Abstract
This study aimed to determine the efficacy of PEMF (pulsed electromagnetic field) treatment in experimental osteochondral defect healing in a rabbit model. The study was conducted on 12 New Zealand white rabbits. Six rabbits formed the study group and six rabbits the control group. The right knee joints of all 12 animals were exposed and a 3.5-mm diameter osteochondral defect was created in the trochlear groove. The defect was filled with calcium phosphate scaffold. Six animals from the study group were given PEMF of one hour duration once a day for six weeks with set parameters for frequency of 1 Hz, voltage 20 V, sine wave and current ±30 mA. At six weeks the animals were sacrificed and histological evaluation was done using H&E, Safranin O, Maissons trichrome staining and immunohistochemistry for type 2 collagen. The quality of the repair tissue was graded and compared between groups with the Wakitani histological grading scale and a statistical analysis was done. The total histological score was significantly better in the study group (p = 0.002) with regeneration similar to adjacent normal hyaline cartilage. Immunohistochemistry for collagen type II was positive in the study group. PEMF stimulation of osteochondral defects with calcium phosphate scaffold is effective in hyaline cartilage formation. PEMF is a non-invasive and cost effective adjuvant treatment with salvage procedures such as abrasion chondroplasty and subchondral drilling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulsed electromagnetic field therapy is used as an adjuvant therapy in the management of un-united fractures and in osteoarthritis. This treatment has well documented physiological effects on cells and tissues such as the upregulation of gene expression of members of the TGF-β (transforming growth factor) super family [1, 2]. It also enhances chondrogenic proliferation, differentiation and synthesis of cartilage extracellular matrix proteins [8, 9]. It additionally promotes subchondral bone healing which in turn augments cartilage regeneration [12]. In vitro culture models have shown an increase in growth factor synthesis when human chondrocytes are exposed to magnetic field [9]. Although in vitro effects of PEMFs on articular cartilage are proven, there are very few in vivo studies proving its efficacy [7, 11].
In a previous study, local expression of TGF-β by treatment with pulsed magnetic field therapy resulted in better bone healing in animal study osteotomy models compared to controls [6]. Our hypothesis was that in full thickness articular cartilage defects resulting in marrow stimulation, growth factors delivered locally by pulsed electromagnetic field (PEMF) should help in the formation of hyaline cartilage. This study aimed to determine the efficacy of PEMF in the treatment of experimental osteochondral defect healing in a rabbit model.
Materials and methods
A total of 12 male adult New Zealand white rabbits were used. The study was approved by the Institutional Animal ethics committee (approval number 02/2008). Six animals formed the study group and six more the control group. The rabbits were anaesthetised using a combination of intramuscular ketamine (35 mg/kg) and xylazine (5 mg/kg). The right knee joint of all 12 animals was exposed through a midline skin incision and a medial parapatellar arthrotomy. The patella was dislocated laterally to expose the trochlear groove. A 3.5-mm diameter osteochondral defect was created in the trochlear groove to a depth of 5 mm. The defect created was filled with calcium phosphate scaffold. The patella was relocated and the wound was closed in layers with absorbable sutures. Postoperatively all animals were given intramuscular enrofloxacin (10 mg/kg) and meloxicam (5 mg/kg) for three postoperative days.
The animals were housed separately. Principles of laboratory animal care were followed. Food and water were given ad libitum. They were randomised into two groups of six as study and control animals by an independent observer not involved in the study. Six animals from the study group were given PEMF of one hour duration once a day for six weeks from day one. Six animals from the control group were sham treated with only immobilisation for one hour without PEMF. They were also allowed free movement out of the cage and within the room regularly.
At six weeks the animals were sacrificed with lethal doses of intramuscular ketamine. The distal femur from the supracondylar region was excised and fixed in 10% buffered formalin for three days. The specimen was sent for histopathological evaluation to an independent observer who was blinded.
Apparatus
The pulsed magnetic field enclosure used for this experiment was designed and fabricated according to the parametrical equations of Fansleau and Brauenbeck. Pulsed electric current from a signal generator energises the coil system such that the frequency, strength and waveform of the output current can be controlled to any set of desired values, thus offering a pulsating magnetic field of any desired frequency, intensity and wave form along the axis of the coil system.
The animals were housed directly inside this apparatus, which was made up of two larger (inner) coil frames and two smaller (outer) coil frames in a completely nonmetallic environment. The four coils had the same number of turns and were connected in “series-aiding” configuration. This system is a modified version of the classical Helmholtz coil. The set parameters for the PEMF were frequency of 1 Hz, voltage 20 V, sine wave and current ±30 mA [6].
Histopathology and immunohistochemistry evaluation
All specimens were decalcified in 10% formic acid, processed in automatic tissue processor Leica ASP300 (Leica Micro-systems GmbH, Wetzlar, Germany) and embedded in paraffin. Longitudinal sections of 5-μm thickness were prepared (Leica RM2255, Wetzlar, Germany) and stained (Leica Autostainer XL, Wetzlar, Germany) with haematoxylin and eosin to assess tissue morphology, and with Safranin O-fast green and Masson’s trichrome to identify the presence of proteoglycan-rich matrix. The quality of the repair tissue in the articular defect that was treated with PEMF (study animals) was compared with that of the untreated defects (control animals) by the modified Wakitani histological grading scale [25].
The sections were graded according to (1) cell morphology, (2) matrix staining (metachromasia), (3) surface regularity, (4) thickness of cartilage, and (5) integration of donor with adjacent host cartilage; a score was assigned from 0 to 14 by two observers who were blinded of the group to which the animals belonged.
Immunohistochemistry analysis was performed in accordance with the manufacturer’s protocol mentioned in the Super Sensitive Polymer-HRP Detection Kit HRP/DAB (BioGenex, USA). All slides were deparaffinised, rehydrated and pre-treated with Proteinase K (20 µg/ml in Tris-EDTA buffer pH-8.0) (Axygen, India) at 37°C for 30 minutes. Slides were washed with PBS (Gibco). To reduce endogenous peroxidase activity all sections were incubated with Peroxide Block (Biogenex, USA) for ten minutes. All sections were then treated with Power Block (Biogenex, USA) for ten minutes prior to primary antibody incubation. Monoclonal mouse anti-human collagen type II (Acris Antibodies GmbH, Germany) antibody was diluted (10 µg/ml) with deionised water and applied to the sections which were then incubated for 90 minutes at room temperature in a humidified chamber. After washing with PBS, super enhancer (Biogenex, USA) was applied to the slides for 25 minutes at room temperature. Slides were then incubated with poly-HRP reagent (Biogenex, USA) for 30 minutes. After washing with PBS, the signal was finally visualised as a brownish precipitate, using freshly prepared chromogen substrate 3,3′-diaminobenzidine (DAB) (Biogenex, USA). Sections were counterstained with Mayer’s haematoxylin and mounted using low viscosity permanent mounting medium (cytoseal TM60) (Electron Microscopy Sciences, USA).
Statistical methods
SPSS 11.0 (SPSS, Inc., Chicago, IL, USA) for Windows was used for statistical analysis with the help of a statistician. Median and range were presented for both groups separately. Mann-Whitney test was used to compare between the test and control groups. A p-value less than 0.05 was considered statistically significant.
Results
There were no infections or postoperative complications and all animals were used for evaluation of final outcome.
Histopathology
Macroscopic observation
There was no evidence of intra-articular infection or arthrofibrosis. There was no synovitis and osteophyte formation. In most of the study animals the scaffold transplant area, although seen, resembled the adjacent normal cartilage. The surface of the treated group was smooth and the contour was normal. In the control group the scaffold could still be identified. There was a fibrinous cover seen in all the control groups.
Histological findings
Healing of the osteochondral defects was graded by the modified Wakitani histological score after the osteochondral defect areas were examined by means of histological staining, namely, H&E, Safranin O and Masson’s trichrome.
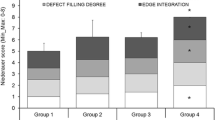
The total histological score was significantly better in the animals treated with PEMF than the untreated group (p = 0.002) (Table 1). Cell morphology in the study animals was mostly hyaline cartilage compared to controls which had non-cartilage only (p = 0.002). The cells were round with morphology of cartilage in the study animals. The cartilage type was mostly hyaline in the PEMF-treated group and non-cartilage, i.e. fibrous tissue or fibro cartilage, in the control group (Fig. 1a, b). The matrix staining in study animals was normal or slightly reduced compared to controls which had no metachromatic stain (p = 0.002) (Fig. 2a, b). The surface was smooth in all animals except in two control animals which had irregular surfaces, and this score was significantly better in the study animals (p = 0.041). The surface was also uniformly regular in the study animals. Cartilage thickness in the study group was more than two thirds compared to controls which had less than one third, and this was also significant (p = 0.002). There was no significant difference between study and controls in integration of donor cartilage with adjacent host cartilage (p = 0.937). Although the integration was similar in both groups, it could not be identified in the study groups due to formation of hyaline cartilage similar to adjacent normal cartilage. The transplant site in the study group was identified based on the presence of subchondral bone remodelling and presence of few areas of scaffold. Notably, subchondral bone regeneration with subsequent scaffold resorption was pronounced in the PEMF-treated group compared to the non-treated controls.
a Control animal with predominantly fibrous cartilage (H&E stain). The edges are clearly visible (red arrows) and there are still remnants of scaffold (black arrows) in the subchondral bone in the control animal. b Study animal with regeneration of cartilage and subchondral bone (H&E stain). The edge of the study animal is well integrated (red arrows) and there are few remnants of scaffold (black arrows) in the subchondral bone in the study animal
Analysis of collagen type II in the defect region of the PEMF-treated group and non-treated animal group at six weeks post-treatment revealed collagen type II in all specimens mainly localised at the pericellular and inter-territorial matrix. Collagen type II exhibited moderate to strong immunopositivity at the specific region of scaffold transplant in the study group, but in the control group it showed no immunopositivity (Fig. 3a, b).
Discussion
This study was done to determine the efficacy of pulsed electromagnetic field treatment in healing of experimental osteochondral defects in rabbit knees, and it was found that there was complete healing of osteochondral defects in study animals treated with PEMF. The total histological score for cartilage healing was significant and better in study animals. The score for integration of repair tissue with adjacent normal cartilage was however not significant. This was because the scoring was not different for fibrocartilage and hyaline cartilage since it only considers integration at both ends.
Our study has a few limitations. The number of animals used in the study was small. The short-term outcome was checked at six weeks. Another set of animals treated and evaluated for long-term outcome would be beneficial. The rationale to check outcome at six weeks was because PEMF treatment as a modality is followed up for bone healing for six weeks only. Also, in a previous study on rabbit models, the authors claimed healing of 6-mm defects using mesenchymal stem cells in type I collagen scaffold after two weeks of treatment [25]. Hence, we were justified in our six-week treatment.
PEMFs were tested in human and animal monolayer chondrocyte cultures. These in vitro studies have shown that chondrocyte proliferation and matrix synthesis are significantly enhanced by PEMF stimulation [8, 9]. In vivo, it up-regulates the gene expression of growth factor TGF-β and prevents the catabolic effects of inflammatory cytokines [1, 2, 9].
Growth factors have been known to help in cartilage healing [13, 19, 20]. The TGF-β super family has been used in animal models of cartilage healing [13]. TGFß-1 stimulates prostaglandin and collagen II synthesis. It also counteracts pro-inflammatory cytokine production [15]. Growth factors have the ability to convert mesenchymal stem cells into chondrogenic lineage and hence should be useful in drilling and micro fracture. They also prevent loss of chondrogenicity by terminal differentiation [16]. The other advantage is that it does not allow terminal dedifferentiation to stem cells, which is a potential problem.
The function of each growth factor in articular cartilage is varied. Ideally, a combination of factors would exist which could control various stages of healing. PEMF has shown an increase of multiple growth factor levels. A microarray assay would reveal all growth factors expressed and this would be helpful to know since healing occurs due to an interrelation of growth factor expression at different time intervals. In a previous study quantitative PCR analysis showed increased expression of TGF-β1 and TGF-β2 after six weeks of PEMF stimulation [6].
Cell-based techniques, when translated to human conditions, have the disadvantage of two surgical interventions, one for harvest of cells and the other for re-implantation of the cultured cartilage. Allogenic culture and transplant can avoid the disadvantage of two operations. However, there is risk of infection and antigenicity. Although the risk of antigenicity seems to be absent in few studies, it needs further evaluation [23]. Osteochondral autograft transfer (OAT) also has the disadvantage of inferior mechanical properties and donor site morbidity although only a single surgical procedure is required [5]. In a comparative study between ACI to micro fracture technique no difference was found in outcome at five years [14].
Full thickness defects penetrate the subchondral bone and result in healing by bone marrow derived mesenchymal cells. The predominant repair tissue is fibro cartilage with few areas of hyaline cartilage [18, 24]. A marrow stimulation procedure produces fibro cartilage unlike cultured cell transplantation since the quantity of stem cells available during marrow stimulation is less [21]. Tissue engineering strategies are now employed to achieve cartilage healing. We introduced the same strategy by using a three dimensional scaffold after marrow stimulation and then adding growth factor by PEMF stimulation. This was evident by the formation of hyaline cartilage in the study animals. PEMF also has the advantage of being a cost effective alternative to costly growth factors.
The scaffold used provides the structural support for cells from the marrow to migrate and regenerate. The histological superiority of micro fracture with scaffold augmentation compared to only microfracture is documented [10]. It is also known that cartilage cells can redifferentiate better in a three dimensional multilayer culture [17]. We used calcium phosphate scaffold with an average pore size of 200–400 µm, which is considered ideal for cell integration. This scaffold can also withstand the joint pressure until the cells integrate and cartilage regenerates [3]. The disadvantages of periosteal arthroplasty such as hypertrophy of the defect area or bump formation were avoided. The scaffold could be wedged inside the defect created. It was also stable in joint motion. In all the animals the scaffold was intact in the transplanted site. Our procedure is similar to autologous matrix induced chondrogenesis with additional enhancement with growth factors provided through PEMF.
PEMF influences healing in nonunions and similarly it helps in healing the subchondral bone. In our study we found that subchondral bone healing was a key factor in cartilage regeneration. This was also demonstrated in a previous study in which they considered the basis of early osteochondral graft stabilisation and later integration [4]. Increased subchondral bone formation also protects cartilage loss [12, 26]. It also prevents excess stress on the cartilage. Hence, it is important to heal the subchondral bone. PEMF will definitely add to the quality of repair tissue. It is non-invasive with hardly any morbidity. No radiation hazards have been reported at this extremely low intensity of magnetic field [22].
Beneficial effects of PEMF are now evident in both in vitro and in vivo studies. With regard to in vivo studies, publications so far are on the same animal model [7, 11, 12]. Further studies in large animals are required. Once proven this can be translated to humans since abrasion chondroplasty and drilling are common cartilage healing procedures. In conclusion, PEMF is effective in hyaline cartilage formation in full thickness articular cartilage defects filled with calcium phosphate scaffold.
References
Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ (2004) Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res 419:30–37
Aaron RK, Wang S, Ciombor DM (2002) Upregulation of basal TGFbeta1 levels by EMF coincident with chondrogenesis—implications for skeletal repair and tissue engineering. J Orthop Res 20:233–240. doi:10.1016/S0736-0266(01)00084-5
Al-Munajjed AA, O’Brien FJ (2009) Influence of a novel calcium-phosphate coating on the mechanical properties of highly porous collagen scaffolds for bone repair. J Mech Behav Biomed Mater 2:138–146
Benazzo F, Cadossi M, Cavani F, Fini M, Giavaresi G, Setti S, Cadossi R, Giardino R (2008) Cartilage repair with osteochondral autografts in sheep: effect of biophysical stimulation with pulsed electromagnetic fields. J Orthop Res 26:631–642. doi:10.1002/jor.20530
Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J (2003) A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br 85:223–230
Boopalan P, Chittaranjan SB, Balamurugan R, Nandakumar N, Sabareeswaran A, Mohanty M (2009) Pulsed electromagnetic field (PEMF) treatment for fracture healing. Curr Orthop Practice 20:423–428
Ciombor DM, Aaron RK, Wang S, Simon B (2003) Modification of osteoarthritis by pulsed electromagnetic field—a morphological study. Osteoarthr Cartil 11:455–462
Ciombor DM, Lester G, Aaron RK, Neame P, Caterson B (2002) Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J Orthop Res 20:40–50. doi:10.1016/S0736-0266(01)00071-7
De Mattei M, Caruso A, Pezzetti F, Pellati A, Stabellini G, Sollazzo V, Traina GC (2001) Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect Tissue Res 42:269–279
Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S (2005) Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials 26:3617–3629
Fini M, Giavaresi G, Torricelli P, Cavani F, Setti S, Cane V, Giardino R (2005) Pulsed electromagnetic fields reduce knee osteoarthritic lesion progression in the aged Dunkin Hartley guinea pig. J Orthop Res 23:899–908
Fini M, Torricelli P, Giavaresi G, Aldini NN, Cavani F, Setti S, Nicolini A, Carpi A, Giardino R (2008) Effect of pulsed electromagnetic field stimulation on knee cartilage, subchondral and epyphiseal trabecular bone of aged Dunkin Hartley guinea pigs. Biomed Pharmacother 62:709–715
Hunziker EB, Driesang IM, Morris EA (2001) Chondrogenesis in cartilage repair is induced by members of the transforming growth factor-beta superfamily. Clin Orthop Relat Res 391Suppl:S171–S181
Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O (2007) A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 89:2105–2112
Martel-Pelletier J (1999) Pathophysiology of osteoarthritis. Osteoarthr Cartil 7:371–373
Mastrogiacomo M, Cancedda R, Quarto R (2001) Effect of different growth factors on the chondrogenic potential of human bone marrow stromal cells. Osteoarthritis Cartilage 9 Suppl A:S36–S40
O’Brien FJ, Harley BA, Yannas IV, Gibson LJ (2005) The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 26:433–441
Pecina M, Jelic M, Ivkovic A, Hudetz D (2006) Gene therapy applications in orthopaedics. Int Orthop 30:215–216. doi:10.1007/s00264-005-0047-x, author reply p 217
Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S (2002) Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop 26:131–136. doi:10.1007/s00264-002-0338-4
Pecina M, Vukicevic S (2007) Biological aspects of bone, cartilage and tendon regeneration. Int Orthop 31:719–720. doi:10.1007/s00264-007-0425-7
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Schenck JF (2000) Safety of strong, static magnetic fields. J Magn Reson Imaging 12:2–19
Shangkai C, Naohide T, Koji Y, Yasuji H, Masaaki N, Tomohiro T, Yasushi T (2007) Transplantation of allogeneic chondrocytes cultured in fibroin sponge and stirring chamber to promote cartilage regeneration. Tissue Eng 13:483–492
Shapiro F, Koide S, Glimcher MJ (1993) Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am 75:532–553
Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM (1994) Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am 76:579–592
Wang Y, Ebeling PR, Hanna F, O’Sullivan R, Cicuttini FM (2005) Relationship between bone markers and knee cartilage volume in healthy men. J Rheumatol 32:2200–2204
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boopalan, P.R.J.V.C., Arumugam, S., Livingston, A. et al. Pulsed electromagnetic field therapy results in healing of full thickness articular cartilage defect. International Orthopaedics (SICOT) 35, 143–148 (2011). https://doi.org/10.1007/s00264-010-0994-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-010-0994-8