Abstract
We studied the biochemical characteristics of human knees with deficient anterior cruciate ligaments (ACL) and analysed their relationship to the time after ligamentous injury. Thirty-two patients with isolated ACL-injured knees and six healthy volunteers were enrolled. Synovial fluid samples were centrifuged after aspiration during arthroscopic examination, and aliquots of supernatant were frozen and stored at −80°C. The samples were analysed for interleukin (IL)-1β, tumour necrosis factor (TNF)-α, IL-6, matrix metalloproteinase (MMP)-3, and tissue inhibitor of metalloproteinase (TIMP)-1 using commercially available sandwich enzyme-linked immunosorbent assay. In fluid from ACL-injured knees, the average concentrations of IL-6, MMP-3 and TIMP-1 were highly elevated in comparison with normal controls. There was a statistically significant correlation between the concentrations of MMP-3 and IL-6. The IL-6 and TIMP-1 concentrations were interrelated. The concentration of MMP-3 remained high, independent of the duration since the injury, whereas the TIMP-1 and IL-6 levels decreased. The results suggest that the timing of the treatment of an ACL-injured knee might be of importance.
Résumé
Nous avons étudié les caractéristiques biochimiques des genoux humains avec un ligament croisé antérieur défectueux (LCA) et avons analysé leur rapport au temps après la lésion ligamentaire. Trente-deux malades avec une lésion isolée du LCA et six volontaires sains ont été enrôlés. Des échantillons du liquide synovial ont été centrifugés après aspiration pendant un examen arthroscopique, et le surnageant a été congelé et entreposé à −80 degrés Celsius. Les échantillons ont été analysés pour l'interleukine (IL)-1β, le facteur de nécrose tumorale (TNF), IL 6, la métalloprotéinase de la matrice (MMP)-3 et l'inhibiteur tissulaire de la métalloproteinase (TIMP)-1. Dans le liquide des genoux traumatisés les concentrations moyennes de IL 6, MMP-3 et TIMP-1 étaient très élevées en comparaison des contrôles normaux. Il y avait une corrélation statistiquement significative entre les concentrations de MMP-3 et IL 6. Les concentrations de IL 6 et TIMP-1 ont été mises en corrélation. La concentration de MMP-3 est restée élevée, indépendante du délai de la lésion ligamentaire, alors que le niveau de TIMP-1 et IL6 a diminué. Les résultats suggèrent que le délai d'intervention sur un genou ayant une rupture du croisé antérieur peut être un élément d'importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The natural course of the anterior cruciate ligament (ACL) deficient-knee has been well documented. Injury to the ACL often leads to the development of degenerative osteoarthritis (OA) [8, 17]. In addition, patients with ACL injury and posttraumatic OA are on average 15–20 years younger than patients with primary OA when they become symptomatic [5, 17]. Restoring knee-joint stability through ACL reconstruction has not been shown to decrease the incidence of posttraumatic OA in this patient population. However, some reports indicate that 50–60% of patients with ACL-reconstructed knees have radiographic evidence of OA after five years [1, 3]. These data suggest that something other than, or in addition to, a biomechanical disturbance is responsible for the osteoarthritic changes seen after ACL rupture.
Various cytokines are involved in the aetiology of rheumatoid arthritis and osteoarthritis, and clinical trials using cytokines as arthritis markers for the staging of diseases have been performed [19]. Cartilage destruction progresses due to the activation of various proteases, and matrix metalloproteinases (MMPs) are key enzymes in cartilage destruction [7]. Studies concerning interactions between various cytokines and MMPs have also been carried out, and the mechanisms responsible for cartilage destruction and its prevention are being clarified. However, few reports [11, 12] exist concerning the concentrations of MMPs and tissue inhibitors of metalloproteinase (TIMP) in human knees with ACL injury, and there has been no study on their relationships with cytokines.
The purpose of this study was to investigate the biochemical characteristics of human knees with ACL injury, including the concentrations of cytokines, MMPs, and TIMP in synovial fluid (SF), and to evaluate their relationships with each other. We also studied the relationships of their concentration profiles with the time after ACL injury to evaluate the appropriate timing for the treatment of ACL injury based on biochemical parameters.
Materials and methods
Patient profiles
We included 32 knees with complete ACL rupture confirmed arthroscopically in our study. The patients were 20 males and 12 females with a mean age of 26.5 (17–42) years. The patients did not have any previous history of knee injury. The mean period from injury to the sampling of SF was 32 (2–134) weeks. Although injury to the meniscus was noted arthroscopically in eight knees (lateral in seven, medial in one), no ligament other than the ACL was damaged. Radiography of the knee was normal in 29 knees and grade I in three knees according to the International Knee Documented Committee (IKDC) scale [9]. None of the patients had received intra-articular injection of steroid or hyaluronic acid.
Control samples of six healthy volunteers comprising five males and one female with no history of knee injury with a mean age 24.3 (15–37) years were also evaluated (Table 1).
Experimental protocol
Synovial fluid was aseptically sampled from the patients and volunteers. The samples were immediately centrifuged at 8,000 rpm for ten minutes, and the supernatants were preserved at −80°C after the removal of cell components and tissue debris. The interleukin (IL)-1β, tumour necrosis factor (TNF)-α, IL-6, MMP-3, and TIMP-1 concentrations were determined by sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Amersham Biosciences, Piscataway, NJ, USA).
Statistics
A nonparametric method, the Mann-Whitney U test, was used to calculate statistical differences between unpaired groups. The two-tailed Student’s t-test was used for comparisons within and between the groups, and Pearson’s correlation coefficients were calculated. All statistical procedures were performed using Stat-View software. A P value of less than 0.05 was considered to indicate statistical significance.
Results
Characteristics of cytokine levels in ACL-injured knees
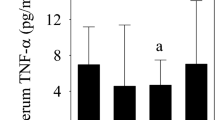
The IL-6, MMP-3, and TIMP-1 concentrations were higher in the knees with ruptured ACL than in the normal knees. TNF-α was not detected in the normal knees but was noted at a mean level of 10.4 pg/ml in the knees with ruptured ACL. The IL-1β concentration was below the detectable range in all normal knees and in 25 knees with ruptured ACL (Table 2).
In the knees with ruptured ACL, the synovial fluid MMP-3 concentration was significantly correlated with the IL-6 concentration among various cytokines (P<0.01), but not with IL-1β and TNF-α (Table 3). The MMP-3 concentration showed a strong positive correlation (P<0.001) with the TIMP-1 concentration (Fig. 1), and a significant positive correlation (P<0.01) was also noted between the IL-6 and TIMP-1 concentration (Fig. 2). Moreover, in the ruptured ACL knees the synovial fluid MMP-3 concentration was elevated regardless of the time after injury. The TIMP-1 concentration tended to decrease with time, but it remained high regardless of the time after injury without a statistically significant decrease. The IL-6 concentration was high in many knees within 50 weeks after ACL injury but decreased thereafter (P<0.01) (Fig. 3).
The relationship between cytokine levels and other joint disorders
In the ACL-injured knees, the mean concentrations of each cytokine in the eight cases with meniscal tears were 42,250.0±21,942.3 ng/ml for MMP-3, 1,354.0±1,066.6 ng/ml for TIMP-1, and 2,201.3±1,252.9 ng/ml for IL-6. In contrast, the mean concentrations in 24 knees without meniscal tear were 27,617.5±23,336.6 ng/ml for MMP-3, 1,232.2±955.2 ng/ml for TIMP-1, and 1,057.5±1,435.0 ng/ml in IL-6. The IL-6 concentration in the cases of a meniscal tear was significantly higher than in the cases without tear (P<0.05). The mean concentration of each cytokine in the three cases with radiographic degenerative changes was 44,933.3±28,517.8 ng/ml for MMP-3, 730.0±258.7 ng/ml for TIMP-1, and 438.7±370.6 ng/ml for IL-6. On the other hand, in the 29 cases without radiographic changes they were 29,862.8±23,096.6 ng/ml for MMP-3, 1,317.8±999.4 ng/ml for TIMP-1, and 1,437.1±1,502.5 ng/ml for IL-6, respectively. There was no significant difference between radiographic changes and cytokine concentrations.
Discussion
MMP-3, also called proteoglycanase, is a key enzyme involved in cartilage destruction [7] because it has the ability to decompose important molecular structures of the cartilage matrix, such as aglycan, type IX collagen, and fibronectin, and is involved in the activation of other MMPs [13]. Many cells including synovial fibroblasts produce MMP-3. Chondrocytes also have the ability to produce MMP-3, and its production is induced and promoted by the action of cytokines such as IL-1 and TNF [4, 18]. Furthermore, immunohistochemical studies have shown that a positive correlation exists between the intensity of MMP-3 staining and matrix degradation in cartilage tissues [16]. Since MMP-3 plays a central role in the destruction of cartilage matrix, clinical attempts to use it as an arthritis marker for the staging of arthritic diseases have been reported [19]. The MMP-3 concentration in synovial fluid is higher in patients with rheumatoid arthritis than in those with osteoarthritis [20], suggesting an association with the degree of progression of synovitis and joint destruction. Lohmander et al. also found that the MMP-3 concentration in the synovial fluid of patients with osteoarthritis of the knee increases as the disease progresses, suggesting that MMP-3 is an index of cartilage destruction [11]. TIMP, on the other hand, acts as a regulator of MMP activity and as a protective factor against metastasis and invasion of malignant neoplasms [2, 10]. In cartilage, TIMP-1 not only prevents matrix destruction as an MMP-inhibiting protein but also contributes to matrix maintenance by promoting cell proliferation.
In this study, the synovial fluid MMP-3 concentration was markedly higher in the knees with ruptured ACL than in the normal knees, indicating that an increase in MMP-3 may have a destructive effect on the cartilage matrix. Synovial fluid MMP-3 concentration is increased in the knees with ruptured ACL similarly to knees with osteoarthritis and is markedly higher than in normal knees [11], and our results support this. Similarly, the TIMP-1 concentration was markedly increased in the knees with ruptured ACL compared with the normal knees. In addition, a strong positive correlation was observed between the MMP-3 and TIMP-1 concentration. Lohmander et al. found a correlation between the MMP-3 and TIMP-1 concentrations with a molar ratio of 1 or less in normal knees [11], suggesting a protective reaction of TIMP-1 against MMP-3. In this study, the MMP-3/TIMP-1 ratio (molar ratio) was markedly increased to 17 in the knees with a ruptured ACL. This imbalance between MMP-3 and TIMP-1 may make the environment of the knee with a ruptured ACL more susceptible to cartilage destruction.
Of the cytokines examined in this study, only IL-6 was increased in the knees with a ruptured ACL compared with the normal knees. IL-6 was discovered as a cytokine that promotes B-cell differentiation and proliferation and induces antibody production, but was subsequently shown to be produced by a variety of cells including chondrocytes and synovial cells [6]. Since IL-6 has biological activities similar to those of IL-1, such as inducing the production of pyrogenic factors and acute phase proteins, and is elevated in morbid synovial fluid, such as that of patients with rheumatoid arthritis, it initially attracted attention due to its possible relationship with cartilage destruction. In addition, IL-6 is a pre-inflammatory cytokine, and an IL-6 receptor antagonist is being used as an anti-inflammation drug for RA [14, 15]. In this study, the IL-6 concentration was high in many knees within 50 weeks after ACL injury but decreased thereafter, while the MMP-3 concentration was elevated regardless of the time after injury. We suggest that, to prevent the development of OA after ACL injury, it is important to start treatment of the ACL-injured knee as soon as possible because cytokines such as IL-6 and MMP-3, induce the cartilage destruction. However, this study was designed in a cross-sectional manner and was performed at random times after ACL injury. Changes in cytokines levels with time are still unknown. In the future, a biochemical study based on cohort analysis after ACL injury would clarify the relationship between the ACL-injured knee and the development of OA.
References
Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR (1994) Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med 22:632–644
Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF Jr (1989) Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest 84:678–685
Ferretti A, Conteduca F, De Carli A, Fontana M, Mariani PP (1991) Osteoarthritis of the knee after ACL reconstruction. Int Orthop 15:367–371
Frisch SM, Ruley HE (1987) Transcription from the stromelysin promoter is induced by interleukin-1 and repressed by dexamethasone. J Biol Chem 262:16300–16304
Graham GP, Fairclough JA (1988) Early osteoarthritis in young sportsmen with severe anterolateral instability of the knee. Injury 19:247–248
Guerne PA, Carson DA, Lotz M (1990) IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol 144:499–505
Hasty KA, Reife RA, Kang AH, Stuart JM (1990) The role of stromelysin in the cartilage destruction that accompanies inflammatory arthritis. Arthritis Rheum 33:388–397
Hawkins RJ, Misamore GW, Merritt TR (1986) Followup of the acute nonoperated isolated anterior cruciate ligament tear. Am J Sports Med 14:205–210
Hefti F, Muller W, Jakob RP, Staubli HU (1993) Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc 1:226–234
Hicks NJ, Ward RV, Reynolds JJ (1984) A fibrosarcoma model derived from mouse embryo cells: growth properties and secretion of collagenase and metalloproteinase inhibitor (TIMP) by tumor cell lines. Int J Cancer 33:835–844
Lohmander LS, Hoerrner LA, Lark MW (1993) Metalloproteinases, tissue inhibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum 36:181–189
Lohmander LS, Roos H, Dahlberg L, Hoerrner LA, Lark MW (1994) Temporal patterns of stromelysin-1, tissue inhibitor, and proteoglycan fragments in human knee joint fluid after injury to the cruciate ligament or meniscus. J Orthop Res 12:21–28
Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJ (1987) Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J 248:265–268
Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, Nishimoto N (2003) Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum 48:1521–1529
Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Hashimoto J, Azuma J, Kishimoto T (2004) Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 50:1761–1769
Okada Y, Shinmei M, Tanaka O, Naka K, Kimura A, Nakanishi I, Bayliss MT, Iwata K, Nagase H (1992) Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest 66:680–690
Sherman MF, Warren RF, Marshall JL, Savatsky GJ (1988) A clinical and radiographical analysis of 127 anterior cruciate insufficient knees. Clin Orthop 227:229–237
Shinmei M, Okada Y, Masuda K, Naramatsu M, Kikuchi T, Harigai M, Shimomura Y (1990) The mechanism of cartilage degradation in osteoarthritic joints. Semin Arthritis Rheum 19:16–20
Thonar EJ, Shinmei M, Lohmander LS (1993) Body fluid markers of cartilage changes in osteoarthritis. Rheum Dis Clin North Am 19:635–657
Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y (2000) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 59:455–461
Acknowledgements
This study was partly presented at the 69th annual meeting of the American Academy of Orthopaedic Surgeons. We thank Kiichi Osawa, Technician, for his help in preparing the figures. This study was supported by Grant of Japan Sports Medicine Foundation, in 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Higuchi, H., Shirakura, K., Kimura, M. et al. Changes in biochemical parameters after anterior cruciate ligament injury. International Orthopaedics (SICO 30, 43–47 (2006). https://doi.org/10.1007/s00264-005-0023-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-005-0023-5