Abstract
Gene therapy presents a novel approach to biological treatment. Several orthopaedic diseases can cause changes in biological signalling at the tissue level that potentially can be repaired or modified by inserting genes into the cells or tissues to modulate gene expression. Impaired bone healing, need for extensive bone formation, cartilage repair and metabolic bone diseases are all conditions where alterations of the signalling peptides involved may provide cure or improvement. In orthopaedic oncology, gene therapy may achieve induction of tumour necrosis and increased tumour sensitivity to chemotherapy. In the last decade, extensive improvements have been made to optimise gene therapy and have been tested on several orthopaedic conditions. How far this development has come in orthopaedics is highlighted in this paper.
Résumé
La thérapie génique représente une nouvelle approche comme traitement biologique. Plusieurs maladies orthopédiques peuvent causer des changements au niveau des tissus qui potentiellement peuvent être réparés ou modifiés en insérant des gènes dans les cellules pour moduler l’expression du gène. Les maladies métaboliques sont des conditions ou l’altération de certains peptides particuliers peuvent fournir une guérison ou une amélioration. En oncologie la thérapie gènique peut améliorer la nécrose tumorale et accroitre la sensibilité de la tumeur à la chimiothérapie. Des améliorations importantes ont été faites dans la dernière décennie pour optimiser cette thérapie qui a été testée dans plusieurs conditions orthopédiques. Cet article envisage les développements actuels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Principles of gene therapy
Gene therapy represents a novel principle of biochemical alteration, which can modify cellular or tissue gene expression in order to either repair genetic defects or enhance biological responses to cope with disease states.
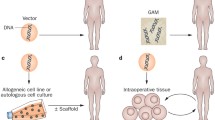
Generally gene therapy aims at introducing one or more specific genes into an organism or tissue. The vehicles that encapsulate therapeutic genes for delivery are called vectors. These vectors can be either viral or non-viral. Viral vectors are normally modified viruses, which cannot replicate but still have the capacity to carry genes into the cells. In principle gene delivery is performed in two settings. “Ex vivo”, the gene is transferred outside the body to a cell or tissue culture whereas during “in vivo” gene transfer, the gene is transferred directly to the host. For orthopaedic applications, the genes of interest are typically encoded for peptide growth factors that are able to enhance or initiate bone formation, repair cartilage or other tissues or induce programmed cell death (apoptosis) in tumour tissue (Table 1). In ex vivo gene therapy, the modified cells function as a drug delivery system providing increased and prolonged local extra-expression of bioactive peptides. In genetic diseases such as osteogenesis imperfecta, gene therapy provides the possibility of inserting a gene that contains a correct code to replace the defective collagen genetic code causing the disease.
Delivery systems
Viral delivery systems
Viruses are nature’s own gene therapy organism with their ability to invade cells and deliver genetic material to the nucleus for incorporation into the genome. Several types of viruses are used for gene therapy purposes and have various advantages and disadvantages. Viral gene transfer is called transduction whereas non-viral transfer is called transfection.
Retroviruses are highly efficient for gene delivery into dividing cells and were the vectors in earliest use. However, they do not infect non-dividing cells, and one case of death during a clinical trial has been reported [11], these viruses probably have limited usage in future clinical practice.
Adenoviruses readily infect both dividing and non-dividing cells but unlike retroviruses, do not incorporate genes into host DNA. In terms of safety, this is a potential advantage since the risk of mutagenesis is diminished. However, adenoviral transduction often leads to unstable gene expression, and since adenoviral genes often enter into cells along with therapeutic genes, immunogenic stimulation is a problem.
Adeno-associated viruses, like retroviruses, integrate genetic material into infected host DNA. They are non-pathogenic and provide stable expression of the transferred gene. They are also able to infect non-dividing cells. Their disadvantage is the fact that they are only able to carry small amounts of genetic material, which limits the size of genes to be transported [22].
Non-viral delivery systems
Non-viral delivery systems are generally less efficient than viral techniques since no biological system actively incorporates the desired DNA material into the genome. The techniques can be physical, mechanical or chemical. Electroporation is a method where electrical energy increases cell membrane permeability to facilitate DNA intracellular flux. Particle bombardment with DNA-coated gold onto cell layers (Gene-gun) and micro-injection into single cells are examples of mechanical methods. Chemical methods may be used to modify DNA material in order to facilitate cellular uptake. Lipofection is a method where DNA material is coated with cationic lipids, which are readily taken up by cells due to their electrochemical properties. Calcium phosphate binding to DNA material can also facilitate cellular uptake, as can binding of specific proteins to DNA [3, 13].
Applications
Bone healing and repair
Impaired or inadequate bone repair is still a problem in several conditions, such as fracture non-union, implant loosening and large bone defects after trauma or tumour resections. Recombinant growth factor treatment has recently been demonstrated to provide some solutions to these problems in clinical settings [5, 6, 8]. However, local growth factor applications do not provide sustained delivery of active substances and are expensive.
Therapy with genes encoded for osteoinductive factors could potentially provide long-term bone formation activity. In vivo gene transfer techniques have been used successfully in bone induction. An adenovirus vector containing the BMP-2 growth factor was shown to induce formation of bone in muscles of mice [15]. A similar vector also induced healing of critical-sized bone defects in rat femurs [2]. However, in vivo gene transfer with viral vectors induces immune responses that limit the duration and effectiveness of the treatment. To overcome this problem, non-viral methods with collagen-based gene-activated matrix containing genes for BMP-4 and PTH 1-34 have been tested in a model with critical sized defects of 5 mm. These methods induce healing, and double-gene stimulation proved more efficient than single-gene stimulation. However, a similar study demonstrated insufficient bone formation to induce healing of critical-sized defects [4]. Ex vivo gene therapy methods for bone repair offer the advantage of selecting cells for delivery, which independently contribute to the healing response (bone marrow cells or stem cells). Ex vivo methods are also safer since no viral particles or DNA need to be inserted into the patient.
Ex vivo gene therapy has been investigated for bone induction using several different cell types and genes. Lieberman [12] pioneered this work by demonstrating that marrow cells transduced with the adenovirus vector BMP-2 gene could induce healing of critical defects in rat femurs and that the bone formation was more consistent than that seen when recombinant BMP-2 was used. Muscle, fat-derived stem cells and skin fibroblasts have also been used successfully to induce bone formation when transduced with BMP genes [19, 23, 28].
In the study by Peng [19], muscle-derived stem cells were transduced with retroviral vectors containing both BMP-4 and the angiogenic growth factor gene VEGF. Double-gene therapy induced more bone formation than either of the two single genes. These pre-clinical studies demonstrate that gene therapy regimens can induce bone formation. However, consistent and safe methods that can be used in clinical practice still needs to be developed.
Spinal fusion
Fifty percent of all bone graft procedures are said to take place in spinal fusion, where failure to form solid fusion is still a significant problem. A pivotal clinical trial has shown good results using recombinant and purified osteoinductive proteins for spinal fusion [5]. Limitations of recombinant proteins include the high cost and carrier problems. Gene therapy strategies could potentially solve some of these problems.
Genes encoded for osteoinductive proteins within the BMP family could all be potentially used for cell-based gene therapy [10, 21]. One interesting gene, which has been used successfully for gene therapy in spine fusion, is LIM mineralization protein 1 (LMP-1). This gene is an intracellular signalling peptide, which is thought to regulate expression of numerous growth factors in the BMP family. Relatively low transduction rates by non-viral methods have been sufficient to induce significant bone-forming responses and have been used to form solid spinal fusion in rabbits [3]. It has been speculated that LMP-1 initiates cascades of growth factor expression resulting in increased biological response. The possibility of using non-viral gene transfer methods makes LMP-1 gene therapy a good candidate for clinical trials with osteoinductive gene therapy.
Cartilage repair
Repair of both localised cartilage defects and more particularly of generalised degenerative lesions represent very challenging problems. Avascularity, complex tissue organisation and low endogenous healing capacity all contribute to the difficulties of stimulating cartilage regeneration.
Approaches using cell-based gene therapy to stimulate cartilage repair have mainly focused on growth-factor genes that participate in cartilage matrix homeostasis and chondrogenesis. These growth factors are insulin-like growth factor (IGF), transforming growth factor-beta (TGF-beta) and bone morphogenetic proteins (BMP). These genes have been transduced into various types of stem cells for cell-based gene therapy. Due to the poor endogenous repair and remodelling capacity of cartilage, gene-therapy cells have typically been combined with various scaffolds to mimic cartilage tissue architecture. This concept has been proven to give good cartilage repair responses in rabbits using periosteal mesenchymal stem cells (MSC) transfected with BMP-7 and Sonic hedgehog genes [9]. The combination of gene-modified cells and scaffold has been termed “gene-enhanced tissue engineering”.
Degenerative disc disease
Degenerative disc disease (DDD) is a chronic condition characterised by loss of proteoglycans and water in the disc tissue, especially in the nucleus pulposus. These pathological changes alter the biomechanical properties of disc leading to reduced resistance to loads, which in turn leads to tissue degeneration. Gene therapy may restore proteoglycan and collagen synthesis and ultimately rebuild the disc tissue leading to relief of symptoms or, at best, cure by restoring the biomechanical capacity. Several growth factors are able to improve protein synthesis in cells of the intervertebral disc and nucleus pulposus, and such growth-factor genes may be candidates for treatment of DDD using gene therapy. TGF-beta, IGF, BMP’s and platelet derived growth factors (PDGF) posses such abilities [25]. Therapeutic gene transduction into both animal and human intervertebral disc cells has recently demonstrated that proteoglycan synthesis can be stimulated by gene therapy principles using TGF-beta as the therapeutic gene [14, 17]. Studies in a rabbit model have also shown that direct gene transfer of TGF-beta in an adenovirus vector was able to increase TGF levels and proteoglycan levels in intervertebral disc tissue [16]. Another possible approach to the treatment of DDD is inhibition of extra cellular matrix degradation enzymes. Tissue inhibitor of metalloproteinase 1 (TIMP-1) is such an enzyme, and in vitro studies using human nucleus pulposus cells transduced with adenoviral vectors carrying the TIMP-1 gene have demonstrated increased proteoglycan synthesis [27]. Although promising approaches based on gene therapy principles have been established, clinical treatment measures are still a future perspective.
Systemic bone diseases
Osteoporosis
Osteoporosis results in bone loss and osteopenia. Two types of osteoporosis exist. Type 1 is characterised by increased osteoclastogenesis due to due oestrogen depletion and type 2 characterised by decreased osteogenesis due to senescence of marrow stem cells. Treatment for type 1 osteoporosis can be attempted by decreasing osteoclastogenesis. The receptor activator of NF-kappa beta ligand (RANKL) is the most important factor for osteoclastogenesis, and this factor can be inhibited by osteoprotegerin (OPG) [7]. Marrow stem cells transfected with the gene for OPG and re-introduced into an osteoporotic organism could result in systemic inhibition of osteoclastogenesis. Gene therapy for type 2 osteoporosis can be accomplished by ex vivo principles where marrow stem cells from osteoporotic donors are transduced with adenoviral vectors carrying the BMP-2 gene, a potent bone-inducing growth factor. Such cells have been shown to increase osteogenic activity in vivo [26].
Osteopetrosis
Osteopetrosis is a genetic disorder with the opposite phenotype to osteoporosis and characterised by excessive bone formation, eventually resulting in bone marrow obliteration. The excessive bone formation is due to decreased osteoclastogenesis resulting from a genetic defect in the gene coding for colony stimulating factor 1 (CSF-1). Gene therapy could be used to incorporate marrow stem cells that over-express the CSF-1 gene leading to increased osteoclastogenesis. This principle has been successful in a transgenic mouse model [1].
Osteogenesis imperfecta (OI)
Osteogenesis imperfecta (OI) is a genetic disease with a mutation in the procollagen alfa 2 (I)-chain. It results in reduced bone strength and susceptibility to fractures. Gene-therapy-based delivery of the correct procollagen gene should, in principle, be able to correct the biochemical disorder. Using a transgenic mouse model, in vitro studies have shown that transfection of marrow stromal cells with an adenovirus carrying the gene of the correct procollagen alfa 2 (I) chain could result in both expression of the correct procollagen alfa 2 (I) chain and correct assembly with other procollagen chains [18]. Thus, principles of gene therapy can be used for systemic bone diseases so far evidenced in vitro and in small, animal in vivo studies.
Oncology
In orthopaedic oncology, gene therapy has been tested in the treatment of both primary bone tumours and bone metastases. Gene therapy has been used to accelerate tumour-tissue necrosis by inserting genes that induce programmed cell death (apoptosis). This has been accomplished in bone tumour cell cultures where genes coding for different interleukins resulted in increased immunologically induced death in the transduced cells [20]. Gene therapy can also improve cancer treatment by increasing cellular sensitivity to chemotherapy. The gene TNF-related apoptosis-inducing ligand (TRAIL) was able to accelerate bone tumour cell death during exposure to different chemotherapeutic agents [24].
Conclusion
Gene therapy has proven its feasibility for the treatment of various orthopaedic problems and disorders. Gene therapy primarily offers the opportunity to provide prolonged local drug delivery, which in combination with different cell types can stimulate synergistic tissue responses. Repair of genomic defects is also a fascinating possibility. Genetically modified cells might provide solutions for some of the complex problems that still exist, especially within the area of tissue engineering.
Until now, the effectiveness of gene therapy has primarily been shown in vitro and in non-clinical settings. Some studies have shown that effects seen in lower animals cannot be reproduced in higher animals. There is therefore a considerable need for continuing research before regimens are developed to a degree where they can be tested in clinical orthopaedics. As with all biological techniques, there must be sufficient impact on clinical outcomes if new, expensive treatments are to be justified. In orthopaedics, gene therapy will primarily be used for non-lethal conditions, and patient safety is therefore of maximal importance. An increased morbidity or mortality will be unacceptable in orthopaedic gene therapy.
Abbreviations
- MSC:
-
Mesenchymal stem cell
- IGF:
-
Insulin-like growth factor
- BMP:
-
Bone morphogenetic protein
- TGF-beta:
-
Transforming growth factor beta
- OPG:
-
Osteoprotegerin
- RANKL:
-
Receptor antagonist of the NF kappa beta ligand
- CSF-1:
-
Colony stimulating factor-1
- TIMP-1:
-
Tissue inhibitor of metalloproteinase
- DDD:
-
Degenerative disc disease
- PDGF:
-
Platelet-derived growth factor
- LMP-1:
-
LIM mineralization protein 1
- TRAIL:
-
TNF-related apoptosis-inducing ligand
References
Abboud SL, Woodruff K, Liu C, Shen V, Ghosh-Choudhury N (2002) Rescue of the osteopetrotic defect in op/op mice by osteoblast-specific targeting of soluble colony-stimulating factor-1. Endocrinology 143:1942–1949
Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzani SC, Robbins PD, Evans CH (2000) Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther 7:734–739
Boden SD, Titus L, Hair G, Liu Y, Viggeswarapu M, Nanes MS, Baranowski C (1998) Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1). Spine 23:2486–2492
Bonadio J, Smiley E, Patil P, Goldstein S (1999) Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med 5:753–759
Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA (2002) Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine 27:2396–2408
Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S (2001) Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am 83 (Suppl 1):S151–S158
Goater JJ, O’Keefe RJ, Rosier RN, Puzas JE, Schwarz EM (2002) Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthoptera Res 20:169–173
Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 84:2123–2134
Grande DA, Mason J, Light E, Dines D (2003) Stem cells as platforms for delivery of genes to enhance cartilage repair. J Bone Joint Surg Am 85 (Suppl 2):111–116
Helm GA, Alden TD, Beres EJ, Hudson SB, Das S, Engh JA, Pittman DD, Kerns KM, Kallmes DF (2000) Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. J Neurosurg Spine 92:191–196
Lehrman S (1999) Virus treatment questioned after gene therapy death. Nature 401:517–518
Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, Kabo JM, Finerman GA, Berk AJ, Witte ON (1999) The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am 81:905–917
Lollo CP, Banaszczyk MG, Chiou HC (2000) Obstacles and advances in non-viral gene delivery. Curr Opin Mol Ther 2:136–142
Moon SH, Gilbertson LG, Nishida K, Knaub M, Muzzonigro T, Robbins PD, Evans CH, Kang JD (2000) Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer: implications for the clinical management of intervertebral disc disorders. Spine 25:2573–2579
Musgrave DS, Bosch P, Ghivizzani S, Robbins PD, Evans CH, Huard J (1999) Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone 24:541–547
Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, Robbins PD, Evans CH (1999) Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine 24:2419–2425
Nishida K, Kang JD, Suh JK, Robbins PD, Evans CH, Gilbertson LG (1998) Adenovirus-mediated gene transfer to nucleus pulposus cells. Implications for the treatment of intervertebral disc degeneration. Spine 23:2437–2442
Niyibizi C, Smith P, Mi Z, Phillips CL, Robbins P (2001) Transfer of proalpha2(I) cDNA into cells of a murine model of human Osteogenesis Imperfecta restores synthesis of type I collagen comprised of alpha1(I) and alpha2(I) heterotrimers in vitro and in vivo. J Cell Biochem 83:84–91
Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J (2002) Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest 110:751–759
Ramnaraine M, Pan W, Goblirsch M, Lynch C, Lewis V, Orchard P, Mantyh P, Clohisy DR (2003) Direct and bystander killing of sarcomas by novel cytosine deaminase fusion gene. Cancer Res 63:6847–6854
Riew KD, Wright NM, Cheng S, Avioli LV, Lou J (1998) Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcif Tissue Int 63:357–360
Robbins PD, Ghivizzani SC (1998) Viral vectors for gene therapy. Pharmacol Ther 80:35–47
Rutherford RB, Moalli M, Franceschi RT, Wang D, Gu K, Krebsbach PH (2002) Bone morphogenetic protein-transduced human fibroblasts convert to osteoblasts and form bone in vivo. Tissue Eng 8:441–452
Suzuki H, Hotta T, Koyama T, Komagata M, Imakiire A, Yanase N, Yoshimoto T, Mizuguchi J (2003) Retrovirus-mediated transduction of TRAIL and chemotherapeutic agents co-operatively induce apoptotic cell death in both sarcoma and myeloma cells. Anticancer Res 23:3247–3253
Thompson JP, Oegema TR Jr, Bradford DS (1991) Stimulation of mature canine intervertebral disc by growth factors. Spine 16:253–260
Turgeman G, Pittman DD, Muller R, Kurkalli BG, Zhou S, Pelled G, Peyser A, Zilberman Y, Moutsatsos IK, Gazit D (2001) Engineered human mesenchymal stem cells: a novel platform for skeletal cell mediated gene therapy. J Gene Med 3:240–251
Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P, Gilbertson LG, Kang JD (2003) Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine 28:2331–2337
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lind, M., Bünger, C. Orthopaedic applications of gene therapy. International Orthopaedics (SICOT) 29, 205–209 (2005). https://doi.org/10.1007/s00264-005-0650-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-005-0650-x