Abstract
We retrospectively reviewed the outcome of posterolateral fusion (PLF) in 136 patients with lumbar spondylolisthesis (LS), who had undergone posterior decompression laminectomy with foraminotomy and PLF using laminectomy bone chips as bone graft, with reduction of the slipped vertebra with transpedicle screws, between 1993 and 2003. Diagnosis of LS was confirmed by plain lumbar radiography, with computed tomography (CT) scan or magnetic resonance imaging (MRI) studies performed to confirm an associated condition, such as ruptured disc and spinal stenosis. The outcome of spinal fusion was good with 129 (94.85%) patients attaining solid fusion, while failed fusion was noted in seven (5.15%) patients. None of our patients complained of excessive postoperative wound pain. Additionally, no complications, such as wound infection, were encountered. Proper decortication of the posterior paravertebral gutters with an osteotome and removal of all soft tissues from the laminectomy bone chips are significant factors contributing to the successful outcome of the laminectomy bone chips in PLF. The fusion rate obtained with this type of autogenous bone graft is comparable to that of the iliac bone crest autogenous graft; hence, it is a good substitute for the iliac crest bone autogenous graft in performing PLF in treating lumbar spondylolisthesis.
Résumé
Nous avons analysé de façon rétrospective le devenir de la greffe postéro latérale (PLF) chez 136 patients présentant un spondylolisthésis (LS) et ayant bénéficié d’une décompression postérieure par laminectomie avec foraminotomie, la greffe postéro latérale utilisant des fragments de lames en forme de «chips» osseuses après réduction de la vertèbre olisthésique par des vis transpédicullaires. Ces patients ont été traités de 1993 à 2003. Le diagnostic de spondylolisthésis a été confirmé par les radiographies lombaires et le scanner ainsi que l’IRM de façon à analyser les lésions discales et les lésions de sténose canalaire. La fusion vertébrale a été considérée comme excellente pour 129 patients (94.85%) et a été un échec chez sept patients (5.15%). Aucun de ces patients ne s’est plaint de douleurs post opératoires importantes. Nous n’avons rencontré aucune complication importante et pas d’infection profonde. La décortication du plan vertébral postérieur à l’ostéotome, associée à l’ablation des tissus mous avec greffes par « chips » osseuses est un facteur important de succès pour la consolidation. Le taux de fusion obtenu avec ce type de greffe autogène est comparable à celui obtenu avec une greffe iliaque. Il s’agit là d’une bonne technique de substitution évitant la greffe iliaque et donnant un bon résultat dans le traitement des spondylolisthésis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lumbar spondylolisthesis, which is the forward slippage of one vertebra over the vertebra below it, has several aetiologies that can lead to spinal instability and subluxation, producing symptoms such as persistent dull low back pain with radiculopathy, low back stiffness, tight hamstrings and intermittent claudication. Initial treatment is usually conservative, including rest, non-steroidal anti-inflammatory drugs, wearing of a body brace and physical therapy. Main indications for surgery are: (1) intractable pain, (2) progression of symptoms with radicular involvement, or (3) progression of the slip [3]. There are many different operative procedure reported in the literature, but all must comply to the principle of adequate decompression, repositioning, fusion and adequate fixation in order to achieve a good outcome [3]. These operative procedures can be performed via either an anterior or posterior approach, with a choice of autogenous graft and allograft can be used for fusion. Usually an autogenous graft is the most commonly preferred, because it provides a much better outcome, and the most popular donor site is the iliac crest bone. However, many studies have shown that the iliac crest autogenous graft harvest is not risk-free, reporting an overall complication rate ranging from 9.4% to 49% [2]. We have undertaken this retrospective study to assess the outcome of posterolateral fusion (PLF) using laminectomy bone chips for the treatment of lumbar spondylolisthesis.
Materials and methods
From January 1993 to December 2003, a total of 136 patients (98 females and 38 males; aged 16–76 years, with an average of 46 years) diagnosed with lumbar spondylolisthesis (LS) by plain lumbar radiographs, treated and followed-up well at our Orthopaedic Division were included in this study. All patients presented with persistent low-back pain with radiculopathy and intermittent claudication. Computed tomography (CT) scans or magnetic resonance imagings (MRIs) were performed in all patients to identify other associated lesions, such as ruptured disc and spinal stenosis. Each and every patient underwent a near total posterior decompression laminectomy with foraminotomy and PLF with laminectomy bone chips (Fig. 1) as bone graft, followed by reduction of the slipped vertebra with transpedicle screws and the A-O or Trifix Reduction spinal system implants. The main preoperative radiographic characteristics are summarised in Table 1. All patients fulfilled the following criteria: (1) intractable low-back pain and/or sciatic pain; (2) failed previous conservative treatment; (3) progressive radiculopathy; (4) radiologically proven instability. An informed consent was obtained prior to operation. Spinal fusion was then assessed by plain lumbar spine radiographs at 4, 8, and 24 months after operation. Additional plain lumbar spine radiographs were performed on 60 patients showing solid fusion mass after removal of the spinal implants.
Results
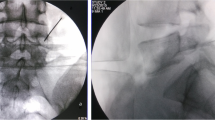
All patients underwent the procedure smoothly, with average operative time for one-level lesions being 1 h and 30 min, while 2 h and 15 min was spent for two-level lesions. Blood transfusion was routinely given for patients with two-level lesions and only to one-level lesion patients whose preoperative haemoglobin levels were less than 11 g/dl. One-hundred and twenty-nine cases (94.85%) developed solid fusion mass at 8 months post-operation (Figs. 2, 3) with failed fusion noted in seven cases (5.15%). Fusion rate for the one-level lesion group was 93% (84/90) and 97% (45/46) for the two-level lesion group. Fusion was assessed based on the criteria summarised in Table 2. No mortality nor morbidity was encountered in our series and our patients were discharged on the fifth post-operative day with a Knight-Taylor body brace. All of the 129 patients that developed solid fusion after the operation claimed to have relief of symptoms (low-back pain with radiculopathy and intermittent claudication) noted immediately after the procedure, and throughout the follow-up period. Sixty patients returned for removal of the spinal implants four years after operation, repeat plain lumbar radiographs (Fig. 4) after removal of implants showed solid fusion mass between the involved vertebrae.
Discussion
Lumbar spondylolisthesis was originally described as a cause of obstruction in labour by Herbiniaux, a Belgian obstetrician in 1782 [1, 6, 7], and its was Rokitansky who is credited for describing it as a pathological entity [1]. Since then, many authors have dedicated their time and effort to the study and search for the aetiology of spondylolisthesis. But it was not until in 1963, when Newman in his review of 319 cases, classified spondylolisthesis into five distinct groups [1, 7]. This classification was revised in 1976 by Wiltse et al. [9], and has since become the most widely accepted classification. Other than the previous five distinct groups by Newman, a sixth group has been added. Thus, present classification of spondylolisthesis is as follows: dysplastic (congenital) spondylolisthesis, isthmic (spondylolitic) spondylolisthesis, degenerative spondylolisthesis, traumatic spondylolisthesis, pathologic spondylolisthesis and iatrogenic spondylolisthesis (following lumbar surgery via laminectomy) [8, 9].
The most common type of spondylolisthesis found in patients less than 50 years of age is the isthmic (spondylolytic) type [5, 10]. It is believed that “biomechanical stress”, such as repetitive mechanical strain from heavy work and sports, causes a fatigue fracture to the pars interarticularis that allows the defective vertebra to move forward in relationship to the vertebra below [1, 4]. However, with the aging population found in an industrial country like Taiwan, the prevalence of degenerative spondylolisthesis has grown. The pathology of degenerative spondylolisthesis is different from that of isthmic spondylolisthesis; that is, the pars in degenerative spondylolisthesis remains intact, with the forward slippage caused by arthritic changes in the zygapophyseal joints between two vertebrae associated with degeneration of the disc at that level [9–11]. The most frequent site of pathology is between L4 and L5, with L3 next in order of occurrence [4]. No matter what the aetiology of the LS, patients usually present with a persistent dull low-back pain with radiculopathy, which increases with activity and decreases with rest, low-back stiffness, tight hamstrings and intermittent claudication. The mainstay of treatment is conservative, with rest, use of NSAIDs, physical therapy and the wearing of a body brace. Surgical intervention is only performed when there is failure of conservative treatment for at least one year. Surgical treatment of LS may be done via either an anterior or posterior approach. After the introduction of instrumentation for spinal reduction in the 1960s, operative management for LS is commonly performed via a posterior decompression laminectomy with posterolateral fusion and reduction of the slipped vertebra with spinal instrumentation.
Fusion is the most important factor in the successful treatment of LS, with autogenous, allograft, dimineralised bone matrix (DBM) and other graft extenders, such as calcium phosphate, as options to achieve this objective. Overall, present studies show that an autogenous graft provides the best fusion, because of its osteogenic, osteoconductive, and osteoinductive properties. An allograft bone, which has low or no osteogenicity and weak osteoinductive properties, is very poor in stimulating fusion. It is also reported that an allograft bone has increased immunogenicity, increasing its risk for disease transmission and resorbs more rapidly than an autogenous graft. The DBM and graft extenders contain proteins that stimulate bone formation and have successfully fused spines in animal studies, but at present there is no sufficient information to prove that they effectively stimulate successfull fusion in the human spine. They are also expensive, and are not recommended for use without addition of the patient’s own bone. So far, the most popular donor site for autogenous graft is the iliac crest. In our study, we discovered that laminectomy bone chips are excellent for PLF, both in quantity and quality. In the case of iliac crest bone harvest, the donor site is at risk of complications, such as large haematoma, wound infection, disabling donor wound pain, unsightly scars, meralgia paraesthesia, pelvic fracture (high in patients with osteoporosis), herniation at the harvest site, suture rejection with prolonged sterile drainage and seroma. All of these can lengthen hospital stay and may require additional surgery, leading to additional cost of treatment [2]. However, these co-morbidities were found to be extremely variable by different authors. As for the technique we have describe, our patients did not encounter any of the above mentioned co-morbidities with a short operative time and minimal blood loss noted.
In our series, we achieved a fusion rate of 94.85% (129/136) compared with the fusion rate from iliac crest bone graft of 97% reported in the literature. Although the fusion rate achieved from our technique is acceptable, some cases of failed fusion occured during the early stages of this study. The cause of failed fusion was investigated, and found out to be due to technical error; that is, decortication of the posterior paravertebral gutters was performed with a mechanical burr, prior to graft implantation. We later send a piece of the decorticated bone for pathological examination and burn osteonecrosis was noted. Since then, we have performed decortication of the paravertebral gutters with an osteotome, and with the removal of all soft tissues attached to the laminectomy bone chips prior to graft implantation, we were able to achieve successful fusion for the rest of our cases. It is important to note that these bone-chip grafts used for PLF contain spongious bone surrounded by cortical bone, which we believe provides better spinal fusion.
Conclusion
In conclusion, proper decortication of the posterior paravertebral gutters, with removal of all possible soft tissues attached to the laminectomy bone chips prior to the implantation of the graft, are significant factors contributing to the feasibility and successful outcome of the use of laminectomy bone chips in PLF. We also found out that performing decortication of the paravertebral gutters with a mechanical burr causes burn osteonecrosis of the fusion bed, which can lead to failed fusion. Furthermore, none of the possible complications from iliac crest bone harvest were encountered in our patients, allowing them to return to their daily activities at an earlier date. The fusion rate obtained with this type of autogenous graft is comparable to that of the iliac bone crest autogenous graft, and hence should be a good substitute for the iliac crest bone autograft for performing PLF in treating lumbar spondylolisthesis, especially in the degenerative type of spondylolisthesis.
References
Amundson G, Edwards C, Garfin S (1999) Spondylolisthesis. In: Herkowitz HN, Garfin SR, Balderston RA, Eismont FJ, Bell GR, Wiesel SW (eds) Rothman-Simeone The spine, 4th edn. WB Saunders, Philadelphia, pp 835–885
Banwart JC, Asher MA, Hassanein R (1995) Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine 20:1055–1060
Csécsei G, Klekner A, Dobai J, Lajgut A, Sikula J (2000) Posterior interbody fusion using laminectomy bone and transpedicular screw fixation in the treatment of lumbar spondylolisthesis. Surg Neurol 53:2–7
Lombardi JS, Wiltse LL, Reynolds J, Widell EH, Spencer C III Degenerative spondylolisthesis. Spine 10:821–827
Moe JH et al (1978) Scoiliosis and other spinal deformities. WB Saunders, Philadelphia
Newell RLM (1995) An historical review. Spine 20:1950–1956
Newman PH (1963) The aetiology of spondylolisthesis. J Bone Joint Surg 45:39–59
Puschak TJ, Sasso RC (2005) Spondylosis-spondylolisthesis. In: Vaccaro AR (ed) Orthopedic knowledge, update 8: Philadelphia, Pennsylvania, pp 553–563
Wiltse LL, Newmna PH, Macnab I (1976) Classification of spondylosis and spondylolisthesis. Clin Orthop 117:23–29
Wiltse LL, Widell EH Jr, Jackson DW (1975) Fatigue fracture: the basic lesion is isthmic spondylolisthesis. J Bone Joint Surg Am 57:7–22
Yochum TR, Rowe LJ (1996) Essentials of skeletal radiology, 2nd edn. Williams & Wilkin, Baltimore
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ka-Siong Kho, V., Chen, WC. Posterolateral fusion using laminectomy bone chips in the treatment of lumbar spondylolisthesis. International Orthopaedics (SICO 32, 115–119 (2008). https://doi.org/10.1007/s00264-006-0274-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-006-0274-9