may develop after just one hour in a severe crush situation, but usually requires four to six hours of compression for the systemic manifestations to appear. Early stages offer only very subtle local clues. Clinically detectable vascular compromise may present as the injury advances, however, with ischaemic changes in the affected muscles causing rhabdomyolysis and leading to muscle cell death [11]. Such symptoms can include pronounced swelling, skin redness or pallor, pain with passive movement, parasthesia, and motor deficit. If and when circulation is restored to the affected limb, the toxic byproducts of rhabdomyolysis can cause myoglobinuria with resultant renal failure, and hyperkalemia, which can cause fatal dysrhythmias.
An extremity trapped under the rubble following a disaster can suffer either crush or compartment syndrome with blood outflow from the involved area impaired and the toxic metabolic byproducts not a part of the victim’s systemic circulation. But restoration of perfusion can lead to reperfusion injuries—with associated cardiac, renal, circulatory, and electrolytes abnormalities—up to and including death.
For this reason, treatment of crush syndrome is primarily focused on the preservation of a patient’s cardiac, renal, metabolic and circulatory fluid volumes. The priority is saving life over limb.
Local treatment of crush syndrome is different from the treatment of acute compartment syndrome. The role of fasciotomy in treating crush syndrome is more controversial than some realise. Some physicians believe there is a chance to reverse tissue damage if fasciotomy is preformed at presentation after the crush [51]. And there are some good results reported in cases of crush syndrome of a single extremity with early presentation [52, 53]. In the presence of fracture, however, stabilisation of the affected extremity—either by use of a splint or spanning external fixation—is indicated, and any open reduction and fixation methods that convert closed fractures to open ones are associated with high rates of infection [28].
Also, oft-reported field experience in different disaster zones indicates high rates of infection and associated amputation no matter what the circumstances when fasciotomies are performed in the presence of crush syndrome [9–11, 17–25]. In hypoxic tissues, infection control and healing mechanisms are impaired, increasing the risk of infection, and problems with wound healing are more common than after other injuries. Although fasciotomy is a main treatment for acute compartment syndrome, its use in crush syndrome should be reserved for cases where peripheral circulation of the involved limb is compromised and compartment decompression must be done to re-establish peripheral blood flow. Fasciotomy is also recommended when compartment syndrome is suspected in cases involving open fractures, or when initial compartment pressure is normal and delayed compartment syndrome develops [6, 9].
Indications for amputation
Amputation is performed in field hospitals or at the scenes of disaster for the primary purpose of saving patients’ lives when no delay or transfer is possible. Irreparable vascular injury, completion of a partial amputation, and overwhelming sepsis are the main indications for such amputations. However, since losing an extremity can make normal living near to impossible in some countries and even shorten a person’s life, physicians should also take cultural imperatives into consideration.
Amputation in a setting of crush injury should be considered in all cases of severe soft-tissue damage—with or without fractures—deteriorating renal and cardio-respiratory function, and sepsis. Limb salvage, however, has become a more realistic alternative in recent years with improvement in fracture stabilisation methods [12] and advanced vascular techniques. Attempts to codify the limb-salvage or amputation decision process have produced a number of scoring systems over the years [54, 55]. Not a single one has proven to be useful.
The decision for or against amputation is based on a patient’s factors, including vascular, neurological, the affected limb’s soft tissue and bone condition, as well as the patient’s general health and associated systemic involvement. The facility, post-operative follow up, rehabilitation, and prosthetic care should all be considered part of the decision-making process.
Amputation: local aspects
Clinical factors like blood loss, category of crush injury, and contamination of the wound should contribute to determination of the level of amputation. While maximal limb length should be preserved if possible [31–33], adequate debridement is of primary importance to minimise complications, especially wound infection and sepsis.
A careful examination of the neurovascular function and bone and soft tissue condition of the injured extremity is essential. If X-ray assessment is possible, it can help to evaluate the integrity of the bone and the presence of foreign bodies, especially in the case of blast injuries.
If the mechanism of injury is a blast or open crush injury, the wound care strategy should focus on preservation of all viable tissues—since the exact level of soft tissue damage is impossible to assess in the immediate post-injury setting. The “open length-preserving amputation” procedure, formerly “open circular amputation” or “Guillotine amputation” (Fig. 3) [5, 36, 37, 56], does not actually preserve limb length [37, 39] and can introduce challenges to residual limb healing and rehabilitation. It should be evaluated as a solution in that light. Skin traction (Fig. 4), often advocated to prevent soft tissue retraction and minimise local swelling [2, 56], can be unnecessarily cumbersome in an emergency setting [58]. It should be replaced by vacuum-assisted closure if available.
If there is significant bleeding, immediate direct pressure at the site of the bleeding should be applied to control the haemorrhage. This should be followed immediately by placement of a tourniquet above the site of the bleeding. The use of tourniquets is necessary to ensure that amputations are not compromised in advance. The Combat Application Tourniquet (CAT) used in combat settings by the US Army has improved survival rates by 23 % relative to later application in emergency departments [3, 29, 34–36, 57]. Double ligation of transacted arteries, however, is often advocated as an early solution to secure bleeding from the larger vessels. A sterile haemostatic dressing should then be applied. Secondary examination should be performed to exclude other possible injuries.
Early aggressive debridement of the skin—usually within the first two hours—should be performed with a skin incision made as distal as possible through skin and fascia. All viable tissue should be preserved for use during definitive reconstruction if and when needed.
In an acute setting, skin vascularity can be assessed by a trial skin incision [30, 38]. Skin incisions should be made along the limb axis and should not be short [40]. When crossing flexing creases, incisions should either be oblique or transverse to avoid future flexion contractures. When definitive amputation is performed, clinical assessment guides the surgeon in establishing skin viability. Successful use of other diagnostic modalities, such as ankle brachial pressure index, transcutaneous PO2 measurements [30], arterial Doppler studies, Xenon 133, laser Doppler, and thermography have been reported but are not practical in a disaster zone.
Subcutaneous fat should be excised within 2–3 cm of the wound’s edges, being careful to avoid undermining myocutaneous flaps. Fascia can be widely excised to prevent muscle herniation. Muscle viability should be established based on contractility more then the rate of bleeding [4, 30].
After initial debridement, the wound should be left open and covered with a sterile dressing or negative-pressure dressing such as vacuum-assisted closure (VAC) if available. Based on the level of available care, fasciotomy and revascularisation—using shunts or definitive vascular reconstruction—and skeletal stabilisation with internal or external fixation can be done if needed.
Another debridement should be performed within 48–72 hours and repeated as needed. Definitive soft-tissue flaps should be fashioned in later stages, since soft-tissue viability is difficult to assess at the initial stage. Skin traction and transportation casts [36, 37, 56] are rarely used today because of improvements in wound management and transportation times. Various soft-tissue coverage techniques, including local flaps [38–41], free tissue transfer [42–46], and split-thickness skin grafts [47, 48] are often used successfully. Multiple operative debridements should be performed prior to definitive, delayed wound closure.
Comminuted and devascularised, and stripped of soft tissue attachments, bone fragments should be removed to avoid future local infection due to sequestrum formation. Large bone fragments with soft tissue attachment and preserved blood supply should be stabilised using either external or internal fixation, to allow preservation of the longest optimal amputation stamp.
Bone cutting should be carried out with full consideration of soft-tissue coverage, so that when the wound closure is performed, the skin will not be under tension.
During initial wound care, both nerve and tendon length should be preserved as much as possible to allow for future reconstructions. Their ends should be tagged with non-absorbable sutures.
Primary or premature wound closure is associated with wound dehiscence and infection is not recommended in natural disaster or mass casualty settings [28, 49, 50, 59].
Results of below-knee amputations performed in a Peshawar, Pakistan, hospital operated by the International Committee of the Red Cross (ICRC) were reported by Simper [58]. In his retrospective study of 111 patients, most of whom had explosion injuries and had undergone amputation, primary closures were always delayed. The median delay time from amputation to closure was 6.4 days. Ninety-six of the stumps (87 %) healed without complications. If the closure was done within one week, 94 % of the stumps healed without complications. But if closure was later than one week, the success rate dropped to 72 % (p < 0.05).
Delayed wound closure should be performed after five to six days. After eight days, delayed primary closure is less favourable and more challenging [58].
Hyperbaric oxygen (HO) therapy has been reported to improve oxygen delivery to the local tissues, reduce oedema and infection rates, improve both wound healing after fasciotomy and skin grafting [7, 8]. Availability of the HO in the disaster zone and its use during mass casualty situations is not very likely.
Although specific upper and lower extremity amputation techniques are beyond the scope of this review, in general the weight-bearing areas of the residual limb should be sensate and actively controlled and scars should not be located in those weight-bearing areas [59].
Saving a life vs. saving a limb
Mass casualties from recent natural, military, and terrorist disasters present a serious treatment challenge to civilian medical communities all over the world. In the recent Haitian earthquake, more than 50 % of the injuries were to extremities and a high percentage of those were crush injuries. Crush injuries, crush syndrome, delayed presentation of the injured, amputations, and infections overwhelmed the region’s medical community.
Amputation of extremities during natural disasters—and specifically during earthquakes—is a common, complex and controversial issue. When amputation must be performed, especially if presentation is delayed, the most successful surgical technique is often based on a staged approach that includes repeated debridement and delayed wound closure to minimise infection and sepsis.
There is increasing evidence from both natural disaster—mainly earthquake-related—and war zone mass-casualty sites, that treating crush syndrome by addressing renal and cardiac systems with fasciotomy should be done sparingly in the light of the high rate of infection in fasciotomised limbs. A staged approach to amputation with wound closure done in a delayed fashion after appropriate debridements—preferably within a week of the initial insult—can achieve optimal results with minimal risk of such complications.
To prevent this life-threatening complication and minimise risk of infection, some physicians recommend early amputation [51]. It is also recommended as a last resort when necessary to facilitate extraction of victims with limbs trapped under the rubble [28, 50].
Conclusion
The choice to perform an amputation is one of the most challenging decisions orthopaedic surgeons face. Given the current geopolitical situation, it is very likely that surgeons will continue to be confronted with these complex decisions. As a rule, the decision to amputate should be seen not as a failure of treatment but as a life-saving, function-preserving operation.
Technical, cultural, facility, and surgical skill factors should all play significant roles in the decision-making process when amputation is considered. Given what we have learned to date, a staged approach to amputation should be implemented whenever possible to minimise the risk of local and systemic infection. Since field amputation is an evolving medical skillset that will inevitably grow with the increasing incidence of disaster, education in its purposes, techniques, planning, and approaches, it should be of critical importance to all orthopaedic surgeons.
References
Lebel E, Blumberg N, Gill A, Merin O, Gelfond R, Bar-On E (2011) External fixator frames as interim damage control for limb injuries: experience in the 2010 Haiti earthquake. J Trauma 71(6):E128–31
Borden Institute (2004) Emergency war surgery handbook. 3rd United States Revision 2004. Borden Institute. Walter Reed Army Medical Center, Washington, D.C., chapter 22. Soft Tissue Injuries 22:1–14
Lakstein D, Blumenfeld A, Sokolov T et al (2003) Tourniquets for hemorrhage control on the battlefield: a 4-year accumulated experience. J Trauma 54(5 suppl):S221–S225
Scully R, Artz C, Sako Y (1956) An evaluation of the surgeon’s criteria for determining the viability of muscle during debridement. Arch Surg 73:1031–1035
Fitzmaurice-Kelly M (1916) The flapless amputation. Br J Surg 3:676–681
Better OS, Rubinstein I, Reis ND (2003) Muscle crush compartment syndrome: fulminant local oedema with threatening systemic effects. Kid Int 63:1155–7
Garcia-Covarrubias L, McSwain NE Jr, Van Meter K, Bell RM (2005) Adjuvant HBOT in the management of crush injury and traumatic ischemia: an evidence based approach. Am Surg 71(2):144–51
Philips JC (2005) Understanding HBOT and its use in the treatment of compromised skin grafts and flaps. Plast Surg Nurs 25(2):72–80
Reis ND, Better OS (2005) Mechanical muscle-crush injury and acute muscle-crush compartment syndrome: with special reference to earthquake casualties. J Bone Joint Surg Br 87(4):450–3
Reis ND, Michaelson M (1986) Crush injury to the lower limbs: treatment of the local injury. J Bone Joint Surg Am 68-A:414–18
Better OS, Rubinstein I, Reis DN (2003) Muscle crush compartment syndrome: fulminant local edema with threatening systemic effects. Kidney Int 63(3):1155–1157
Lerner A, Soudry M (2011) Armed conflict injuries to the extremities: A treatment manual. Springer, New York
Mubarak SJ, Owen CA (1975) Compartment syndrome and its relation to the crush syndrome: a spectrum of disease. Clin Orthop 113:81–89
Jagodzinski NA (2010) Crush injuries and crush syndrome — a review. Part 1: the systemic injury. Trauma 12:69–88
Matsen FA III (1980) Compartmental syndromes. Grune & Stratton, Inc., New York
Elliott KGB, Johnstone AJ (2003) Diagnosing acute compartment syndrome. J Bone Joint Surg Br 85-B:625–32
Bywaters EGL, Beal D (1941) Crush injuries with impairment of renal function. Br Med J I:427–32
Bywaters EGL, Delory GE, Rimington C, Smiles J (1941) Myohaemoglobin in air raid casualties with crushing injury. Biochem J 35:1164–8
Sever SM, Vanholder R, Lameire N (2006) Management of crush-related injuries after disasters. N Engl J Med 354(10):1052–10
Sever SM, Erek E et al (2002) Clinical findings in the renal victims of a catastrophic disaster: the Marmara earthquake. Nephrol Dial Transplant 17:1942–9
Nadjafi I, Atef MR, Broumand B, Rastegar A (1997) Suggested guidelines for treatment of acute renal failure in earthquake victims. Ren Fail 19:655–64
Tetsuya M, Toshiharu Y et al (2002) Long term physical outcome of patients who suffered crush syndrome after the 1995 Hanshin-Awaji earthquake: prognostic indicators in retrospect. J Trauma 52:33–9
Duman H, Kulahci Y, Sengezer M (2003) Fasciotomy in crush injury resulting from prolonged pressure in an earthquake in Turkey. Emerg Med J 20(3):251–2
Demirkiran O, Dikmen Y, Utku T, Urkmez S (2003) Crush syndrome patients after the Marmara earthquake. Emerg Med J 20(3):247–50
Huang KC, Lee TS et al (2002) Clinical features and outcome of crush syndrome caused by the Chi-Chi earthquake. J Formos Med Assoc 101:249–56
Bulut M, Fedkar R, Akkose S, Akgoz S, Ozguc H, Tokyay R (2005) Medical experience of a university hospital in Turkey after the 1999 Marmara earthquake. Emerg Med J 22:494–498
Tanaka K (1996) The Kobe earthquake: the system response. A disaster report from Japan. Eur J Emerg Med 3:263–9
Bar-On E, Lebel E, Kreiss Y, Merin O, Benedict S, Gill A, Lee E, Pirotsy A, Shirov T, Blumberg N (2001) Orthopaedic management in a mega mass casualty situation. The Israel Defense Forces Field Hospital in Haiti following the January 2010 earthquake. Injury 42(10):1053–1059
Bagg MR, Covey DC, Powell ET (2006) Levels of medical care in the global war on terrorism. J Am Acad Orthop Surg 14(10):S7–S9
Depairon M, Krahenbuhl B, Vaucher J (1986) Determination of amputation level by transcutaneous measurement and distal arterial systolic pressure. J Mal Vasc 11(3):229–234
Gonzalez EG, Corcoran PJ, Reyes RL (1974) Energy expenditure in bellow-knee amputees: correlation with stump length. Arch Phys Med Rehabil 55:111–119
Sarmiento A, Warren WD (1969) A reevaluation of lower extremity amputations. Surg Gynecol Obstet 129:799–802
Marsden FW (1977) Amputation surgical technique and postoperative management. Aust NZ J Surg 47:384–394
Husum H, Gilbert M, Wisborg T, Pillgram-Larsen J (2004) Prehospital tourniquets: there should be no controversy. J Trauma 56:214–215
Kragh JF, Walters TJ et al (2008) Practical use of emergency tourniquet to stop bleeding in major limb trauma. J Trauma 64:S38–S50
Emergency War Surgery (2004) Third United States Revision. United States Government Printing Office, Washington, D.C.
Dougherty PJ (2006) War wounds, limb salvage, and traumatic amputations, in Rockwood and Green’s Fractures, 6th edn. Lippincott Wilkins & Williams, Philadelphia, pp 477–496
Jain AS, Stewart CP, Turner MS (1995) Transtibial amputation using a medially based flap. J R Coll Surg Edinb 40:253–265
Bickel WH (1943) Amputation below the knee in occlusive arterial diseases. Surg Clin North Am 23:982–994
Peterson BM (1974) Sagittal incision form bellow-knee amputation in ischemic gangrene. J Bone Joint Surg Br 56:110–114
Robinson K (1982) Skew flap myoplastic below—knee amputation: a preliminary report. Br J Surg 69:554–557
Burgess EM (1968) The below-knee amputation. Bull Prosthet Res 10:19–25
Ruckley CV, Stonebridge PA, Prescott RJ (1991) Skew flap versus long posterior flap in below-knee amputations: multicenter trial. J Vasc Surg 13:423–427
Chen L, Yang F, Zhang Z-X et al (2008) Free fillet foot flap for salvage of below-knee amputation stump. Chin J Traumatol 11(6):380–384
Sadhotra LP, Singh M, Singh SK (2004) Resurfacing of amputation stumps using free tissue transfer. MJAFI 60(2)
Gallico GG III, Ehrlichman RJ, Jupiter J et al (1987) Free flaps to preserve below-knee amputation stumps: long-term evaluation. Plast Reconstr Surg 79(6):871–878
Pinzur M (2004) Knee disarticulation: surgical management. In: Smith DG, Michael JW, Bowker JH (eds) Atlas of amputations and limb deficiencies, 3rd edn. AAOS, Rosemont, pp 517–524
Burgess EM (1977) Disarticulation of the knee: a modified technique. Arch Surg 112:1250–1255
Naylor CS, Eaton JT et al (1998) Structure of the key toxin in gas gangrene. Nat Struct Biol 5(8):738–746
Gonzales D (2005) Crush syndrome. Crit Care Med 33(suppl 1):S 34–41
Malinoski D, Slater M, Mullins R (2004) Crush injury and Rhabdomyolysis. Crit Care Clin 20:171–92
Taisuke S, Takima M (2004) A case of crush syndrome resulting from continues compression of legs by the car. Cent Jpn J Orthop Surg Traumatol 47(6):1251–52
Mrsic V, Nasek AV et al (2008) Acute rhabdomyolysis case report and literature review. Acta Med Croatica 62(3):317–22
Bosse MJ, MacKenzie E, Kellam JF et al (2001) A prospective evaluation of the clinical utility of the lower-extremity injury-severity scores. J Bone Joint Surg Am 83:3–14
McNamara MG, Heckman JD, Corley FG (1994) Severe open fractures of the lower extremity: a retrospective evaluation of the Mangled Extremity Severity Score (MESS). J Orthop Trauma 8:81–87
Dougherty PJ (2004) Wartime amputee care. In: Smith DG, Michael JW, Bowker JH (eds) Atlas of amputations and limb deficiencies: surgical, prosthetic and rehabilitation principles, 3rd edn. American Academy of Orthopaedic Surgeons, Rosemont, pp 77–97
Kalish J, Burke P et al (2008) The return of tourniquets: Original research evaluates the effectiveness of prehospital tourniquets for civilian penetrating extremity injuries. J Emerg Med Serv 33(8):44–54
Simper LB (1993) Below knee amputation in war surgery: a review of 111 amputations with delayed primary closure. J Trauma 34(1):96–98
Wolfson N, Schecter S (2010) Amputation in combat trauma, Chapter 21. In: Lerner A, Soudry M (eds) Armed conflict injuries to the extremities. Springer, New York, pp 335–354
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolfson, N. Amputations in natural disasters and mass casualties: staged approach. International Orthopaedics (SICOT) 36, 1983–1988 (2012). https://doi.org/10.1007/s00264-012-1573-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-012-1573-y