Abstract
The use of pedicled vascularised bone grafts from the distal radius makes it possible to transfer bone with a preserved circulation and viable osteoclasts and osteoblasts. Experiments performed at the basic science level has provided substantial evidence that low-intensity ultrasound can accelerate and augment the fracture healing process. Only an adequate double-blind trial comparing treatment by ultrasound stimulation in patients treated by similar surgical techniques can provide evidence of the true effect of ultrasound. This paper describes the results of such a trial. From 1999 to 2004, 21 fractures of the scaphoid with established non-union treated with vascularised pedicle bone graft were selected for inclusion in a double-blind trial. All patients were males, with an average age of 26.7 years (range 17–42 years) and an average interval between injury and surgery of 38.4 months (range 3 months–10 years). Low-intensity ultrasound was delivered using a TheraMed 101-B bone-growth stimulator (30 mW/cm2, 20 min/day), which was modified to accomplish double-blinding. These modifications did not affect the designated active units. The placebo units were adjusted to give no ultrasound signal output across the transducer. Externally, all units appeared identical but were marked with individual code numbers. Patients were randomly allocated to either an active or placebo stimulation. Follow-up averaged 2.3 years (range 1–4 years). All patients achieved fracture union (active and placebo groups), but compared with the placebo device (11 patients), the active device (ten patients) accelerated healing by 38 days (56±3.2 days compared with 94±4.8 days, p<0.0001, analysis of variance).
Résumé
L’utilisation de greffes pediculées de l’extrémité distale du radius est possible. Elles peuvent être utilisées comme transferts osseux pourvu que l’on préserve la vascularisation garant d’une bonne circulation avec des osteoclastes et osteoblates bienvivants. De nombreuses expérimentations ont également montré que l’utilisation d’ultrasons de basse intensité peut accélérer et augmenter le processus de consolidation osseuse. Seule une étude en double aveugle stimulant l’ostéogènèse par ultrasons sur des patients opérés avec des techniques identiques peut mettre en évidence le véritable effet de ceux-ci. Le but decetravail est de décrire les résultats d’un tel essai. Entre 1999 et 2004 21 fractures du scaphoide avec pseudarthrose ont été traitées par une greffe pédiculée et sélectionnée pour cet essai en double aveugle. Tous les patients étaient de sexe masculin, l’âge moyen était de 26 ans (17 à 42 ans) et le temps moyen entre le traumatisme et l’intervention chirurgicale a été de 38,4 mois (3 mois à 10 ans). Des ultrasons de basse intensité ont été utilisés avec un stimulateur de type TheraMed 101-B (30 mW/cm2, 20 minutes par jour) en double aveugle. Un appareil de type placébo a été également utilisé. L’apparence externe des deux appareils était absolument identique. Les patients ont été randomisés entre ceux recevant la stimulation placébo et ceux recevant une stimulation active d’ultrason. Après un suivi moyen de 2,3 ans (de 1 à 4 ans), tous les patients ont consolidé, aussi bien dans la série avec ultrasons que dans la série placébo. Si l’on compare la série avec appareil de type placébo (11 patients) à la série utilisant un appareil délivrant des ultrasons (10 patients), la guérison a été plus rapide dans cette dernière série de 38 jours (56±3,2 jours, versus 94±4,8 jours; p<0.0001 en analyse devariance).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ideal treatment of non-union of the scaphoid remains unsolved and controversial. Displaced fracture fragments and soft tissue interposition can prevent union of acute fractures of the scaphoid carpal bone by interrupting the blood supply. The rate of osseous unions seems to be related to the stability and vascularity of the proximal fragment.
Only an adequate double-blind trial comparing treatment by ultrasound stimulation in patients treated with similar surgical techniques can provide evidence of the true effect of ultrasound. This paper describes the results of such a trial.
Materials and methods
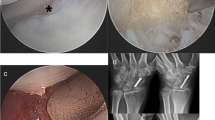
From 1999 to 2004, 21 fractures of the scaphoid with established non-unions treated with vascularised pedicle bone graft [1] (Fig. 1) were selected for inclusion in a double-blind trial.
All patients were males, with an average age of 26.7 years (range 17–42 years) and an average interval between injury and surgery of 38.4 months (range 3 months–10 years). Nine patients had fractures through the scaphoid waist, and 12 had fractures in the proximal third. All patients had a history of dorsiflexion injury of the wrist. Ten patients had sustained their fractures in simple falls, four during sports activity. The dominant wrist was affected in six patients.
Fracture fixation was with K-wires. All patients were initially immobilized with a long-arm thumb spica cast, followed by a short-arm cast until healing was demonstrated by X-rays.
Low-intensity ultrasound was delivered using a TheraMed 101-B bone-growth stimulator [2] (Fig. 2), which was modified to accomplish double-blinding. These modifications did not affect the designated active units. The placebo units were adjusted to give no ultrasound signal output across the transducer. Externally, all units appeared identical but were marked with individual code numbers. Patients were randomly allocated to either an active or placebo stimulation.
Neither the patient nor the surgeon knew whether the unit was active or a dummy because they were indistinguishable in external appearance and in use.
The patients were familiarised with the apparatus and instructed in its use. After the operation with the use of a template and a standardised technique, a window was created in the volar-radial aspect of the cast over the scaphoid. A low-intensity pulsed ultrasound signal of 30 mW/cm2 (ISATA), was applied transdermally for 20 min/day. A water-based gel was used as coupler between the transducer device and the skin to facilitate effective ultrasonic wave transmission to the fracture site.
The parameters that were evaluated included pain, active range of motion of the wrist, radiographic evidence of union, carpal height index, and scapholunate-capitolunate angles.
Pain was assessed by using a visual analog scale that ranged from one point (no pain) to ten points (severe pain).
Active range of motion of the wrist in the saggital and frontal planes and rotation of the forearm were measured by the author.
Standardised radiographs were made in the posteroanterior, lateral, and oblique views. Radiographs were made every 4 weeks for the first 6 months to assess union.
The defined end point of the study was a healed nonunion. The following definition of healing was developed before the assessments.
Time to healed non-union was defined as the interval, in days, between the date of the operation and the time when the fracture site (non-union) was healed both clinically (solid and not causing tenderness or pain) and radiographically (evidenced by complete bridging cortices).
For the patients who had complied with the study protocol and had complete follow-up, time to healing was assessed with the use of one statistical method, analysis of variance, to compare the mean times to healing for the two treatment groups.
Results
Follow-up averaged 2.3 years (range 1–4 years). Fracture union was achieved in all cases (21 patients) treated with vascularised pedicle bone graft. Fourteen patients were very satisfied with the results of surgery, 15 had occasional pain or discomfort with strenuous activity, 11 returned to their previous occupation and leisure activity with mild limitations, and 17 demonstrated a flexion/extension arc averaging 93° and a radial/ulnar deviation arc of 44°. The scapho-lunate and capito-lunate angles averaged 58.2° and 8.6°, respectively. The average carpal height index was 0.54.
Comparing the active device (ten patients) with the placebo device (11 patients), ultrasound treatment led to a reduced time to overall (clinical and radiographic) healing by 38 days (average of 56±3.2 days compared with 94±4.8 days; p<0.0001; analysis of variance; Fig. 3a–c).
No adverse reactions or complications were attributable to the device, and no contraindications to its use were reported during the study.
Discussion
Scaphoid non-unions are challenging because they may not always be symptomatic in their early stages; therefore, at delayed presentation, they can have greater bone loss, carpal collapse, and loss of blood supply. The natural history of non-unions is that eventual carpal collapse and degenerative arthrosis will ensue, usually within 10 years. When non-unions are recognised in stable position, bone grafting with the use of supplemental fixation leads to union in almost all cases as long as the proximal pole of the scaphoid is free of osteonecrosis. Proximal pole non-unions accompanied by osteonecrosis require the addition of a vascularised bone graft. Vascularized grafts may also be useful for non-unions that fail to heal after adequate fixation and traditional grafting methods. Salvage procedures, such as radial styloidectomy, scaphoid excision with or without limited midcarpal fusion, proximal row carpectomy, and total wrist fusion, are reserved for cases with severe carpal collapse and arthrosis.
The use of pedicled vascularised bone grafts (VBGs) in the reconstruction of bone defects or osteonecrosis is nearly a century old. In 1905, Huntington transferred a fibula with its nutrient artery pedicle in the reconstruction of a tibial defect [3]. Sixty years later, Roy-Camille transferred a pedicled VBG (scaphoid tubercle) on an abductor pollicis brevis muscle pedicle to a delayed scaphoid fracture union [4]. VBGs, unlike conventional bone grafts, preserve the circulation as well as viable osteoclasts and osteoblasts, allowing primary bone healing without creeping substitution within the dead bone [5]. Use of low ultrasound stimulation in scaphoid non-unions treated by pedicled VBGs may aid or accelerate healing.
Ultrasound, a form of mechanical energy that is transmitted through and into biological tissues as an acoustic pressure wave at frequencies above the limit of human hearing, is used widely in medicine as a therapeutic, operative, and diagnostic tool [6, 7]. Therapeutic ultrasound, and some operative ultrasound, uses intensities as high as 1–3 W/cm2 and can cause considerable heating in living tissues. To take full advantage of this energy absorption, physiotherapists often use such levels of ultrasound acutely to decrease joint stiffness, reduce pain and muscle spasms, and improve muscle mobility. The use of ultrasound as a surgical instrument involves even higher levels of intensity (5–300 W/cm2), and sharp bursts of energy are used to fragment calculi, to initiate the healing of non-unions, to ablate diseased tissues such as cataracts, and even to remove methylmethacrylate cement during revision of prosthetic joints [8]. At the opposite end of the ultrasound-intensity spectrum, much lower magnitudes of 1–50 mW/cm2 are used to drive diagnostic devices that non-invasively image vital organs, foetal development, peripheral blood flow, and metabolic bone diseases such as osteoporosis and to evaluate fracture callus during healing [9, 10]. The intensity level used for imaging, which is five orders of magnitude below that used for surgery, is regarded as nonthermal and nondestructive [11]. Nevertheless, low-intensity ultrasound is still a mechanical force, and it therefore holds the potential to influence bone mass and morphology through bone tissue’s strong sensitivity to physical stimuli.
In 1952, investigators in Italy demonstrated, in a controlled, paired study of radial fractures in rabbits, that continuous-wave ultrasound could stimulate the formation of bone callus [12]. These findings led to the first clinical use of ultrasound to stimulate fracture healing, and in 1953 the same investigators found, in a study of eight patients, that the treatment was safe and produced an increase in periosteal callus [13]. More than 30 years later, Dyson and Brookes [14], in a study of bilateral fibular fractures in rats, demonstrated accelerated fracture healing when treatment with 500 mW/cm2 of pulsed ultrasound was compared with no therapy. These investigators found that ultrasound treatment was most effective during the early stages of healing. Extrapolating these data to the clinical setting, Xavier and Duarte [15] reported in a Brazilian orthopedic journal that 70% of 26 non-unions healed after brief exposure (20 min/day) to very low-intensity ultrasound (30 mW/cm2 ).
These original findings were soon supported by Reuter et al. [16, 17], who found positive effects in bone in a series of animal studies that involved using a continuous ultrasound signal an order of magnitude higher than that used by Duarte [18]. Klug et al. [19, 20] demonstrated that ultrasound treatment delivered at an intensity of 200 mW/cm2 accelerated the healing of closed lower-extremity fractures in rabbits by 18%. Pilla et al. [21], in a placebo-controlled study of midshaft tibial osteotomies in rabbits, found that brief periods (20 min/day) of pulsed ultrasound (a 200-μs burst of 1.5-MHz sine waves, repeated at 1 kHz), delivered at a low intensity of 30 mW/cm2, accelerated the recovery of torsional strength and stiffness.
The mechanical stimulation inherent to ultrasound translates into a biological response. Wide-ranging studies both in vitro and in vivo have been used to investigate the biological mechanisms responsible for the observed influence of ultrasound on fracture healing. In one of the first such studies, Chapman et al. [22] reported that ultrasound induced a change in the rates of influx and efflux of potassium ions in rat thymocytes. Ryaby et al. [23–25] later reported that low-intensity ultrasound increased calcium incorporation in both differentiating cartilage and bone cell cultures, reflecting a change in cell metabolism. This increase in second-messenger activity was paralleled by the modulation of adenylate cyclase activity and transforming growth factor-B synthesis in osteoblastic cells. The influence of ultrasound on second-messenger activity in primary chondrocytes was also reported by Parvizi et al. [26], who found, using a real-time assay, that the application of ultrasound at 50 mW/cm2 increased the release of cellular calcium. Kokubu et al. [27] showed that low-intensity ultrasound (30 mW/cm2) increased prostaglandin-E2 production through the induction of cyclo-oxygenase-2 mRNA in mouse osteoblasts, and concluded that ultrasound exerts its influence in a manner similar to that of fluid shear stress and tensile force stimuli. More recently, Ito et al. [28] studied the effect of low-intensity ultrasound on growth factor secretion in a coculture of human osteoblastic and endothelial cells and found that ultrasound increased the release of platelet-derived growth factor in the conditioned media.
Not all of the impact of ultrasound need be identified at the molecular mechanistic level in order to ultimately benefit healing. Rawool et al. [29] demonstrated that low-intensity ultrasound, delivered over a 10-day period, stimulated a greater degree of vascularity at the site of ulnar osteotomies in dogs.
These data suggest that, in addition to modulating gene expression (molecular interaction), ultrasound may increase blood flow through the dilation of capillaries (structural intervention) and the enhancement of angiogenesis (cellular interaction). It is generally believed that greater blood flow serves as a principal factor in the acceleration of fracture healing. Indeed, one of the main biological goals of the inflammatory response is to reestablish blood flow to the injured area.
The orthopaedic literature contains several references to alternative healing methods based in most cases, on the therapeutic methods of various forms of electricity, on extracorporal shock wave therapy, and on ultrasound. These methods have been developed to accelerate the healing process of fresh fractures and to treat delayed union and pseudarthroses. These methods have not as yet been widely used, and one reason for this may be the fact that these publications only include a small number of cases. Conversely, there are only a few publications reporting on the surgical treatment of delayed union and non-union on large numbers of patients.
Low-intensity pulsed ultrasound has been principally investigated as a technique to accelerate healing of fresh fractures, but more recently as a treatment of fracture non-unions [30–33]. Ultrasound can be delivered non-invasively to the skin surface overlying the fracture site. Ultrasound treatment can be self-administered with one daily 20-min treatment, continuing until the fracture has healed. The mechanism of action at the cellular level is not precisely known but is thought to be related to a mechanical effect on cell deformation or indirectly by an electrical effect caused by cell deformation [31]. The ultimate effect on fracture healing may be mediated by enhanced vascularity at the fracture site or enhanced chondrocyte maturation [32].
In this study comparing the placebo group (11 patients) with the active group (10 patients), non-union healing was accelerated by 38 days.
Our data analysis suggests that ultrasound therapy may be beneficial to the healing of non-union of the scaphoid after treatment by vascularised pedicle bone graft. This finding is of considerable importance because treatment with a low-intensity pulsed ultrasound signal may reduce healing time and could yield substantial cost savings and decreased disability associated with delayed union and non-union of scaphoid fractures. It is important to limit the conclusion of the present study to the specific type of fracture studied and to the specific surgical treatment used. Further clinical trials are needed to determine the optimal role of ultrasound therapy in fracture healing.
References
Kawai H, et al (1988) Pronator quadratus pedicled bone graft for old scaphoid fractures. J Bone Joint Surg 70:829–831
Rodríguez O, Chong J, Monreal R (2004) Stimulation of tissue healing by ultrasound: physical mechanisms of action. AIP Conf Proc 724(1):106
Huntington TW (1905) Case of bone transference: use of a segment of fibula to supply a defect in the tibia. Ann Surg 41:249–251
Roy-Camille R (1965) Fractures et pseudarthroses du scaphoide moyen: utilisation d’un greffo pedicule. Actual Chir Ortho R Poincare 4:197–214
Barth A (1895) Histologische untersuchungen über knochenimplantationen. Beitr Pathol Anat Allg Pathol 17:65–142
Maylia E, Nokes LD (1999) The use of ultrasonics in orthopaedics—a review. Technol Health Care 7:1–28
Ziskin MC (1987) Applications of ultrasound in medicine—comparison with other modalities. In: Rapacholi MH, Grandolfo M, Rindi A (eds) Ultrasound: medical applications, biological effects, and hazard potential. Plenum Press, New York, pp 49–59
Wells PNT (1985) Surgical applications of ultrasound. In: Nyborg WL, Ziskin MC (eds) Biological effects of ultrasound. Churchill Livingstone, New York, pp 157–167
Moed BR, Kim EC, van Holsbeeck M, Schaffler MB, Subramanian S, Bouffard JA, Craig JG (1998) Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: histologic correlation using a canine model. J Orthop Trauma 12:200–205
Moed BR, Subramanian S, van Holsbeeck M, Watson JT, Cramer KE, Karges DE, Craig JG, Bouffard JA (1998) Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: clinical results. J Orthop Trauma 12:206–213
St John Brown R (1984) How safe is diagnostic ultrasonography? J Can Med Assoc 131:307–311
Corradi C, Cozzolino A (1952) [The action of ultrasound on the evolution of an ex-perimental fracture in rabbits]. Minerva Ortop 55:44–45
Corradi C, Cozzolino A (1953) Ultrasound and bone callus formation during function. Arch Ortop 66:77–98
Dyson M, Brookes M (1983) Stimulation of bone repair by ultrasound. Ultrasound Med Biol Suppl 2:61–66
Xavier CAM, Duarte LR (1983) Stimulation of bone callus by ultrasound. Rev Brasil Ortop 18:73–80
Reuter U, Strempel F, John F, Knoch HG (1984) Modification of bone fracture healing by ultrasound in an animal experimental model. Z Exp Chir Transplant Kunstliche Organe 17:290–297
Reuter U, Strempel F, John F, Dürig E (1987) Modification of fracture healing by ultrasonics in an animal model. 2. Radiologic and histologic results. Z Exp Chir Transplant Kunstliche Organe 20:294–302
Duarte LR (1983) The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg 101:153–159
Klug W, Franke WG, Schulze M (1986) Animal experimental scintigraphic obser-vations of the course of secondary fracture healing without and with ultrasound stimulation. Z Exp Chir Transplant Kunstliche Organe 19:115–185
Klug W, Franke WG, Knoch HG (1986) Scintigraphic control of bone-fracture healing under ultrasonic stimulation: an animal experimental study. Eur J Nucl Med 11:494–497
Pilla AA, Mont MA, Nasser PR, Khan SA, Figueiredo M, Kaufman JJ, Siffert RS (1990) Non-invasive low-intensity pulsed ultrasound accelerates bone healing in the rabbit. J Orthop Trauma 4:246–453
Chapman IV, MacNally NA, Tucker S (1980) Ultrasound-induced changes in rates of influx and efflux of potassium ions in rat thymocytes in vitro. Ultrasound Med Biol 6:47–58
Ryaby JT, Bachner EJ, Bendo JA, Dalton PF, Tannenbaum S, Pilla AA (1989) Low intensity pulsed ultrasound increases calcium incorporation in both differentiating cartilage and bone cell cultures. Trans Orthop Res Soc 14:15
Ryaby JT, Mathew J, Pilla AA, Duarte-Alves P (1991) Low-intensity pulsed ultrasound modulates adenylate cyclase activity and transforming growth factor beta synthesis. In: Brighton CT, Pollack SR (eds) Electromagnetics in medicine and biology. San Francisco Press, San Francisco, pp 95–100
Ryaby JT, Mathew J, Duarte-Alves P (1992) Low intensity pulsed ultrasound affects adenylate cyclase activity and TGF-B synthesis in osteoblastic cells. Trans Orthop Res Soc 7:590
Parvizi J, Wu CC, Lewallen DG, Greenleaf JF, Bolander ME (1999) Low-intensity ultrasound stimulates proteoglycan synthesis in rat chondrocytes by increasing aggrecan gene expression. J Orthop Res 17:488–494
Kokubu T, Matsui N, Fujioka H, Tsunoda M, Mizuno K (1999) Low intensity pulsed ultrasound exposure increases prostaglandin E2 production via the induction of cyclooxygenase-2 mRNA in mouse osteoblasts. Biochem Biophys Res Commun 256:284–287
Ito M, Azuma Y, Ohta T, Komoriya K (2000) Effects of ultrasound and 1,25– dihydroxyvitamin D3 on growth factor secretion in co-cultures of osteoblasts and endothelial cells. Ultrasound Med Biol 26:161–166
Rawool D, Goldberg B, Forsberg F, Winder A, Talish R, Hume E Power (1998) Doppler assessment of vascular changes during fracture treatment with low-intensity ultrasound. Trans Radiol Soc North Am 83:1185
Xavier C, Duarte L (1987) Treatment of non-unions by ultrasound stimulation: first clinical applications. Read at the meeting of the Latin-American Orthopedic Association and the annual meeting of the American Academy of Orthopaedic Surgeons, San Francisco, January 25 1987
Duarte L, Choffie M (1994) Low intensity pulsed ultrasound and effects on ununited fractures. Presented at the Orthopaedic Health Conference, University Hospital, University of Sao Paulo, Brazil, June 1994
Hadjiargyrou K, McLeod K, Ryaby J, Rubin C (1998) Enhancement of fracture healing by low intensity ultrasound. Clin Orthop Oct 355:216–219
Mayr S, Wagner M, Ecker M, Ruter A (1999) Ultrasound therapy for non-unions. Three case reports. Unfallchirug 102(3):191–196
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ricardo, M. The effect of ultrasound on the healing of muscle-pediculated bone graft in scaphoid non-union. International Orthopaedics (SICO 30, 123–127 (2006). https://doi.org/10.1007/s00264-005-0034-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-005-0034-2