Abstract
This prospective six-year longitudinal study reviews the clinical outcome of patients undergoing autologous chondrocyte implantation (ACI) and a porcine type I/III collagen membrane cover for deep chondral defects of the knee. We present 57 patients (31 male, 26 female) with a mean age of 31.6 years (range 15–51 years) that have undergone ACI since July 1998. The mean size of the defect was 3.14 cm2 (range 1.0–7.0 cm2). All patients were assessed annually using seven independent validated clinical rating scores with the data analysed using ANOVA. ACI using a porcine type I/III collagen membrane cover produced statistically significant improvements (p < 0.001), maintained for up to six years, in knee symptoms compared to pre-operative levels. This study provides evidence of the medium-term benefit achieved by transplanting autologous chondrocytes to osteochondral defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Articular cartilage is an avascular, aneural and relatively hypocellular tissue with unique biomechanical properties [1]. The production of a collagen-proteoglycan extracellular matrix by chondrocytes is essential to maintain optimal tissue architecture. Cartilage is devoid of undifferentiated cells capable of migration and proliferation with chrondrocytes being encased within the dense extracellular matrix [2]. The biosynthetic capacity of chondrocytes is restricted to maintaining the extracellular matrix of non-pathological tissue [3]. However, chondrocytes do not produce sufficient collagen or proteoglycan to regenerate hyaline cartilage following significant chondral defects. Thus, articulating joint surfaces with cartilage defects, either focal or generalised, have little intrinsic capability for repair and fibrocartilage is commonly formed. Fibrocartilage affords a lower load-bearing capacity and has inferior biomechanical qualities in comparison to hyaline cartilage. Treatment modalities aiming to stimulate fibrocartilage production, including abrasion arthroplasty, drilling and microfracture, have variable short-term outcomes [4]. The regeneration of hyaline-like cartilage from autologous chondrocytes aims to recreate articular-type cartilage with normal biomechanical and histological features.
Advances in cell culture and tissue scaffolds have lead to the development of autologous chondrocyte implantation (ACI) as a treatment option for osteochondral defects on articulating joint surfaces [5, 6]. The delivery of cultured chondrocytes to the joint surface has progressed from using a fluid cell suspension under a periosteal (ACI-P) or a porcine type I/III collagen (ACI-C) cover to the use of tissue scaffolds seeded with cultured chondrocytes [7].
Chondral defects in the knee joint have proved a challenge for clinicians and researchers, with young active patients placing high demands upon all current treatment modalities [8]. The use of cultured autologous chondrocytes has increased over the last 15 years with increasing evidence demonstrating beneficial clinical outcomes [6, 9].
This prospective six-year longitudinal study details the sequential annual clinical outcome from patients who have undergone autologous chondrocyte implantation using the ACI technique.
Methods
Study design
This prospective cohort study was conducted to evaluate articular cartilage repair using autologous chondrocyte implantation (ACI) performed within a single centre by a single surgeon (TWRB). The study was approved by the Joint Research and Ethics committee of the Royal National Orthopaedic Hospital, and written consent from all patients, including agreement to yearly follow-up, was obtained prior to surgery.
Indications for surgery included:
-
Pain and mechanical symptoms associated with osteochondral defect >1.0 cm2
-
Age between 18 and 50 years
-
Suitable for structured rehabilitation programme
Exclusion criteria included:
-
Joint instability
-
Gross joint deformity or malalignment
-
Infection
-
Osteoarthritis
-
Kissing lesions
-
Inflammatory joint disease
-
Previous menisectomy, of greater than one third medial or lateral meniscus
-
Withheld consent
Patient demographics
Since 1998, all 57 consecutive patients (31 male, 26 female) with a mean age of 31.6 years (range 15–51 years) undergoing ACI performed by one surgeon (TWRB) were included in this prospective study. The primary surgical indication was persisting pain and/or mechanical symptoms resulting from a discreet osteochondral defect greater than 1 cm2 in the weight-bearing articular surfaces of the knee confirmed with MRI and arthroscopy. All patients entered into a structured, standardised rehabilitation programme.
The osteochondral defects result primarily from trauma, osteochondritis dissecans, or chondromalacia patella, with the vast majority of patients having previously undergone other surgical treatments.
The location of the osteochondral defects was predominantly the medial femoral condyle with a mean area of 3.14 cm2 (range 1.0–7.0 cm2).
Operative technique
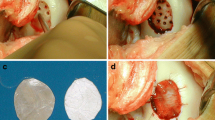
The two stage operative technique has previously been detailed [6, 10]. Following an examination of the knee under anaesthesia, the initial arthroscopy assessed the size, site and containment of the chondral defect and whether there was an adjacent ‘kissing’ lesion. If deemed suitable, arthroscopic chondrocyte harvest from non-load bearing regions of articular cartilage, usually the medial or lateral trochlea, was performed and the tissue cultured in the patient’s serum for approximately six weeks.
The second stage involved a medial or lateral para-patellar arthrotomy under tourniquet and the edges of the chondral lesion were debrided back to normal articular cartilage. A section of porcine type I/III collagen membrane was fashioned to the appropriate size and secured in situ with a combination of 6/0 vicryl sutures and fibrin glue. The chondrocyte cell suspension (ACI) was then injected into the membrane-covered defect using a fine catheter.
The arthrotomy was closed in the usual way and the knee joint maintained in full extension. Touch weight-bearing with the use of crutches was encouraged from the first postoperative day with the knee maintained in extension for the first ten days using a light-weight cylinder cast. From day ten, the patients enter a phased rehabilitation program.
Postsurgery rehabilitation regime
All patients underwent a supervised regime of physiotherapy over the first 12 months following surgery. During the entire regime every patient was prescribed suitable and adequate analgesia. Advice was given on an individual basis as to how to adhere to the rehabilitation plan whilst continuing with activities of daily living. The phased regime was structured as follows:
-
1.
Active dorsiflexion and plantar flexion of the ankle to help reduce oedema and encourage peripheral circulation
-
2.
Isometric quadriceps, hamstrings and gluteal muscle exercises
-
3.
Partial weight-bearing (20%) with crutches for six weeks
-
4.
Closed-chain exercises, initially with and then without a knee brace
-
5.
Isokinetic quadriceps, hamstrings and gluteal muscle exercises
-
6.
Progressive weight-bearing as tolerated and use of crutches as required
-
7.
Increased frequency and duration of walking and isotonic exercises
-
8.
Gradual introduction of open chain exercises such as jogging
-
9.
Resumption of dynamic sporting activities; although sports that necessitate high rotational and shearing forces within the knee were discouraged
Overall, full weight-bearing exercise was aimed for by 12 weeks with open chain exercises and impact recreational activities introduced at the six- to nine-month stage.
Outcome measures
In addition to the normal preoperative investigations, all patients were assessed prior to chondrocyte implantation using the following clinical scoring systems:
-
1.
Modified Cincinnati rating (0–100) [11]
-
2.
Visual analogue score (0–10) [12]
-
3.
Bentley functional rating score (0–4)
-
4.
Lysholm & Gilquist Score (0–100) [13]
-
5.
Patient functional outcome (0–10)
-
6.
Brittberg (poor, fair, good or excellent) [14]
-
7.
Patient rating (better, same, worse)
Annual functional outcome was assessed using a postal questionnaire of the same scoring systems. The response rate was above 85% throughout all annual patient assessments with non-responding patients assigned poor/low outcome scores. Statistical analysis (ANOVA) was performed using SPSS v11.0.
Results
Modified Cincinnati rating score
The modified Cincinnati rating score (MCRS) assesses the following: pain, swelling, giving way, overall activity level, walking, stairs, running activity, jumping or twisting activities. A maximum score is 100, whereby >80 is excellent, 55–79 is good, 30–54 is fair (30 to 54) and <30 is poor.
The mean MCRS improved significantly one year following surgery relative to the pre-operative value (60.1–72.1); see Chart 1.
Visual analogue score
The overall sequential visual analogue score (VAS) (ranging from 1 to 10, good to poor scale) is shown in Chart 2. In comparison to the preoperative VAS, there were sequential significant improvements (p < 0.001, ANOVA) maintained up to six years postsurgery.
Bentley functional rating score
The Bentley functional rating score (BFRS) related pain experienced from 0 (no pain) to 4 (severe pain). There were significant and sequential improvements (p < 0.001, ANOVA) in comparison to their preoperative scores (see Chart 2) maintained up to six years postsurgery.
Patient rating score
The patient rating score (PRS) allowed patients to subjectively score knee function as ‘better’, ‘the same’ or ‘worse’. Chart 3 demonstrates that the majority of patients (>60%) described their knee function as ‘better’ following the procedure, with no patients describing their knee function as ‘worse’ at five or six years following surgery.
Patient functional outcome score
Patient functional outcome score (PFOS) enabled patients to record their overall condition and function from 0 (poor) to 10 (excellent) (see Chart 4). All PFOS scores were significantly improved (unpaired t-test, p < 0.0001) compared to the respective mean preoperative scores and showed continued sequential improvement up to six years postsurgery.
Lysholm & Gilquist
The Lysholm & Gilquist (LG) assessment provides a score from 1 (poor) to 100 (excellent) by rating the following patient factors: the presence of a limp, the need for support, stair climbing, squatting, instability, pain, swelling and thigh atrophy. Sequential annual improvements were demonstrated in the LG scores up to six years following surgery (see Chart 4). Trendlines created from both LG & PFOS scores (see Chart 4), used to quantify the rate of improvement, exhibit a coefficient of determination of greater than 0.87.
Brittberg rating
The Brittberg rating enables patients to subjectively score knee function as poor, fair, good or excellent. Chart 5 shows the sequential Brittberg results with responses being grouped as “excellent or good”, compared with “fair or poor”. Continued sequential increases in beneficial responses (good or excellent) from pre-op were observed for both techniques (bars in Chart 5), whilst there was a corresponding reduction in adverse responses (lines in Chart 5).
Complications
From the original cohort of patients, 24 underwent a check arthroscopy at one year to assess the integrity of the chondral repair. All were found to have a fair to excellent grade of repair (International Cartilage Repair Society classification), and three cases were biopsied showing graft hypertrophy. There were no graft failures. Three patients underwent a manipulation of the knee under anaesthesia with no abnormality found.
Discussion
Early studies suggested ACI was beneficial for large (>2 cm2) symptomatic osteochondral lesions associated with significant baseline functional impairment [15]. A combination of patient-specific (age, higher preoperative function scores and few previous procedures) and lesion-specific factors (lateral femoral condyle and trochlear loci) have now been shown to be important in patient selection [16]. Recent outcome studies have helped refine the indications and the techniques for autologous chondrocyte implantation, demonstrating continued improvement in clinical results [10, 17, 18]. One of the principal developments has been the use of porcine type I/III collagen bi-membranes, seeded with cultured chondrocytes (termed matrix-induced ACI–MACI), with the aim to achieve a more uniform chondrocyte density.
The methodology of cartilage repair studies has previously been criticised and this study has both strengths and limitations that should be acknowledged. There are three principal strengths of this study. First, this study has a high patient number of 57, compared to previous published evidence with a median number of 30 [19]. Second, this study has a relatively long follow-up, i.e. six years in comparison to a median of 33 months in other studies [19]. Lastly, this study incorporates seven independent scoring systems to evaluate the functional outcomes following autologous chondrocyte implantation using the ACI technique for up to six years following surgery. Excluding the qualitative Brittberg and patient rating scores, all scoring schemes demonstrate continued sequential improvement in knee function.
The principal limitation of this study, as in many other cartilage repair studies, is that this is a prospective cohort and not a randomised controlled trial. As has been the case with numerous historical orthopaedic innovations, the initial development of a new technology does not lend itself easily to testing with a blinded randomised control trial. With time a consensus view prevails that results from a collective opinion created from numerous independent studies. Furthermore, whilst this study includes no radiological assessment or evaluation of the chondral repairs undertaken, we feel this omission does not detract from its main conclusion, namely, the ongoing mean functional improvements this technique affords. The histological evaluation of this technique has already been published on a small and separate cohort of patients by this centre [9]. This study detailed the histological findings from 16 patients willing to undergo repeat arthroscopic examinations for research purposes. Further, these patients underwent an initial period of immobilisation in plaster of Paris followed by six weeks of non-weight-bearing. For these reasons, these patients were not included in this study. Published studies have addressed the topic of the MRI evaluation of cartilage repair and no doubt further studies will refine the correlation between MRI and clinical outcomes [20]. Despite these limitations, we feel this study does significantly add to the body of published clinical evidence for autologous chondrocyte implantation (see Table 1).
A review of the current UK national guidelines states the continued need to develop culture methods that increase the percentage of hyaline cartilage and openly questions whether de-differentiated cells are capable of forming hyaline cartilage.
Due to these recommendations, all patients undergoing autologous chondrocyte transplantation should be enrolled into on-going clinical studies and informed of both the uncertain long-term effectiveness and possible risks. These studies should not only provide further evidence to clarify the important prognostic factors of autologous chondrocyte implantation but also help identify the most suitable tissue scaffold.
The results from this study are encouraging and provide further evidence of the benefit in the medium term of transplanting autologous chondrocytes to areas of osteochondral defects in carefully selected patients. In conclusion, these results demonstrate a statistically significant functional improvement over six years compared to preoperative scores in patients undergoing autologous chondrocyte implantation.
References
Mankin HJ, Mow VC, Buckwalter JA (2005) Articular cartilage structure, composition and function. In: Buckwalter JA, Einhorn TA, Simon WH (ed) Orthopaedic Basic Science AAOS, pp 444–467
Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC (1988) Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res 6:777–792
Larsson T, Aspden RM, Heinegard D (1991) Effects of mechanical load on cartilage matrix biosynthesis in vitro. Matrix 11:388–394
Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG (2003) Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy 19:477–484
Brittberg M, Peterson L, Sjogren-Jansson E, Tallheden T, Lindahl A (2003) Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am 85-A(Suppl 3):109–115
Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A (2000) Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res 212–234
Haddo O, Mahroof S, Higgs D, David L, Pringle J, Bayliss M, Cannon SR, Briggs TW (2004) The use of chondrogide membrane in autologous chondrocyte implantation. Knee 11:51–55
Bentley G, Minas T (2000) Treating joint damage in young people. BMJ 320:1585–1588
Briggs TW, Mahroof S, David LA, Flannelly J, Pringle J, Bayliss M (2003) Histological evaluation of chondral defects after autologous chondrocyte implantation of the knee. J Bone Joint Surg Br 85:1077–1083
Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G (2005) Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br 87:640–645
Noyes FR, Barber-Westin SD (1997) Arthroscopic-assisted allograft anterior cruciate ligament reconstruction in patients with symptomatic arthrosis. Arthroscopy 13:24–32
Wewers ME, Lowe NK (1990) A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 13:227–236
Lysholm J, Gillquist J (1982) Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med 10:150–154
Peterson L, Minas T, Brittberg M, Lindahl A (2003) Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 85-A(Suppl 2):17–24
Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A (2002) Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 30:2–12
Krishnan SP, Skinner JA, Bartlett W, Carrington RW, Flanagan AM, Briggs TW, Bentley G (2006) Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br 88:61–64
Bartlett W, Gooding CR, Carrington RW, Skinner JA, Briggs TW, Bentley G (2005) Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft. A preliminary report. J Bone Joint Surg Br 87:330–332
Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J (2003) A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br 85:223–230
Jakobsen RB, Engebretsen L, Slauterbeck JR (2005) An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am 87:2232–2239
Henderson IJ, Tuy B, Connell D, Oakes B, Hettwer WH (2003) Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br 85:1060–1066
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R (2003) Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am 85-A:185–192
Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O (2004) Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 86-A:455–464
Browne JE, Anderson AF, Arciero R, Mandelbaum B, Moseley JB, Jr, Micheli LJ, Fu F, Erggelet C (2005) Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res 237–245
Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A (2006) A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee 13:203–210
Steinwachs M, Kreuz PC (2007) Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy 23:381–387
Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, Lagae K, van der BJ, Vandenneucker H, Yang KG, Jelic M, Verdonk R, Veulemans N, Bellemans J, Luyten FP (2008) Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med 36:235–246
Author information
Authors and Affiliations
Corresponding author
Additional information
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Rights and permissions
About this article
Cite this article
Rogers, B.A., David, L.A. & Briggs, T.W.R. Sequential outcome following autologous chondrocyte implantation of the knee: A six-year follow-up. International Orthopaedics (SICOT) 34, 959–964 (2010). https://doi.org/10.1007/s00264-009-0842-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-009-0842-x